Editor’s Highlight: The aryl hydrocarbon receptor (AhR) holds a high place in the annals of toxicology, but its function still eludes us. In the paper by Wheeler and coworkers (137, 324–334), the authors explore the role of AhR activation on immune system regulation. Specifically, the group treated animals with the synthetic TCDD (a.k.a. dioxin) or the naturally-derived FICZ (photooxidation product of tryptophan) and tested the response to the common respiratory virus, influenza A. One might expect that AhR activation, regardless of the ligand, would lead to similar immunomodulatory response, but this was not the case. In initial studies TCDD altered a range of host immune responses, but FICZ was without effect. After duration of receptor activation was controlled for, there was still a divergent response suggesting that other mechanisms, such as the type of cells in which the receptor is activated, may be involved. Gary W. Miller
Key Words: AhR, TCDD, FICZ, influenza virus.
Abstract
Immune modulation by the aryl hydrocarbon receptor (AhR) has been primarily studied using 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD). Recent reports suggest another AhR ligand, 6-formylindolo[3,2-b]carbazole (FICZ), exhibits distinct immunomodulatory properties, but side-by-side comparisons of these 2 structurally distinct, high-affinity ligands are limited. In this study, the effects of in vivo AhR activation with TCDD and FICZ were directly compared in a mouse model of influenza virus infection using 3 key measures of the host response to infection: pulmonary neutrophilia, inducible nitric oxide synthase (iNOS) levels, and the virus-specific CD8+ T-cell response. By this approach, the consequences of AhR activation on innate and adaptive immune responses to the same antigenic challenge were compared. A single dose of TCDD elicited AhR activation that is sustained for the duration of the host’s response to infection and modulated all 3 responses to infection. In contrast, a single dose of FICZ induced transient AhR activation and had no effect on the immune response to infection. Micro-osmotic pumps and Cyp1a1-deficient mice were utilized to augment FICZ-mediated AhR activation in vivo, in order to assess the effect of transient versus prolonged AhR activation. Prolonged AhR activation with FICZ did not affect neutrophil recruitment or pulmonary iNOS levels. However, FICZ-mediated AhR activation diminished the CD8+ T-cell response in Cyp1a1-deficient mice in a similar manner to TCDD. These results demonstrate that immunomodulatory differences in the action of these 2 ligands are likely due to not only the duration of AhR activation but also the cell types in which the receptor is activated.
It is widely accepted that the aryl hydrocarbon receptor (AhR) influences the development and function of the immune system, although the molecular mechanisms through which AhR does this remain to be fully elucidated. The majority of these studies have used persistent environmental contaminants, including polycyclic aromatic hydrocarbons, halogenated aromatic hydrocarbons, and some polychlorinated biphenyls to activate the AhR (Burchiel and Luster, 2001; Lawrence and Kerkvliet, 2006; Nguyen and Bradfield, 2008). Other studies have used naturally derived chemicals, such as the photodegradation product of tryptophan, 6-formylindolo[3,2-b]carbazole (FICZ), or novel pharmaceutical candidates, such as VAF347, to activate AhR and modulate immune responses (Lawrence et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008). To date, the best characterized and most commonly used AhR ligand is the pollutant 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD). Indeed, the majority of published studies investigating immune modulation by AhR have used TCDD (Lawrence and Kerkvliet, 2006). Reasons for this include TCDD’s high affinity for the AhR, its demonstrated specificity for the receptor, and its long half-life in vivo (Bohonowych and Denison, 2007; Gasiewicz et al., 1983; Mimura et al., 1997; Safe, 1990). However, using TCDD is often criticized because the same properties that make it an excellent tool for activating AhR also contribute to its known toxicity (Nguyen and Bradfield, 2008). Furthermore, given that TCDD is a synthetic chemical, tremendous interest lies in characterizing the immunomodulatory potential of naturally derived chemicals and putative endogenous ligands for the AhR in an effort to better understand the role this receptor plays in immune function.
Based principally on information derived from studies using TCDD, we know the AhR rests as an inactive complex in the cytosol, which upon ligand binding dissociates from cochaperone proteins, translocates into the nucleus, and binds to its heterodimerization partner: hypoxia inducible factor-1β (HIF-1β, also called AhR nuclear translocator). The ligand:AhR:HIF-1β complex binds to aryl hydrocarbon response elements in the upstream regulatory region of AhR target genes (Abel and Haarmann-Stemmann, 2010). Although the AhR has been shown to directly regulate the expression of about 25 different genes (Gasiewicz et al., 2008; Tijet et al., 2006), the phase I drug-metabolizing enzymes, cytochrome p450 (CYP) 1A1, 1A2, and 1B1, are often used as biomarkers of AhR activation (Fujii-Kuriyama and Mimura, 2005; Nebert et al., 2004; Schrenk, 1998; Vanden Heuvel et al., 1993).
