Abstract
Overactivation of calcium-activated neutral protease (calpain) has been implicated in the pathophysiology of several degenerative conditions, including stroke, myocardial ischemia, neuromuscular degeneration, and cataract formation. Alpha-mercaptoacrylate derivatives (exemplified by PD150606), with potent and selective inhibitory actions against calpain, have been identified. PD150606 exhibits the following characteristics: (i) Ki values for mu- and m-calpains of 0.21 microM and 0.37 microM, respectively, (ii) high specificity for calpains relative to other proteases, (iii) uncompetitive inhibition with respect to substrate, and (iv) it does not shield calpain against inactivation by the active-site inhibitor trans-(epoxysuccinyl)-L-leucyl-amido-3-methylbutane, suggesting a nonactive site action for PD150606. The recombinant calcium-binding domain from each of the large or small subunits of mu-calpain was found to interact with PD150606. In low micromolar range, PD15O6O6 inhibited calpain activity in two intact cell systems. The neuroprotective effects of this class of compound were also demonstrated by the ability of PD150606 to attenuate hypoxic/hypoglycemic injury to cerebrocortical neurons in culture and excitotoxic injury to Purkinje cells in cerebellar slices.
Full text
PDF


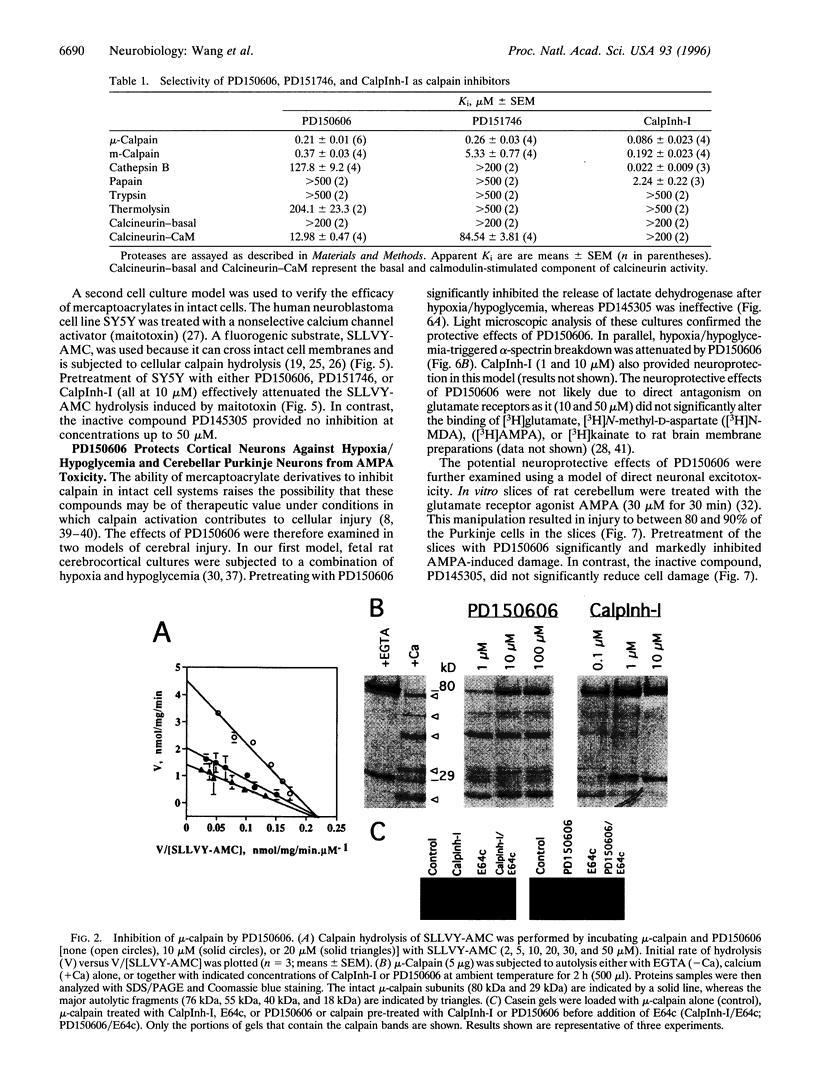
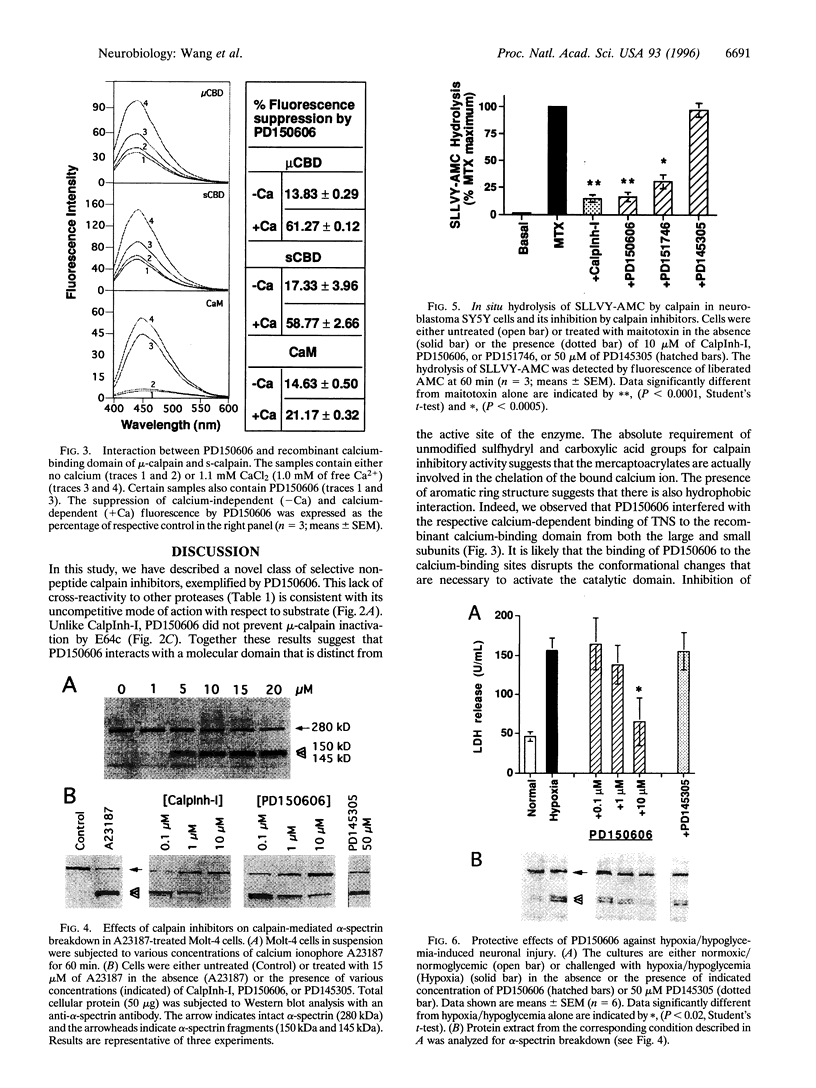
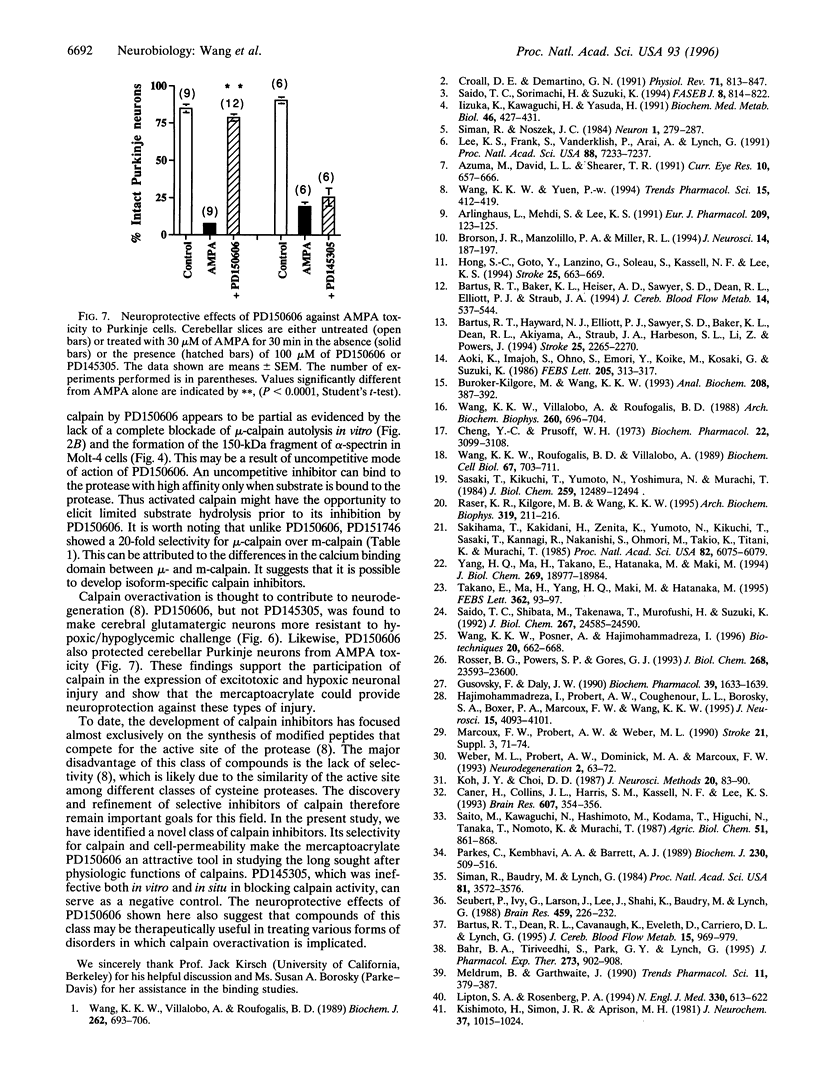
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki K., Imajoh S., Ohno S., Emori Y., Koike M., Kosaki G., Suzuki K. Complete amino acid sequence of the large subunit of the low-Ca2+-requiring form of human Ca2+-activated neutral protease (muCANP) deduced from its cDNA sequence. FEBS Lett. 1986 Sep 15;205(2):313–317. doi: 10.1016/0014-5793(86)80919-x. [DOI] [PubMed] [Google Scholar]
- Arlinghaus L., Mehdi S., Lee K. S. Improved posthypoxic recovery with a membrane-permeable calpain inhibitor. Eur J Pharmacol. 1991 Dec 10;209(1-2):123–125. doi: 10.1016/0014-2999(91)90022-i. [DOI] [PubMed] [Google Scholar]
- Azuma M., David L. L., Shearer T. R. Cysteine protease inhibitor E64 reduces the rate of formation of selenite cataract in the whole animal. Curr Eye Res. 1991 Jul;10(7):657–666. doi: 10.3109/02713689109013857. [DOI] [PubMed] [Google Scholar]
- Bahr B. A., Tiriveedhi S., Park G. Y., Lynch G. Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther. 1995 May;273(2):902–908. [PubMed] [Google Scholar]
