Abstract
Background:
Ventriculoperitoneal (VP) shunts are among the most frequently performed operations in the management of hydrocephalus. Hepatic cerebrospinal fluid (CSF) pseudocyst is a rare but important complication in patients with a VP shunt insertion. In addition to presenting our own case, we performed a PubMed search to comprehensively illustrate the predisposing factors, clinical picture, diagnostic methods, and surgical treatment. This article represents an update for this condition.
Case Description:
A 40-year-old male was admitted to a hospital complaining of fever, abdominal distention, and pain. He had undergone a VP shunt for communicating hydrocephalus caused by a head trauma one year earlier. Laboratory studies showed liver enzymes alterations, and imaging studies demonstrated a well-defined intraaxially hepatic cyst with the shunt catheter placed inside. Staphylococcus epidermis was cultured via CSF. After removing the VP shunt and an adequate antibiotic treatment, the complication of hepatic CSF pseudocyst was resolved.
Conclusion:
Hepatic CSF pseudocyst is a rare complication of a VP shunt. Once the diagnosis is verified and if the CSF is sterile, just simply remove the peritoneal catheter and reposition a new one in the abdomen. We believe that it is not necessary to remove or aspirate the hepatic intraaxial pseudocyst, because of the risk of bleeding. In case of CSF infection, the VP shunt can be removed and/or an external derivation can be made, and after treatment with antibiotics, a new VP shunt is placed in the opposite side of the peritoneum.
Keywords: Cerebrospinal fluid pseudocyst, hepatic pseudocyst, hydrocephalus, ventriculoperitoneal shunt complication
INTRODUCTION
Ventriculoperitoneal (VP) shunt is the standard therapy for the management of hydrocephalus, because the peritoneal cavity is considered the best site for cerebrospinal fluid (CSF) absorption. However, as more patients with hydrocephalus survive and live longer, more complications, which may be seen after VP shunt insertion, are identified. Between approximately 5%[20] and 47%[10] of abdominal complications by VP shunt insertion are reported. One of the less frequent but important complications is the abdominal CSF pseudocysts. Even more uncommon are pseudocysts of parenchymal organs such as the liver.[2,4,5,7,8,14,15,17,18,19,25,27,32,33,34] or spleen.[21]
This paper describes a case of hepatic pseudocysts after VP shunt insertion and presents the clinical picture, radiological findings, and surgical treatment. The literature reviewed in this study was retrieved from the author's files and PubMed (search conducted on June 30, 2013) using the keywords “intraabdominal pseudocyst,” “abdominal cerebrospinal fluid pseudocyst,” “peritoneal pseudocyst,” “intraperitoneal pseudocyst,” “hepatic pseudocyst,” “hepatic cerebrospinal fluid pseudocyst,” “intrahepatic cyst,” and “shunt complications.” Details of history, clinical features, radiological finding, CSF culture, surgical treatment, as well as follow-up period for the reported cases with this condition were analyzed [Table 1].
Table 1.
Summary of reported hepatic pseudocysts associated with ventriculoperitoneal shunt
CASE REPORT
A 40-year-old male patient presented to the hospital with a 7-day fever, abdominal distention, and pain. His medical and surgical history includes VP shunt placement following a head injury from a motor vehicle crash one year earlier. The patient had no history of malignancy and pancreatic or liver disease. An initial physical examination revealed low-grade fever with mild right-upper-quadrant discomfort and a slightly distended abdomen. Bowel sounds were normal, and there were no peritoneal signs. A central nervous system (CNS) examination was within normal limits. Blood test on admission showed mild leukocytosis (WBC, 13,200/mm3), anemia (hemoglobin, 9.0 g/dL; hematocrit, 26.9%), elevation of C-reactive protein levels (15.70 mg/dl) and erythrocyte sedimentation rate (110 mm/h), as well as glutamic oxaloacetic transaminase levels (255 U/L), glutamic pyruvic transaminase levels, (186 U/L) gamma-glutamyl transferase levels (275 U/L), and alkaline phosphatase levels (162 U/L). Bilirubin was normal. Examination of the CSF indicates VP shunt infection, and a microbiological analysis showed Staphylococcus epidermidis. The ultrasonographical and abdominal computed tomography (CT) evaluation of the abdomen showed a cystic lesion in the hepatic segment V measuring 81 × 74 × 62 mm, with the shunt catheter placed inside [Figures 1 and 2]. The cystic mass did not show contrast enhancement. After this, we took out the proximal and distal VP shunt catheter from the previous cranial and abdominal incision (right lower abdominal quadrant), and an adequate antibiotic treatment was administrated for 14 days. A follow-up brain CT scan revealed no enlargement of the ventricular size, and a CT of the abdomen showed a regression of the cystic form [Figures 3] with a marked decrease in abdominal symptoms within 3 days and normalization of the abdominal condition within 10 days after the removal of the VP shunt.
