Abstract
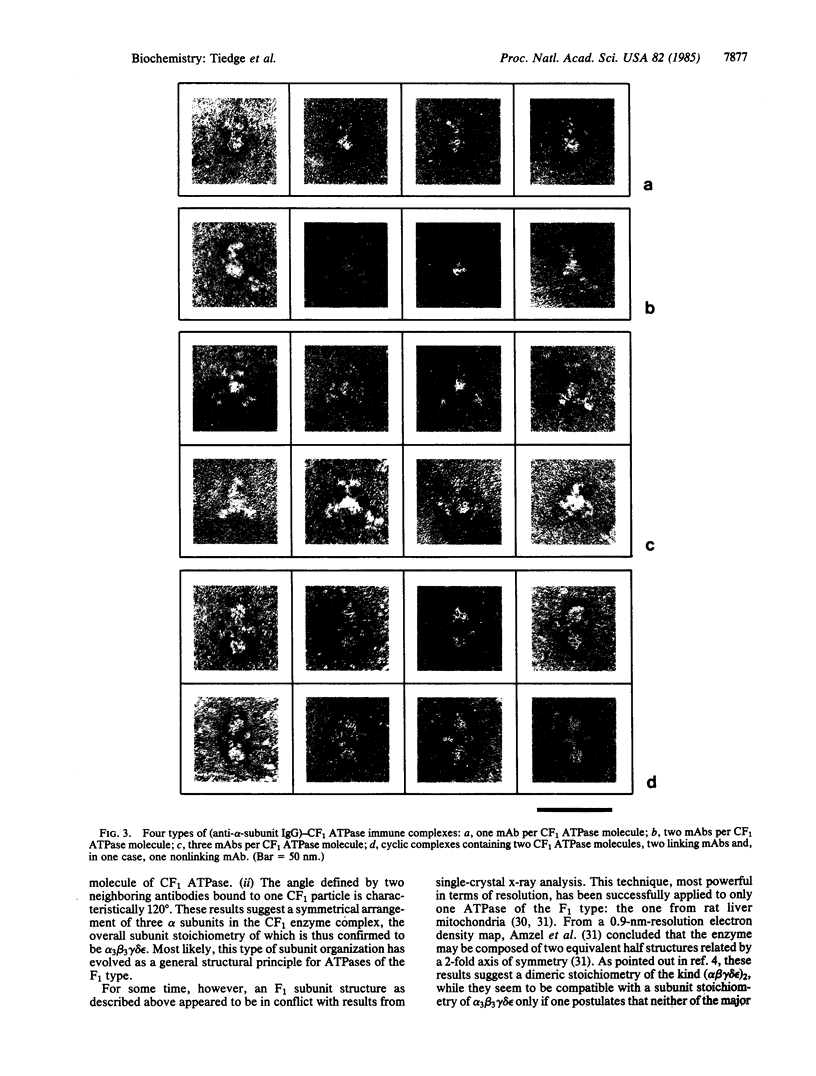
Monoclonal antibodies specific to the α subunits of the photosynthetic coupling factor 1 (CF1) were used as marker molecules in an electron microscopic analysis of the subunit organization of this enzyme. Immune complexes were obtained by incubation of CF1 with saturating amounts of anti-α-subunit IgG, isolated by gel filtration, and visualized by electron microscopy. The maximum number of antibodies bound to a CF1 molecule was three, the angle defined by a neighboring pair of antibodies characteristically being 120°. These results are interpreted as direct evidence for the presence of three α subunits in the CF1 complex, the relative orientation of them being described by 3-fold rotary symmetry. Our observations thus favor an overall subunit stoichiometry of α3β3γδε.