Consistent immunomodulatory effects of AhR activation by TCDD have been reported in assorted rodent models using a variety of different antigens and measuring altered functions of several immune cell types (reviewed in Stevens, 2009). Epidemiological reports reveal the adverse effects of man-made dioxins and dioxin-like chemicals on human health (Dallaire et al., 2006; Stolevik et al., 2011; Van Den Heuvel et al., 2002). However, several studies have shown that the nonanthropogenic AhR ligand FICZ can also alter immune function. For example, FICZ treatment skews CD4+ T-cell differentiation and enhances the severity of experimental autoimmune encephalomyelitis (EAE), a mouse model that mimics aspects of multiple sclerosis (Quintana et al., 2008; Veldhoen et al., 2008). Intriguingly, in the same disease model, TCDD-mediated AhR activation promoted regulatory T-cell (Treg) differentiation and reduced the development of EAE (Quintana et al., 2008). These findings raise the possibility that depending on the type of ligand used to activate the AhR, dramatic differences in immune outcomes can occur. However, it has not been established whether differential immune modulation is unique to particular disease models, such as EAE, or to CD4+ T-cell responses, or whether this dichotomy would be observed in other disease model systems or in the context of the responses of other types of cells.
In the work reported here, we directly compared the consequences of AhR activation by the pollutant TCDD and the potential endogenous AhR ligand FICZ on 3 key immune responses to infection with human influenza A virus: pulmonary neutrophilia, inducible nitric oxide synthase (iNOS) levels in the lung, and virus-specific CD8+ cytotoxic T lymphocytes (CTL). Modulation of these responses to infection is mediated specifically through AhR activation (Lawrence et al., 2006; Neff-LaFord et al., 2007; Teske et al., 2008). Moreover, these particular responses play critically important roles in host resistance to primary influenza A virus infection (Akaike et al., 1996; Flynn et al., 1998; Tate et al., 2011) and represent 3 distinct pathways through which AhR modulates host responses to infection (Lawrence et al., 2006; Wheeler et al., 2013). Further, using micro-osmotic pumps and Cyp1a1-deficient mice, we investigated the consequences of the duration and nature, or “quality,” of AhR activation on these responses to influenza virus infection in an effort to better characterize specifically how these 2 structurally distinct AhR ligands impact viral immunity.
MATERIALS AND METHODS
Animals and treatment.
C57BL/6 mice (female, 6–10 weeks of age) were purchased from either the Jackson Laboratories or the National Cancer Institute. Breeding stock for Cyp1a1-deficient (Cyp1a1 −/−) mice were kindly provided by Dr Daniel Nebert (University of Cincinnati) and were backcrossed onto the C57BL/6 background (Dalton et al., 2000). All mice were housed in pathogen-free microisolator cages and maintained on a 12-h light/dark cycle, and provided food and water ad libitum. Unless indicated otherwise, mice were gavaged with either 10 μg TCDD/kg body weight (≥ 98% pure, Cambridge Isotope Laboratories) dissolved in anisole and diluted in peanut oil or 10 μg FICZ/kg body weight (Enzo Life Sciences, Farmingdale, New York) dissolved in dimethysulfoxide (DMSO) and diluted in peanut oil. Anisole diluted in peanut oil was used as the vehicle control. To overcome transient AhR activation by FICZ, for some experiments, micro-osmotic pumps (Alzet, Cupertino, California, Model 1007D) were implanted 1 day prior to infection. The pumps continuously release FICZ at a rate of 10 μg/kg body weight/h until the day of sacrifice; thus, mice are exposed to FICZ continuously. For the micro-pumps, FICZ was dissolved in DMSO and diluted in 45% hydroxypropyl-β-cyclodextrin (HPβCD; wt/vol, water) or vehicle control (diluted HPβCD). One day after gavage or pump implantation, mice were anesthetized with Avertin (2,2,2-tribromoethanol) and infected intranasally with 120 hemagglutinating units influenza virus, strain A/HKx31 (x31, H3N2). This dose of virus is typically sublethal in vehicle-treated, immunocompetent mice (Vorderstrasse et al., 2003). The University of Rochester Institutional Animal Care and Use and Institutional Biosafety Committees preapproved all procedures involving laboratory animals and infectious agents.
Immune cell isolation.