Figure 1.
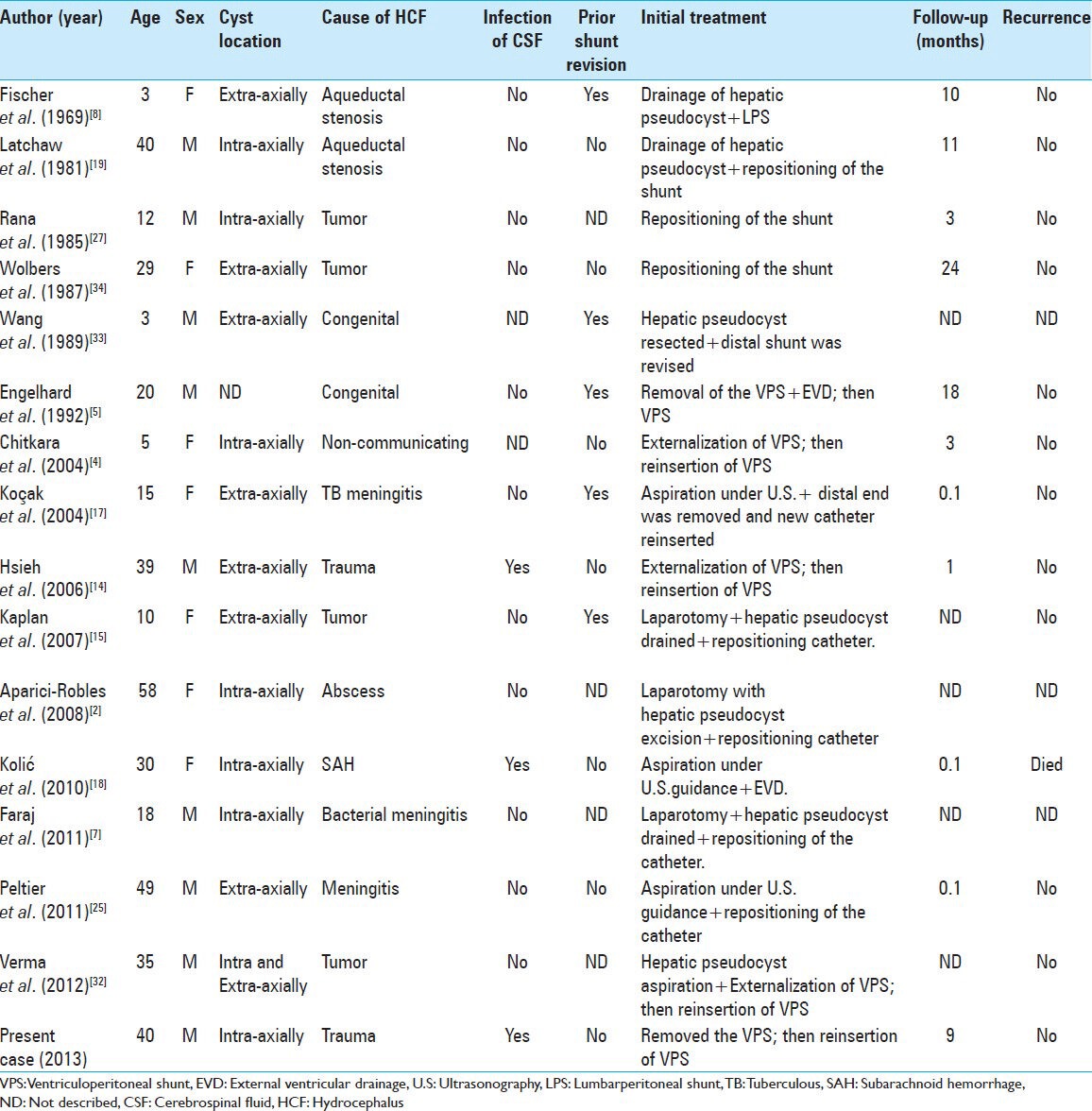
The CT topogram shows that the distal part of VP shunt is lying in the right-upper-quadrant superimposed on the hepatic silhouette
Figure 2.