Keywords: spinach chloroplasts, quaternary protein structure, hybridoma technique
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akey C. W., Crepeau R. H., Dunn S. D., McCarty R. E., Edelstein S. J. Electron microscopy and single molecule averaging of subunit-deficient F1-ATPases from Escherichia coli and spinach chloroplasts. EMBO J. 1983;2(8):1409–1415. doi: 10.1002/j.1460-2075.1983.tb01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzel L. M., McKinney M., Narayanan P., Pedersen P. L. Structure of the mitochondrial F1 ATPase at 9-A resolution. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5852–5856. doi: 10.1073/pnas.79.19.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzel L. M., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. Crystallization and x-ray diffraction studies of the F1-component of the enzyme. J Biol Chem. 1978 Apr 10;253(7):2067–2069. [PubMed] [Google Scholar]
- Amzel L. M., Pedersen P. L. Proton atpases: structure and mechanism. Annu Rev Biochem. 1983;52:801–824. doi: 10.1146/annurev.bi.52.070183.004101. [DOI] [PubMed] [Google Scholar]
- Baird B. A., Hammes G. G. Chemical cross-linking studies of beef heart mitochondrial coupling factor 1. J Biol Chem. 1977 Jul 10;252(13):4743–4748. [PubMed] [Google Scholar]
- Bowien B., Mayer F. Further studies on the quaternary structure of D-ribulose-1, 5-bisphosphate carboxylase from Alcaligenes eutrophus. Eur J Biochem. 1978 Jul 17;88(1):97–107. doi: 10.1111/j.1432-1033.1978.tb12426.x. [DOI] [PubMed] [Google Scholar]
- Delacroix D., Vaerman J. P. Simple purification of goat IgG1 and IgG2 subclasses by chromatography on protein A-sepharose at various pH. Mol Immunol. 1979 Oct;16(10):837–840. doi: 10.1016/0161-5890(79)90164-0. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Grubmeyer C., Penefsky H. S. Cooperatively between catalytic sites in the mechanism of action of beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1981 Apr 25;256(8):3728–3734. [PubMed] [Google Scholar]
- Holmes N. J., Parham P. Enhancement of monoclonal antibodies against HLA-A2 is due to antibody bivalency. J Biol Chem. 1983 Feb 10;258(3):1580–1586. [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Function of the "quantasome" in photosynthesis: structure and properties of membrane-bound particle active in the dark reactions of photophosphorylation. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1261–1268. doi: 10.1073/pnas.58.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. X. Correlation of morphology and function in submitochondrial particles. J Biol Chem. 1966 May 25;241(10):2475–2482. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kohlbrenner W. E., Boyer P. D. Probes of catalytic site cooperativity during catalysis by the chloroplast adenosine triphosphate and the adenosine triphosphate synthase. J Biol Chem. 1983 Sep 25;258(18):10881–10886. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lünsdorf H., Ehrig K., Friedl P., Schairer H. U. Use of monoclonal antibodies in immuno-electron microscopy for the determination of subunit stoichiometry in oligomeric enzymes. There are three alpha-subunits in the F1-ATPase of Escherichia coli. J Mol Biol. 1984 Feb 15;173(1):131–136. doi: 10.1016/0022-2836(84)90408-x. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Moradi-Ameli M., Godinot C. Characterization of monoclonal antibodies against mitochondrial F1-ATPase. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6167–6171. doi: 10.1073/pnas.80.20.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. Structure and function of chloroplast ATPase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):314–338. doi: 10.1016/0304-4173(76)90003-3. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Wise J. G. The proton-ATPase of bacteria and mitochondria. J Membr Biol. 1983;73(2):105–124. doi: 10.1007/BF01870434. [DOI] [PubMed] [Google Scholar]
- Shavit N. Energy transduction in chloroplasts: structure and function of the ATPase complex. Annu Rev Biochem. 1980;49:111–138. doi: 10.1146/annurev.bi.49.070180.000551. [DOI] [PubMed] [Google Scholar]
- Snyder B., Hammes G. G. Structural mapping of chloroplast coupling factor. Biochemistry. 1984 Nov 20;23(24):5787–5795. doi: 10.1021/bi00319a018. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Hesse H., Edelmann K. Quantitative determination of coupling factor CF1 of chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):202–210. doi: 10.1016/0005-2728(73)90135-7. [DOI] [PubMed] [Google Scholar]
- Tiedge H., Lücken U., Weber J., Schäfer G. High-affinity binding of ADP and of ADP analogues to mitochondrial F1-ATPase. Eur J Biochem. 1982 Oct;127(2):291–299. doi: 10.1111/j.1432-1033.1982.tb06869.x. [DOI] [PubMed] [Google Scholar]
- Tiedge H., Schäfer G., Mayer F. An electron microscopic approach to the quaternary structure of mitochondrial F1-ATPase. Eur J Biochem. 1983 Apr 15;132(1):37–45. doi: 10.1111/j.1432-1033.1983.tb07322.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuprun V. L., Mesyanzhinova I. V., Kozlov I. A., Orlova E. V. Electron microscopy of beef heart mitochondrial F1-ATPase. FEBS Lett. 1984 Feb 27;167(2):285–290. doi: 10.1016/0014-5793(84)80144-1. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T., Kubota M., Yoshida M., Kagawa Y. Structure of ATPase (coupling factor TF1) from a thermophilic bacterium. J Mol Biol. 1977 Dec 5;117(2):515–519. doi: 10.1016/0022-2836(77)90140-1. [DOI] [PubMed] [Google Scholar]
- Younis H. M., Winget G. D., Racker E. Requirement of the delta subunit of chloroplast coupling factor 1 for photophosphorylation. J Biol Chem. 1977 Mar 10;252(5):1814–1818. [PubMed] [Google Scholar]