- Bartus R. T., Baker K. L., Heiser A. D., Sawyer S. D., Dean R. L., Elliott P. J., Straub J. A. Postischemic administration of AK275, a calpain inhibitor, provides substantial protection against focal ischemic brain damage. J Cereb Blood Flow Metab. 1994 Jul;14(4):537–544. doi: 10.1038/jcbfm.1994.67. [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Dean R. L., Cavanaugh K., Eveleth D., Carriero D. L., Lynch G. Time-related neuronal changes following middle cerebral artery occlusion: implications for therapeutic intervention and the role of calpain. J Cereb Blood Flow Metab. 1995 Nov;15(6):969–979. doi: 10.1038/jcbfm.1995.123. [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Hayward N. J., Elliott P. J., Sawyer S. D., Baker K. L., Dean R. L., Akiyama A., Straub J. A., Harbeson S. L., Li Z. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994 Nov;25(11):2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- Brorson J. R., Manzolillo P. A., Miller R. J. Ca2+ entry via AMPA/KA receptors and excitotoxicity in cultured cerebellar Purkinje cells. J Neurosci. 1994 Jan;14(1):187–197. doi: 10.1523/JNEUROSCI.14-01-00187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buroker-Kilgore M., Wang K. K. A Coomassie brilliant blue G-250-based colorimetric assay for measuring activity of calpain and other proteases. Anal Biochem. 1993 Feb 1;208(2):387–392. doi: 10.1006/abio.1993.1066. [DOI] [PubMed] [Google Scholar]
- Caner H., Collins J. L., Harris S. M., Kassell N. F., Lee K. S. Attenuation of AMPA-induced neurotoxicity by a calpain inhibitor. Brain Res. 1993 Apr 2;607(1-2):354–356. doi: 10.1016/0006-8993(93)91531-v. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Gusovsky F., Daly J. W. Maitotoxin: a unique pharmacological tool for research on calcium-dependent mechanisms. Biochem Pharmacol. 1990 Jun 1;39(11):1633–1639. doi: 10.1016/0006-2952(90)90105-t. [DOI] [PubMed] [Google Scholar]
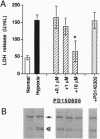
- Hajimohammadreza I., Probert A. W., Coughenour L. L., Borosky S. A., Marcoux F. W., Boxer P. A., Wang K. K. A specific inhibitor of calcium/calmodulin-dependent protein kinase-II provides neuroprotection against NMDA- and hypoxia/hypoglycemia-induced cell death. J Neurosci. 1995 May;15(5 Pt 2):4093–4101. doi: 10.1523/JNEUROSCI.15-05-04093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. C., Goto Y., Lanzino G., Soleau S., Kassell N. F., Lee K. S. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke. 1994 Mar;25(3):663–669. doi: 10.1161/01.str.25.3.663. [DOI] [PubMed] [Google Scholar]