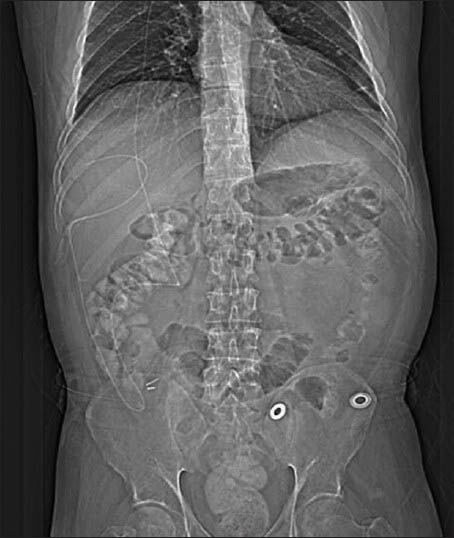
(a) Abdominal CT scans with oral contrast demonstrates an intrahepatic fluid collection (asterisk) measuring 8.1 × 7.4 cm in the right hepatic lobe. (b) The VP shunt catheter tip is seen inside the pseudocyst (arrow)
Figure 3.
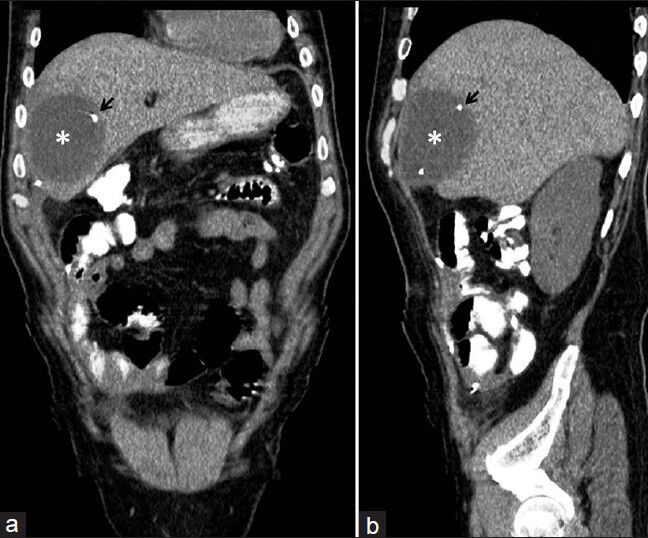
(a) Preoperative and (b) follow-up abdominal CT scan at 19 days after VP shunt removal. Axial abdominal CT scan shows the complete resolution of the hepatic CSF pseudocyst
Two weeks after the surgical procedure, a subsequent cranial CT scan showed bilateral ventricular dilatation, and after three consecutive negative CSF cultures, we performed a shunt reinsertion on the opposite side of the peritoneum. On his 20th day in the hospital, the patient was discharged with complete resolution of previous symptoms. Liver enzymes returned to normal levels, and no clinical recurrence of the hepatic CSF pseudocyst was evident during the 9-month follow-up period.
