Abstract
Background:
During the last decades there has been great attention paid to aflatoxins. They are highly toxic, immunosuppressive, mutagenic, teratogenic, and carcinogenic compounds. Aflatoxin M1 (AFM1), a hydroxylated metabolite of aflatoxin B1 (AFB1), is formed in the liver and excreted into the breast milk. It is considered to cause certain hygienic risks for infant health. The aim of this study was to evaluate the presence of the AFM1 in the breast milk using AFM1 in milk as a biomarker for exposure to aflatoxin B1 and determine the level of AFM1 contamination in the lactating mothers in Isfahan, Iran.
Materials and Methods:
This study was carried out on 80 lactating women randomly selected from two urban health centers. Mother's milk samples and information on food intake were collected from the participants using structured food-frequency questionnaire. Breast milk samples were tested for AFM1 by a competitive ELISA technique.
Results:
Our findings showed that only one sample was contaminated with AFM1 with concentrations of 6.8 ng/L. However, the AFM1 level in this sample was lower than the maximum tolerable limit (25 ng/L) accepted by the European Communities and Codex Alimentarius.
Conclusion:
Although the concentration of AFM1 in none of the samples was higher than the acceptable level, the presence of AFM1 in only one of them confirms the need for developing strategies to reduce exposure to aflatoxin in foods and to carry out biological monitoring of aflatoxins as a food quality control measure routinely.
Keywords: Aflatoxin M1, breast milk, Enzyme-Linked Immunosorbent Assay, infant's exposure
INTRODUCTION
Aflatoxins (AFs) are a kind of mycotoxin mostly produced by a variety of moulds such as Aspergillus flavus, Aspergillus Parasiticus, and Aspergillus nomius. These moulds are common contaminants of foodstuffs, particularly in the tropical regions.[1,2] Crops may be contaminated by one or more of the following four sub-types of aflatoxin: B1, B2, G1, and G2. Aflatoxin B1 is the most toxic aflatoxin.[3] Aflatoxin M1 (AFM1) is the main 4-hydroxylated derivative of aflatoxin B1 (AFB1). It is transformed into aflatoxin M1 (AFM1) through enzymatic hydroxylation in liver by means of cytochrome P450-associated enzymes.[1,4]
AFM1 can be detected in milk of lactating mothers 12-24 h after the first ingestion of AFB1 contaminated food such as grain products, milk and milk products, legumes, meat, fish, corn oil, cottonseed oil, dried fruits, and nuts, when the intake of AFB1 is stopped, the AFM1 concentration reduces to undetectable level after 72 h.[5,6,7]
Toxicological studies on aflatoxins have shown that they are highly toxic, immunosuppressive, mutagenic, teratogenic, and carcinogenic compounds. Although AFM1 has been found to be about 10 times less mutagenic and carcinogenic than AFB1, this toxin classified as a Group 2B agent, by the International Agency for the Research on Cancer (IARC).[6,7,8]
The European Commission has determined the legal limit for AFM1 in raw milk, treated milk, and dairy products at 50 ng/kg, and for infant formulae, infant milk, and special food products should not exceed 25 ng/kg (7). Aflatoxins may cause human health disorders including toxic hepatitis and liver fibrosis, hepatocellular carcinoma, aflatoxicosis, and Reye's syndrome.[9,10] Exposure of infants to AFM1 is worrisome, because their capacity for biotransformation of carcinogens is generally slower than that of adults and they are more susceptible to adverse effects of mycotoxins.[5] Although, the occurrence of AFM1 in milk and cheese from Isfahan market has been previously reported,[11,12] evaluation of exposure of Isfahanian infant to this contaminant had not been performed yet.
The aim of this study was to determine the level of AFM1 contamination in lactating mothers in Isfahan, Iran.
MATERIALS AND METHODS
Sample preparation for analysis
Based on the previous studies, a total of 80 breast milk samples were collected from lactating women of Isfahan, Iran, who exclusively breastfed their infants aged 60-120 days during January and February 2011.[6] The research sample included all mothers in the defined population of two urban health centers. The mothers invited to participate in the study were selected from women who were admitted for their infant's vaccination.
The study protocol was approved by the Ethics Committee of Isfahan University of Medical Science. Written informed consent was obtained from all the subjects after having been informed about the aim of the study.
Data collection
For each of the women a questionnaire containing personal data, occupation, eating, and personal habits was completed. For diet and eating habits information we focused on the foods that were more likely to contribute to dietary intake of aflatoxins including different food groups such as grain products (wheat bread, rice and corn), milk and dairy products (cheese, yogurt, and cream), meat (chicken, beef and lamb), legumes (beans and lentils), oils, dried fruits, and nuts.