- Iizuka K., Kawaguchi H., Yasuda H. Calpain is activated during hypoxic myocardial cell injury. Biochem Med Metab Biol. 1991 Dec;46(3):427–431. doi: 10.1016/0885-4505(91)90091-x. [DOI] [PubMed] [Google Scholar]
- Kishimoto H., Simon J. R., Aprison M. H. Determination of the equilibrium dissociation constants and number of glycine binding sites in several areas of the rat central nervous system, using a sodium-independent system. J Neurochem. 1981 Oct;37(4):1015–1024. doi: 10.1111/j.1471-4159.1981.tb04489.x. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987 May;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Frank S., Vanderklish P., Arai A., Lynch G. Inhibition of proteolysis protects hippocampal neurons from ischemia. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7233–7237. doi: 10.1073/pnas.88.16.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Rosenberg P. A. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994 Mar 3;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Parkes C., Kembhavi A. A., Barrett A. J. Calpain inhibition by peptide epoxides. Biochem J. 1985 Sep 1;230(2):509–516. doi: 10.1042/bj2300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser K. J., Posner A., Wang K. K. Casein zymography: a method to study mu-calpain, m-calpain, and their inhibitory agents. Arch Biochem Biophys. 1995 May 10;319(1):211–216. doi: 10.1006/abbi.1995.1284. [DOI] [PubMed] [Google Scholar]
- Rosser B. G., Powers S. P., Gores G. J. Calpain activity increases in hepatocytes following addition of ATP. Demonstration by a novel fluorescent approach. J Biol Chem. 1993 Nov 5;268(31):23593–23600. [PubMed] [Google Scholar]
- Saido T. C., Shibata M., Takenawa T., Murofushi H., Suzuki K. Positive regulation of mu-calpain action by polyphosphoinositides. J Biol Chem. 1992 Dec 5;267(34):24585–24590. [PubMed] [Google Scholar]
- Saido T. C., Sorimachi H., Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994 Aug;8(11):814–822. [PubMed] [Google Scholar]
- Sakihama T., Kakidani H., Zenita K., Yumoto N., Kikuchi T., Sasaki T., Kannagi R., Nakanishi S., Ohmori M., Takio K. A putative Ca2+-binding protein: structure of the light subunit of porcine calpain elucidated by molecular cloning and protein sequence analysis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6075–6079. doi: 10.1073/pnas.82.18.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Kikuchi T., Yumoto N., Yoshimura N., Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984 Oct 25;259(20):12489–12494. [PubMed] [Google Scholar]