DISCUSSION
Since Harsh described in 1954 a periumbilical cyst associated among 12 ventriculofallopian shunts,[11] several reports about this issue were published in different countries. According to international literature, abdominal CSF pseudocysts account for about 0.25%[20,28] to 10%[3] of all VP shunts. Otherwise, a hepatic pseudocyst secondary to VP shunt is very rare. To the best of our knowledge, only 15 cases have been reported in the international literature to date.[2,4,5,7,8,14,15,17,18,19,25,27,32,33,34]
Many authors have reported abdominal CSF pseudocysts in pediatric patients because hydrocephalus is more common in children. However, Rainov et al.[26] reported a child–adult ratio of 1.8:1. In our study, there were seven women and eight men who were aged from 3 to 58 years (mean age 24.4 years) at the time of presentation. Six out of fifteen (40%) patients were aged 15 or younger.
Hepatic CSF pseudocyst is classified as intraaxial when the tip of the shunt can be lodged in the liver and can cause intraaxial pseudocyst formation in the liver parenchyma,[4] or extraaxial when the tip of the catheter can penetrate Glisson's capsule causing subcapsular pseudocyst formation.[33] In the review of literature cases, six patients (40%) with hepatic CSF pseudocysts are intraaxial, as in our case, where the pseudocyst is deep within the liver parenchyma. Verma et al.[32] reported a case with only the small part of the cyst lying within the liver parenchyma and with the larger part lying outside; therefore, what is described is a juxtahepatic CSF pseudocyst with an extension within the liver.
Although the etiology of abdominal CSF pseudocyst is not clear, different authors attribute cyst formation, with no clinical or scientific support, to several predisposing factors-an inflammatory process, either sterile or infectious, being the most frequent.[3,6,8,9] Other predisposing causes are the following: (1) previous abdominal surgeries or multiple revisions of the shunt in the abdomen, (2) malabsorption of CSF, (3) increase of proteins in CSF,[4] allergic reaction to silicone or ethylene oxide, and (5) history of intracranial tumor.[12,15,16,26] All of them could be responsible for the formation of peritoneal adhesions that prevent CSF reabsorption and can cause cyst formation. Consequently, the oncotic pressure of the cyst fluid increases, interstitial fluid passes into the pseudocyst, and the cyst increases in size, which can be formed between a few days to several years after VP shunt implantation.[6] The most important factor causing hepatic CSF pseudocyst is the migration of the peritoneal tip of the shunt to the liver surface, causing the focal injury to the liver and its chronic irritation.[14] A review of the cases presented in the literature reveals that prior shunt revision was found only in 45% of the cases. Six hepatic CSF pseudocyst patients (55%) did not report history of shunt revision, and four patients did not describe this information.
In Mobley's series regarding abdominal CSF pseudocyst,[22] the most common cause of hydrocephalus for which the shunt was inserted were intraventricular hemorrhage, congenital hydrocephalus, Dandy–Walker cyst, and myelomeningocele. In contrast, in patients with hepatic CSF pseudocyst, including our case, the etiologies of hydrocephalus were tumor (25%), meningitis (25%), aqueductal stenosis (12.5%), congenital hydrocephalus (12.5%), trauma (12.5%), and intraventricular hemorrhage (6%). There was no information in 6% of the cases.