Breast milk collection
Breast milk samples (10 mL) were collected into sterile test tubes. The samples were put inside an ice box, transferred to the laboratory and kept at -20°C until analysis.
Analysis of AFM1 in the samples by a competitive enzyme-linked immunosorbent assay
The quantitative analysis of AFM1 in the samples were performed according to the instructions of the competitive enzyme immunoassay using RIDASCREEN Aflatoxin M1 30/15 (Art. No.: R1111, R Biopharm, Darmstadt, Germany) test kit. The detection limit for milk was 5 ng/L. The average recovery rate and coefficient variation of the method were approximately 95% and 14%, respectively.
All of the samples were thawed gradually at 4°C and then vigorously mixed and were centrifuged for 10 min at 3500 g/10°C. The upper creamy layer was completely removed by aspirating through a Pasteur pipette for each test and the skimmed milks (lower phases) was used for the quantitative test.
One hundred μL of standard solutions or the prepared samples in duplicate were added to each wells of the microtiter plate precoated with antibodies for AFM1 then mixed gently and incubated for 30 min at room temperature in the dark. The wells were washed with washing buffer (250 μL) twice. The microwell holder was tapped upside down vigorously against absorbent paper to ensure complete removal of liquid from the wells.
In the next step, 100 μL of the diluted conjugated enzyme was added to and incubated in the dark at room temperature for 15 min. The washing procedure was repeated two times in order to remove any unbound conjugated enzyme. Subsequently, 100 μL of substrate and chromogen were added the plate was incubated for 15 min at room temperature in dark. Bound conjugated enzyme transformed the colorless chromogen into a blue product. Finally, 100 μL of the stop reagent (1 NH2SO4) was added into the wells and the color changed from blue to yellow was measured at 450 nm by ELISA plate reader (Bio-Tek Instruments, USA) within 15 min. The absorption intensity was found to be inversely proportional to AFM1 concentration in the sample.[13,14,15]
Statistical analysis
Descriptive statistical analysis was performed with SPSS version 17 software. Chi-Square analysis and analysis of variance were performed. P < 0.05 was considered statistically significant.
RESULTS
The standard curve for AFM1 detection by competitive ELISA is depicted in Figure 1. It shows that there is a reverse relationship between the concentration of AFM1 and the percentage of absorbance. Maternal descriptive data are shown in the Table 1.
Figure 1.
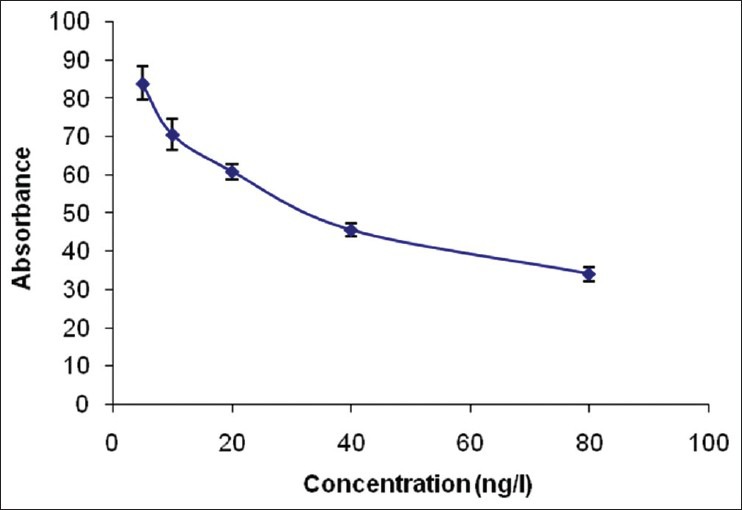
The standard curve for AFM1 by competitive ELISA
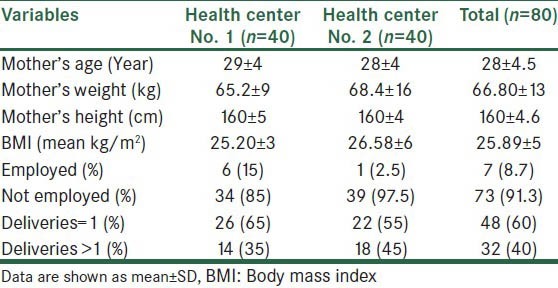
Table 1.