- Seubert P., Ivy G., Larson J., Lee J., Shahi K., Baudry M., Lynch G. Lesions of entorhinal cortex produce a calpain-mediated degradation of brain spectrin in dentate gyrus. I. Biochemical studies. Brain Res. 1988 Sep 6;459(2):226–232. doi: 10.1016/0006-8993(88)90638-5. [DOI] [PubMed] [Google Scholar]
- Siman R., Baudry M., Lynch G. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R., Noszek J. C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988 Jun;1(4):279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Takano E., Ma H., Yang H. Q., Maki M., Hatanaka M. Preference of calcium-dependent interactions between calmodulin-like domains of calpain and calpastatin subdomains. FEBS Lett. 1995 Mar 27;362(1):93–97. doi: 10.1016/0014-5793(95)00219-y. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Posner A., Hajimohammadreza I. Total protein extraction from cultured cells for use in electrophoresis and western blotting. Biotechniques. 1996 Apr;20(4):662–668. doi: 10.2144/19962004662. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Roufogalis B. D., Villalobo A. Characterization of the fragmented forms of calcineurin produced by calpain I. Biochem Cell Biol. 1989 Oct;67(10):703–711. doi: 10.1139/o89-105. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Villalobo A., Roufogalis B. D. Activation of the Ca2+-ATPase of human erythrocyte membrane by an endogenous Ca2+-dependent neutral protease. Arch Biochem Biophys. 1988 Feb 1;260(2):696–704. doi: 10.1016/0003-9861(88)90498-5. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Villalobo A., Roufogalis B. D. Calmodulin-binding proteins as calpain substrates. Biochem J. 1989 Sep 15;262(3):693–706. doi: 10.1042/bj2620693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. K., Yuen P. W. Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol Sci. 1994 Nov;15(11):412–419. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Yang H. Q., Ma H., Takano E., Hatanaka M., Maki M. Analysis of calcium-dependent interaction between amino-terminal conserved region of calpastatin functional domain and calmodulin-like domain of mu-calpain large subunit. J Biol Chem. 1994 Jul 22;269(29):18977–18984. [PubMed] [Google Scholar]