The most common organisms isolated from the CSF culture in patients with abdominal CSF pseudocyst include S. epidermidis and S. aureus. A few isolated cases with Escherichia coli,[6,22] Salmonella typhi,[13] Propionibacterium acnes, Streptococcus faecalis, Acinetobacter sp.,[16] or Pseudomonas aeruginosa were reported.[29] In a retrospective analysis of 27 consecutive patients treated for abdominal CSF pseudocyst, 44% of the patients had a positive culture on presentation.[28] However, in 16 cases of patients with hepatic CSF pseudocyst published in the literature, including our case, only three cases (19%) were reported to have CNS infection on presentation, two cases with CSF culture demonstrated S. epidermidis, and one case demonstrated S. aureus-all cases involved adult patients.[14,18] For this condition, the data suggest that the incidence of infection in hepatic CSF pseudocyst is lower in children than in adults. Otherwise, the abdominal CSF pseudocyst incidence of infection is greater in children than in adults.[24]
Although there are no pathognomonic signs in abdominal CSF pseudocyst, the most common presentations are diffuse abdominal pain, but later more severe and localized in the distal end of the shunt catheter, abdominal distension, often with palpable mass, constipation, intestinal obstruction, and occasional nausea and vomiting rather than neurological or infectious signs.[9] CNS complaints usually appear days or weeks after such abdominal symptoms.[23] The continued slow absorption of CSF, although inadequate to prevent pseudocyst formation, delays CNS symptoms.[1] Our revision found abdominal symptoms and signs (pain, abdominal distention, and right upper quadrant tenderness) in almost all of the cases (14/15), whereas less than 40% of the hepatic CSF pseudocyst patients presented symptoms due to shunt malfunction. However, the clinical presentations in four out of six children with abdominal symptoms were associated with a headache, vomiting, and a decreased level of consciousness related to a shunt malfunction.
The accurate diagnosis of hepatic CSF pseudocysts is difficult based on laboratory examination alone. Several patients with this condition, as in our case, show markedly elevated levels of glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and alkaline phosphatase.[14,27,33] These abnormal findings suggested the presence of an anicteric cholestasis along with hepatic cell necrosis.
For radiological diagnosis, ultrasound is the method of choice because it is noninvasive, easy to perform, inexpensive, and sufficient for a satisfactory diagnosis. Imaging findings may show displacement of the tip of the peritoneal catheter and a large fluid-filled collection. Besides, the double echo of the catheter walls may produce the so-called railroad sign within the pseudocyst.[26] An abdomen CT scan provides a more accurate diagnosis, especially when these abdominal CSF pseudocysts are large and deform the normal architecture of the abdomen.[7,14] In extraaxially growing cases, the hepatic tissue cannot be identified in the periphery wall of the pseudocyst.[15] At times, a CT scan may be a better first choice in the initial evaluation to exclude other causes, such as abdominal abscess,[14] appendicitis, volvulus, or diverticulitis.[1] Furthermore, such hepatic CSF pseudocysts may cause problems in diagnosis where the echinococcosis disease of the liver is endemic due to similarities in clinical presentation and radiological appearance.[7] In two hepatic CSF pseudocyst cases, a radionuclide shunt was performed to show the flow of activity from the shunt into the loculated CSF collection.[27,33]
Regarding the treatment in abdominal CSF pseudocysts, there has been no consensus for handling the VP shunt distal catheter as a pseudocyst itself since the number of cases are limited and because the treatment changes depending on the characteristics of patients, the experience of the surgeon, and the findings during the operation.[31] After the appropriate treatment of infection, the most common definitive procedures include six main categories: (1) repositioning of the distal catheter into the opposite site of the peritoneum, (2) repositioning of the distal catheter into another nonperitoneal space (the atrium, the pleural space, or the gallbladder), (3) exploratory laparotomy with lysis of adhesions and repositioning of the peritoneal catheter in the opposite side of the abdomen, (4) laparoscopic drainage and repositioning of the catheter tip, (5) abdominal CSF pseudocyst aspiration only, and (6) shunt removal or disconnection.[6,22]
In our review of 15 cases, most of the hepatic CSF pseudocyst was treated by cyst aspiration and peritoneal reimplantation of the distal catheter at a different side of the abdomen. In the presence of CSF infection, all cases were treated by external drainage and treatment of infection. In this instance, a new shunt was placed after getting sterile CSF cultures. In our case report, there was an infection in the shunt, and the cyst grew intraaxially. Therefore, we treated the infection with the removal of the entire VP shunt, and once we verified the absence of CSF infection and the hepatic CSF pseudocyst, we placed a new catheter in a different intraabdominal location.