Maternal descriptive data
Our findings showed that only one out of eighty samples (1.25%) was positive for AFM1. AFM1 level in the positive sample was 6.8 ng/L that is lower than the maximum tolerable limit (25 ng/L) accepted by the European Communities and Codex Alimentarius.
The AFM1 positive questionnaire analysis denoted relationship between these values and the mother's lifestyle. There was no significant association between the presence of AFM1 in the breast milk and the consumption of grain products, meat, fish, legumes, oils, dried fruits, and nuts. However, a significant association between daily consumption of sausage and the presence of AFM1 was seen (P < 0.001). Mother, who had contaminated breast milk, had consumed significantly more sausage in her daily diet. The questionnaire analysis did not reveal any relationship between these values and her lifestyle.
DISCUSSION
Children have lower detoxification capacity, rapid growth, and higher intakes of food and water per kilogram body weight. Therefore, early childhood exposure to the environmental toxicants like aflatoxins may be critical determinants of later health effects.[14] AFM1 in breast milk is a biomarker of dietary exposure of the mothers to AFB1 and consequently of aflatoxin exposure to the breastfeeding infants.[6]
The aim of this study was to look at the incidence of AFM1 in the breast milk of urban Isfahanian lactating mothers. From 80 samples collected only one (1.25%) was positive for AFM1. The concentration of AFM1 in the contaminated sample was 6.8 ng/L. The findings indicated low exposure of the lactating mothers to aflatoxin during the time of the study. In addition, in the positive sample the level of AFM1 was lower than the maximum tolerable limit (25 ng/L) accepted by the European Communities and Codex Alimentarius.
The similar rate of contamination of breast milk of Italian women was reported by Turconi et al.[16] However, the concentration of AFM1 reported by this group was much higher (194 ng/L) than that of our study.[16]
The levels of AFM1 reported by several studies are higher than that of this study. The mean AFM1 levels in some of the previous studies are as follow: 6.96 ng/L, Tabriz, Iran,[6] 8.2 ng/L, Tehran, Iran,[13] 6900 ng/L, Ahvaz, Iran,[17] 194 ng/L, Lombardy, Italy,[16] 24 ng/L, Sao Paulo, Brazil,[18] 180 ng/L, Ankara, Turkey,[19] and 71 ng/L, Victoria, Australia.[20]
Although, the Joint FAO/WHO Committee on Food Additives did not establish a tolerable daily intake for genotoxic carcinogenic substances, such as aflatoxins, the strongly recommended that the level of aflatoxins should be as low as possible.[16]
Due to high toxicity and carcinogenic properties, the presence of AFM1 in the breast milk is a concern. As our study was conducted in the winter, the cold weather of the region might be the reason for the low AFM1 level. Also, increasing the number of samples may provide a better interpretation of the situation of AFM1 contamination of breast milk.
Mahdavi et al. from Tabriz, Iran concluded that local cow milk was the major source of aflatoxin exposure for lactating women.[6] Contamination of milk and dairy products with AFM1 has the potential to be a public health problem in Iran. In a study conducted in Isfahan in 2007, the mean concentrations of AFM1 in 236 raw, pasteurized and UHT milk were 68, 56, and 65 ng/L, respectively. 119 samples (55.9%) had concentrations higher than the maximum tolerance accepted by some European countries (50 ng/L).[11] In the various countries including Iran, there is no defined acceptable limit for AFM1 in infant milk products and formulas. In a recent study conducted in Tehran, Iran, 96.6% infant formula samples were found to be contaminated with AFM1 with mean concentration of 7.3 ng/L.[21]
CONCLUSIONS
Based on the findings of this study, it can be concluded that the present status of mycotoxin contamination in Isfahanian mother's milk is not at risk. However, it is needed to develop strategies to reduce exposure to aflatoxin in foods and routinely monitor them as a food quality control measure. Further studies are required for developing strategies to reduce exposure to aflatoxin, including interventions targeted at reducing contamination of foods.