For lung-derived immune cells, the left and right lung lobes were separated at the bronchi. For all experiments, the same side was snap frozen in liquid nitrogen and used for immunoblotting or reverse transcription-PCR (RT-PCR). The other side was used to obtain lung-derived immune cells and lung lavage fluid. Collagenase digestion of lung tissue was performed as previously described (Teske et al., 2005). Immune cell isolation from the lung-draining mediastinal lymph nodes was performed 9 days postinfection as previously described (Lawrence et al., 2006). Erythrocytes were lysed using ammonium chloride and total organ-derived immune cells were enumerated using a Coulter counter (Beckman Coulter Corp., Miami, Florida), TC10 automated cell counter (Bio-Rad, Hercules, California), or hemacytometer.
Immunophenotypic analyses.
Nonspecific staining was blocked using anti-mouse anti-CD16/32 (eBioscience, San Diego, California) and rat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pennsylvania). Influenza A virus (HKx31)–specific CD8+ T cells were identified using an major histocompatibility complex class I tetramer with a viral peptide, Db/NP366–374, as previously described (Lawrence et al., 2006). The NP366–374 peptide is one of the major immunodominant epitopes of HKx31 virus detected by CD8+ T cells from C57BL/6 mice (Townsend et al., 1986). Cells were incubated with the fluorochrome-conjugated tetrameric reagent prior to labeling with fluorochrome-conjugated antibodies directed against CD8, CD44, and CD62L (eBioscience or BD Biosciences, San Jose, California). To identify interferon (IFN)γ-producing cells, isolated cells were restimulated ex vivo for 5h with 12.5U/ml recombinant mouse interleukin-2, 1μM influenza virus nucleoprotein peptide (NP366–374, ASNENMETM), and 5mg/ml brefeldin A (Neff-LaFord et al., 2007). Restimulated cells were stained with fluorochrome-conjugated monoclonal antibodies, fixed, permeabilized, and incubated with a fluorochrome-conjugated anti-IFNγ antibody (eBioscience). Lung-derived immune cells were incubated with fluorochrome-conjugated monoclonal antibodies directed against CD45, CD11b, Gr-1, and CD8 (eBioscience or BD Biosciences). Data were collected using an LSR II 12-color flow cytometer (BD Biosciences; at least 100000 cells were collected for neutrophil phenotypic analyses, and 300000–500000 cells were collected for T-cell analyses) and analyzed using FlowJo software (Treestar, Ashland, Oregon). Neutrophils (and other leukocytes) were also enumerated by differential cell counting of hematoxylin and eosin–stained lung-derived immune cells, as previously described (Teske et al., 2008).
Immunoblotting.
Frozen lung lobes were homogenized in cold homogenization buffer with protease inhibitors using a Tissue Tearor (Biospec Products, Bartlesville, Oklahoma), and the protein concentration of clarified homogenates was determined by bicinchoninic acid protein assay (Pierce, Rockford, Illinois). Samples were boiled in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) buffer for 5min and 50 μg of protein was subjected to SDS-PAGE. After transfer to PVDF membranes, blots were blocked with 5% nonfat dry milk and incubated with anti-iNOS (Cayman, Ann Arbor, Michigan), anti-CYP1A1 (Santa Cruz Biotechnology, Santa Cruz, California), or anti-β-actin (Sigma-Aldrich, St Louis, Missouri) antibodies. After incubation with appropriate horseradish peroxidase–conjugated secondary antibodies, immunoblots were developed with enhanced chemiluminescent reagent (Western Lightning Plus ECL, Pierce) and exposed to x-ray film.
RNA isolation and quantification.
Lungs or livers were homogenized in Trizol and total RNA was isolated (Ambion RNA isolation kit, Life Technologies, Grand Island, New York) using the manufacturer’s instructions. RNA (1 μg) was reverse transcribed using Oligo (dT) Primers (RETROscript, Ambion) and M-MLV enzyme (Ambion). Quantitative RT-PCR was performed using the MyIQ2 with SYBR green (Bio-Rad) using the following primers: Cyp1a1: forward 5′ CTG CCA ATC ACT GTG TCT A 3′; reverse 5′ TTT GGA GCT GGG TTC GAC AC 3′; Gapdh: forward 5′ GAA CAT CAT CCC TGC ATT C 3′; reverse 5′ CCA GTG AGC TTC CCG TTC A 3′; L13: forward 5′ CTA CAG TGA GAT ACC ACA CCA AG 3′; reverse 5′ TGG ACT TGT TTC GCC TCC TC 3′. The fold change in Cyp1a1 was calculated relative to the same-day vehicle control and normalized to the reference gene L13 or Gapdh using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
AhR activity.