As was in our case, the hepatic CSF pseudocyst resorbed spontaneously with the normalization of the abdominal symptoms, and liver enzymes returned to near-normal levels. In the case report by Hsieh et al.,[14] 3 weeks after removing VP shunt, an ultrasonography of the abdomen showed shrinkage of the hepatic pseudocyst, and one month later, it had completely disappeared. We believe that it is not necessary to remove or aspirate the hepatic intraaxial pseudocyst, because of the risk of bleeding, which may increase fibrosis in the area. It is enough to only remove the catheter tip because the cyst usually disappears.
With time, abdominal CSF pseudocysts can recur. This complication varies between 7.1%[26] and 62.5% (30). Erşahin et al.[6] reported 20% of recurrence, concluding that the reposition of the peritoneal catheter seems to carry the risk of recurrence. In contrast, Gaskill et al.[9] did not report pseudocyst recurrence despite the conversion of 58% of the initial shunts to subsequent VP shunt, with follow-up ranging from 3 months to 4 years. In all of the reviewed cases, there was no recurrence of the hepatic CSF pseudocyst following the treatment in a follow-up ranging from 1 to 24 months, and only one death occurred [Table 1]. In our case, no remnant of the hepatic CSF pseudocyst was evident on ultrasound during the 9-month follow-up period.
CONCLUSION
Hepatic pseudocyst is a rare complication of a VP shunt. In patients with abdominal symptoms, liver enzymes alterations, and surgical history of VP shunt insertion, hepatic CSF pseudocyst should be suspect, and thus, an ultrasound or abdominal CT scan should be mandatory. Once the diagnosis is verified and if the CSF is sterile, just simply remove the peritoneal catheter and reposition a new distal catheter in the abdomen. In case of CSF infection, the VP shunt can be removed and/or an external derivation can be made. Then, after treatment with antibiotics and three consecutive negative CSF cultures, a new VP shunt is placed in the opposite side of the peritoneum. In case of recurrence, laparoscopy approach appears to be effective in opening the liver cyst with the aspiration of its contents and the repositioning of the shunt catheter under direct visual control.
ACKNOWLEDGMENTS
The authors thank Carlos F. Dabdoub, MD, for his assistance in preparing this manuscript.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/162/123783
Contributor Information
Carlos B. Dabdoub, Email: carlosdabdoub@hotmail.com.
Emilio A. Fontoura, Email: emiliofontoura@uol.com.br.
Egmond A. Santos, Email: egmond.santos@bol.com.br.
Paulo C. Romero, Email: pcromero@bol.com.br.
Cristiano A. Diniz, Email: cristianodiniz@gmail.com.
REFERENCES
- 1.Anderson CM, Sorrells DL, Kerby JD. Intra-abdominal pseudocysts as a complication of ventriculoperitoneal shunts: A case report and review of the literature. Curr Surg. 2003;60:338–40. doi: 10.1016/S0149-7944(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 2.Aparici-Robles F, Molina-Fabrega R. Abdominal cerebrospinal fluid pseudocyst: A complication of ventriculoperitoneal shunts in adults. J Med Imaging Radiat Oncol. 2008;52:40–3. doi: 10.1111/j.1440-1673.2007.01909.x. [DOI] [PubMed] [Google Scholar]
- 3.Burchianti M, Cantini R. Peritoneal cerebrospinal fluid pseudocysts: A complication of ventriculoperitoneal shunts. Childs Nerv Syst. 1988;4:286–90. doi: 10.1007/BF00271925. [DOI] [PubMed] [Google Scholar]
