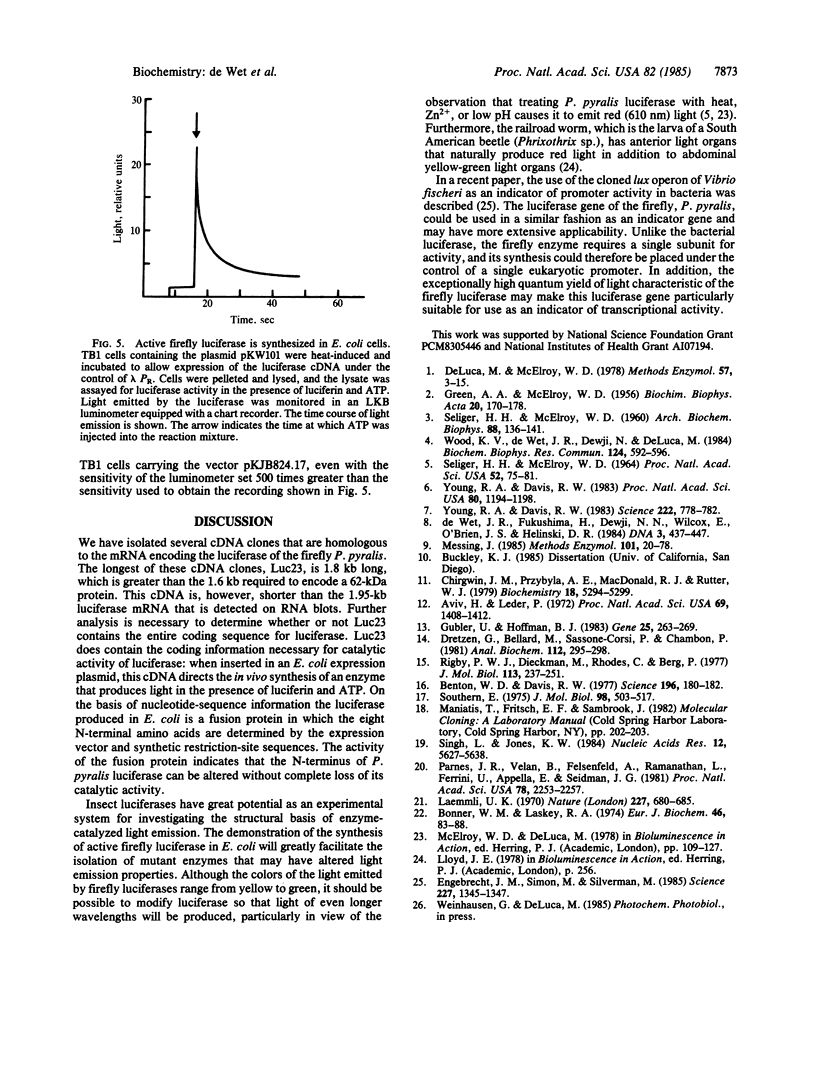
Abstract
A cDNA library was constructed from firefly (Photinus pyralis) lantern poly(A)+ RNA, using the Escherichia coli expression vector lambda gt11. The library was screened with anti-P. pyralis luciferase (Photinus luciferin:oxygen 4-oxidoreductase, EC 1.13.12.7) antibody, and several cDNA clones expressing luciferase antigens were isolated. One clone, lambda Luc1, contained 1.5 kilobase pairs of cDNA that hybridized to a 1.9- to 2.0-kilobase band on a nitrocellulose blot of electrophoretically fractionated lantern RNA. Hybridization of the cloned cDNA to lantern poly(A)+ RNA selected an RNA that directed the in vitro synthesis of a single polypeptide. This polypeptide comigrated with luciferase on NaDodSO4/PAGE and produced bioluminescence upon the addition of luciferin and ATP. A 1.8-kilobase-pair cDNA was isolated by probing the firefly cDNA library with the cDNA from lambda Luc1. This cDNA contained sufficient coding information to direct the synthesis of active firefly luciferase in E. coli.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Simon M., Silverman M. Measuring gene expression with light. Science. 1985 Mar 15;227(4692):1345–1347. doi: 10.1126/science.2983423. [DOI] [PubMed] [Google Scholar]
- GREEN A. A., MCELROY W. D. Crystalline firefly luciferase. Biochim Biophys Acta. 1956 Apr;20(1):170–176. doi: 10.1016/0006-3002(56)90275-x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SELIGER H. H., McELROY W. D. Spectral emission and quantum yield of firefly bioluminescence. Arch Biochem Biophys. 1960 May;88:136–141. doi: 10.1016/0003-9861(60)90208-3. [DOI] [PubMed] [Google Scholar]
- Seliger H. H., McElroy W. D. THE COLORS OF FIREFLY BIOLUMINESCENCE: ENZYME CONFIGURATION AND SPECIES SPECIFICITY. Proc Natl Acad Sci U S A. 1964 Jul;52(1):75–81. doi: 10.1073/pnas.52.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wood K. V., de Wet J. R., Dewji N., DeLuca M. Synthesis of active firefly luciferase by in vitro translation of RNA obtained from adult lanterns. Biochem Biophys Res Commun. 1984 Oct 30;124(2):592–596. doi: 10.1016/0006-291x(84)91595-x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Fukushima H., Dewji N. N., Wilcox E., O'Brien J. S., Helinski D. R. Chromogenic immunodetection of human serum albumin and alpha-L-fucosidase clones in a human hepatoma cDNA expression library. DNA. 1984 Dec;3(6):437–447. doi: 10.1089/dna.1.1984.3.437. [DOI] [PubMed] [Google Scholar]