ACKNOWLEDGMENTS
This study was supported by a grant from the Research Council of Isfahan University of Medical Sciences, Isfahan, Iran. This study would not have been accomplished without the help and participation of the health centers and all the mothers who volunteered testing their milk samples.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sancho GC, Marin S, Ramos AJ, Vicente JP, Sanchis V. Occurrence of aflatoxin M 1 and exposure assessment in Catalonia, Spain. Rev Iberoam Micol. 2010;27:130–5. doi: 10.1016/j.riam.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Tchana AN, Moundipa PF, Tchouanguep FM. Aflatoxin contamination in food and body fluids in relation to malnutrition and cancer status in Cameroon. Int J Environ Res Public Health. 2010;7:178–88. doi: 10.3390/ijerph7010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirestani A, Toghyani M. The effect of aflatoxin levels on milk production, reproduction and lameness in high production Holstein cows. Afr J Biotechnol. 2010;9:7905–8. [Google Scholar]
- 4.Paniel N, Radoi A, Marty JL. Development of an electrochemical biosensor for the detection of aflatoxin M 1 in milk. Sensors (Basel) 2010;10:9439–48. doi: 10.3390/s101009439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez CE, Ramos LL, Ramadan SS, Bulacio LC. Presence of aflatoxin M 1 in milk for human consumption in Argentina. Food Control. 2003;14:31–4. [Google Scholar]
- 6.Williams JH, Phillips DT, Jolly PE, Stiles JK, Jolly CM, Aggaewal D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and intervention. Am J Clin Nutr. 2004;80:1106–22. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 7.Creppy EE. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol Lett. 2002;127:19–28. doi: 10.1016/s0378-4274(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 8.Summary of data reported and evaluation. Vol. 82. CEDEX: Lyon: International Agency for Research on Cancer; 2002. IARC, International Agency for Research on Cancer. Monograph on the evaluation of carcinogenic risk to humans, World Health Organization, some traditional herbal medicines, some mycotoxins, naphthalene and styrene; pp. 171–5. [Google Scholar]
- 9.Dänicke S, Fink-Gremmels J, van Egmond H, Gilbert J, Larsen JC, Leibetseder J, et al. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to Aflatoxin B 1 as undesirable substance in animal feed. EFSA J. 2004;39:1–27. [Google Scholar]
- 10.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153:1677–92. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- 11.Rahimi E, Shakerian A, Jafariyan M, Ebrahimi M, Riahi M. Occurrence of aflatoxin M 1 in raw, pasteurized and UHT milk commercialized in Esfahan and Shahr-e Kord, Iran. Food Sci. 2009;1:317–20. [Google Scholar]
- 12.Fallah AA, Jafari T, Fallah A, Rahnama M. Determination of aflatoxin M 1 levels in Iranian white and cream cheese. Food Chem Toxicol. 2009;47:1872–5. doi: 10.1016/j.fct.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Oveisi M, Jannat B, Sadeghi N. Incidence of aflatoxin M 1 in human breast milk in Tehran, Iran. Food Control. 2009;20:75–8. [Google Scholar]
- 14.Rastogi S, Dwivedi PD, Khanna SK, Das M. Detection of aflatoxin M 1 contamination in milk and infant milk products from Indian markets by ELISA. Food Control. 2004;15:287–90. [Google Scholar]
- 15.Heshmati A, Milani JM. Contamination of UHT milk by aflatoxin M 1 in Iran. Food Control. 2010;21:19–22. [Google Scholar]
- 16.Turconi G, Guarcello M, Livieri S. Evaluation of Xenobiotics in human breast milk and ingestion by the newborn. Eur J Nutr. 2004;43:191–7. doi: 10.1007/s00394-004-0458-2. [DOI] [PubMed] [Google Scholar]
- 17.Heidarinia A. PhD thesis. Ahvaz, Iran: Faculty of Medicine, Ahvaz University; 1995. Detection of aflatoxin M1 in breast milk; pp. 57–63. [Google Scholar]
- 18.Navasi SA, Sabino M, Rodriguez DB. Aflatoxin M 1 and ochratoxin A in a human milk bank in the city of São Paulo, Brazil. Food Addit Contam. 2005;22:457–62. doi: 10.1080/02652030500110550. [DOI] [PubMed] [Google Scholar]
- 19.Gurbay A. Exposure of newborns to aflatoxin M 1 and B 1 from mothers’ breast milk in Ankara, Turkey. Food Chem Toxicol. 2010;48:314–9. doi: 10.1016/j.fct.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 20.El-Nezami H, Nicoletti G, Neal GE, Donohue DC, Ahokas JT. Aflatoxin M 1 in human breast milk samples from Victoria, Australia and Thailand. Food Chem Toxicol. 1995;33:173–9. doi: 10.1016/0278-6915(94)00130-g. [DOI] [PubMed] [Google Scholar]
- 21.Oveisi M, Jannat B, Sadeghi N, Hajimahmoodi M, Nikzad A. Presence of aflatoxin M 1 in milk and infant milk products in Tehran, Iran. Food Control. 2007;18:1216–8. [Google Scholar]