- 4.Chitkara N, Rahul G, Singla SL, Sharma NK. Lower end of ventriculoperitoneal shunt embedding in liver parenchyma. Letter to the editor. Neurol India. 2004;52:405. [PubMed] [Google Scholar]
- 5.Engelhard HH, Miller FB. Abdominal pain resulting from cerebrospinal fluid pseudocyst and cholelithiasis. South Med J. 1992;85:851–2. doi: 10.1097/00007611-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Erºahin Y, Mutluer S, Tekeli G. Abdominal cerebrospinal fluid pseudocysts. Childs Nerv Syst. 1996;12:755–8. doi: 10.1007/BF00261593. [DOI] [PubMed] [Google Scholar]
- 7.Faraj W, Ahmad HH, Mukherji D, Khalife M. Hepatic cerebrospinal fluid pseudocyst mimicking hydatid liver disease: A case report. J Med Case Rep. 2011;5:475. doi: 10.1186/1752-1947-5-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer EG, Shillito J., Jr Large abdominal cysts: A complication of peritoneal shunts. Report of three cases. J Neurosurg. 1969;31:441–4. doi: 10.3171/jns.1969.31.4.0441. [DOI] [PubMed] [Google Scholar]
- 9.Gaskill SJ, Marlin AE. Pseudocysts of the abdomen associated with ventriculoperitoneal shunts: A report of twelve cases and a review of the literature. Pediatr Neurosci. 1989;15:23–6. doi: 10.1159/000120436. [DOI] [PubMed] [Google Scholar]
- 10.Ghidirim G, Mishin G, Zastavnitsky V, Brinza M. Laparoscopic management of associated abdominal complications of ventriculoperitoneal shunt: Case report. Eur Surg. 2010;42:184–6. [Google Scholar]
- 11.Harsh GR., 3rd Peritoneal shunt for hydrocephalus: Utilizing the fimbria of the fallopian tube for entrance to the peritoneal cavity. J Neurosurg. 1954;11:284–94. doi: 10.3171/jns.1954.11.3.0284. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Yokota A, Urasaki E, Tsujigami S, Shimono M. A case of abdominal CSF pseudocyst associated with silicone allergy. Childs Nerv Syst. 2004;20:761–4. doi: 10.1007/s00381-003-0904-0. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-Hernández JG, Martínez-Ordaz JL, Romero-Hernández T, Blanco-Benavides R. Abdominal pseudocyst in a patient with ventriculoperitoneal shunt. A case report. Cir Cir. 2004;72:401–3. [PubMed] [Google Scholar]
- 14.Hsieh CT, Pai CC, Tsai TH, Chiang YH, Su YH. Hepatic cerebrospinal fluid pseudocyst: A case report and review of the literature. Neurol India. 2006;54:86–8. doi: 10.4103/0028-3886.24717. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan M, Ozel SK, Akgun B, Kazez A, Kaplan S. Hepatic pseudocyst as a result of ventriculoperitoneal shunts: Case report and review of the literature. Pediatr Neurosurg. 2007;43:501–3. doi: 10.1159/000108795. [DOI] [PubMed] [Google Scholar]
- 16.Kariyattil R, Steinbok P, Singhal A, Cochrane DD. Ascites and abdominal pseudocysts following ventriculoperitoneal shunt surgery: Variations of the same theme. J Neurosurg. 2007;106:350–3. doi: 10.3171/ped.2007.106.5.350. [DOI] [PubMed] [Google Scholar]
- 17.Koçak A, Baysal T, Çaylı SR, Ateş O, Önal C. An unusual complication of ventriculo-peritoneal shunt: Cerebrospinal fluid cyst in liver. Eur J Radiol Extra. 2004;51:21–4. [Google Scholar]
- 18.Koliæ Z, Kukuljan M, Bonifačić D, Vukas D. CSF liver pseudocyst as a complication of a ventriculoperitoneal shunt. Wien Klin Wochenschr. 2010;122:641–4. doi: 10.1007/s00508-010-1474-2. [DOI] [PubMed] [Google Scholar]
- 19.Latchaw JP, Jr, Hahn JF. Intraperitoneal pseudocyst associated with peritoneal shunt. Neurosurgery. 1981;8:469–72. doi: 10.1227/00006123-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Lortat-Jacob S, Pierre-Kahn A, Renier D, Hirsch JF, Martelli H, Pellerin D. Abdominal complications of ventriculo-peritoneal shunts in children. 65 cases. Chir Pediatr. 1984;25:17–21. [PubMed] [Google Scholar]
- 21.Mata J, Alegret X, Llanger J. Splenic pseudocyst as a complication of ventriculoperitoneal shunt: CT features. J Comput Assist Tomogr. 1986;10:341–2. doi: 10.1097/00004728-198603000-00037. [DOI] [PubMed] [Google Scholar]
- 22.Mobley LW, 3rd, Doran SE, Hellbusch LC. Abdominal pseudocyst: Predisposing factors and treatment algorithm. Pediatr Neurosurg. 2005;41:77–83. doi: 10.1159/000085160. [DOI] [PubMed] [Google Scholar]
- 23.Norfray JF, Henry HM, Givens JD, Sparberg MS. Abdominal complications from peritoneal shunts. Gastroenterology. 1979;77:337–40. [PubMed] [Google Scholar]
- 24.Ohba S, Kinoshita Y, Tsutsui M, Nakagawa T, Shimizu K, Murakami H. Formation of abdominal cerebrospinal fluid pseudocyst–Case report. Neurol Med Chir (Tokyo) 2012;52:838–42. doi: 10.2176/nmc.52.838. [DOI] [PubMed] [Google Scholar]
- 25.Peltier J, Demuynck F, Fichten A, Lefranc M, Toussaint P, Desenclos C, et al. Non-traumatic pseudocyst of Glisson capsule complicating a ventriculoperitoneal shunt. Neurochirurgie. 2011;57:31–3. doi: 10.1016/j.neuchi.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Rainov N, Schobess A, Heidecke V, Burkert W. Abdominal CSF pseudocysts in patients with ventriculo-peritoneal shunts. Report of fourteen cases and review of the literature. Acta Neurochir (Wien) 1994;127:73–8. doi: 10.1007/BF01808551. [DOI] [PubMed] [Google Scholar]
- 27.Rana SR, Quivers ES, Haddy TB. Hepatic cyst associated with ventriculoperitoneal shunt in a child with brain tumor. Childs Nerv Syst. 1985;1:349–51. doi: 10.1007/BF00270822. [DOI] [PubMed] [Google Scholar]
- 28.Roitberg BZ, Tomita T, McLone DG. Abdominal cerebrospinal fluid pseudocyst: A complication of ventriculoperitoneal shunt in children. Pediatr Neurosurg. 1988;29:267–73. doi: 10.1159/000028734. [DOI] [PubMed] [Google Scholar]
- 29.Salomão JF, Leibinger RD. Abdominal pseudocysts complicating CSF shunting in infants and children. Report of 18 cases. Pediatr Neurosurg. 1999;31:274–8. doi: 10.1159/000028875. [DOI] [PubMed] [Google Scholar]
- 30.Sanal M, Laimer E, Haussler B, Hager J. Abdominal cerebrospinal fluid pseudocysts in patients with ventriculoperitoneal shunt: 30 years of experience. J Indian Assoc Pediatr Surg. 2007;12:214–7. [Google Scholar]
- 31.Seçer M, Dalgiçic A, Doğanay M, Altintoprak A rare complication of ventriculoperitoneal shunt; abdominal cerebrospinal pseudocyst. A case report. J Surg Arts. 2011;4:14–6. [Google Scholar]
- 32.Verma A, Mohan S, Gupta A. Ventriculo-peritoneal shunts can cause liver injury, juxta and intrahepatic pseudocysts: Imaging findings and review of literature. Clin Neurol Neurosurg. 2012;114:389–91. doi: 10.1016/j.clineuro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Miller JH. Cerebrospinal fluid pseudocyst presenting as a hepatic mass: A complication of ventriculoperitoneal shunt. Pediatr Radiol. 1989;19:326–7. doi: 10.1007/BF02467305. [DOI] [PubMed] [Google Scholar]
- 34.Wolbers JG, van Zanten TE, van Alphen HA. Ventriculo-peritoneal shunt procedure complicated by liver capsule perforation. A case report. Clin Neurol Neurosurg. 1987;89:55–7. doi: 10.1016/s0303-8467(87)80078-1. [DOI] [PubMed] [Google Scholar]