H1L1.1c2 mouse hepatoma cells (gift of Michael Denison, University of California at Davis) that are stably transfected with an AhR-driven reporter construct were used to directly compare the activity of FICZ and TCDD. H1L1.1c2 cells were propagated and the assay was set up as previously described (Ziccardi et al., 2000). Briefly, confluent monolayers of H1L1.1c2 cells in 96-well plates were treated with the indicated concentrations of TCDD or FICZ. Control wells of H1L1.1c2 cells were cultured in media containing the vehicle (0.1% DMSO), and AhR-driven luciferase was measured 18h later using an automated microplate luminometer (Spectra Max M5, Molecular Devices, Sunnyvale, California).
Statistical analysis.
All statistical analyses were conducted using StatView statistical software (SAS, Cary, North Carolina). Differences between the treatment groups were analyzed using 1-way ANOVA, followed by Bonferroni-Dunn post hoc test. Differences between 2 treatment groups on a single day postinfection were analyzed using a Student’s t test. A value of p ≤ .05 was considered significant.
RESULTS
AhR Activation by FICZ Does Not Elicit the Same Changes in the Immune Response to Influenza Virus Infection That Are Observed With TCDD
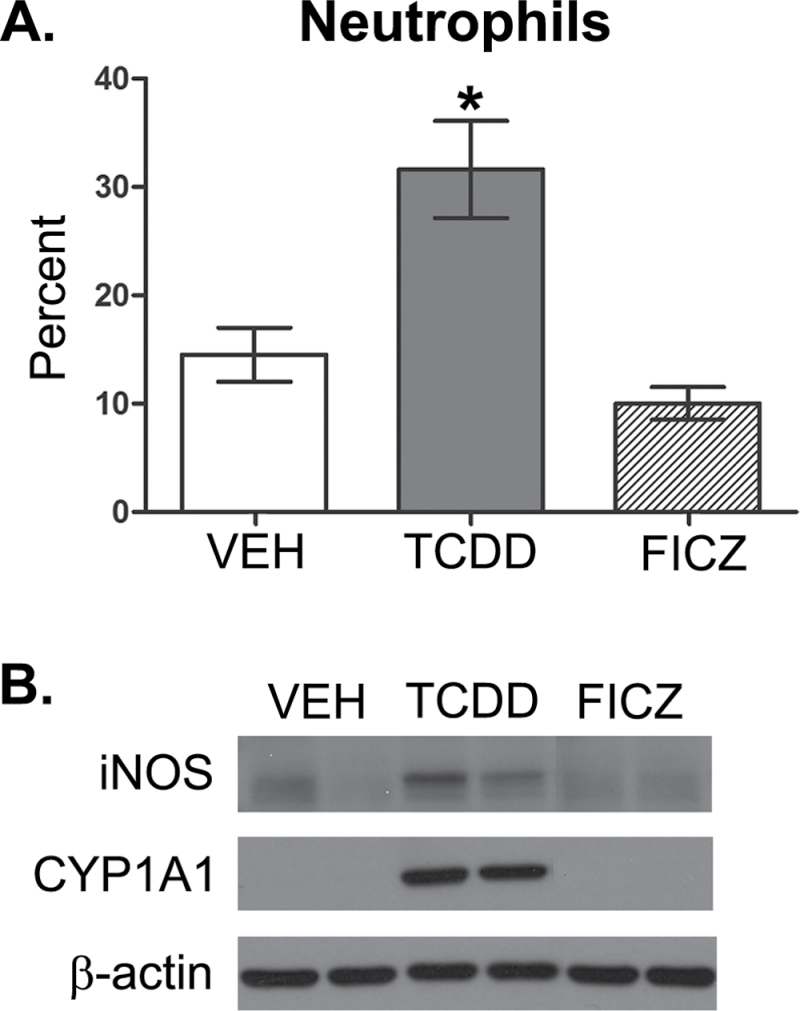
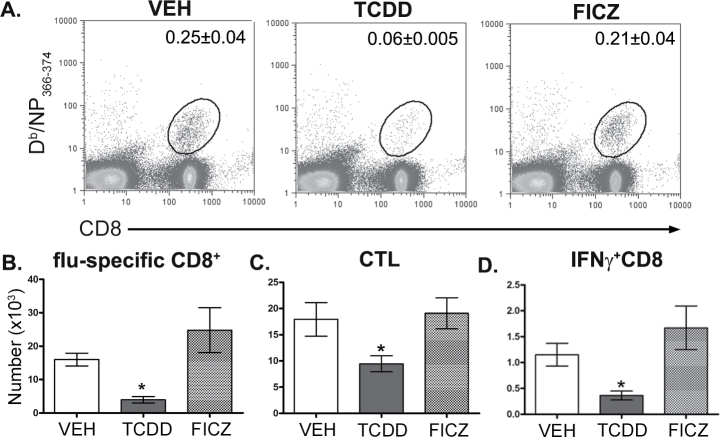
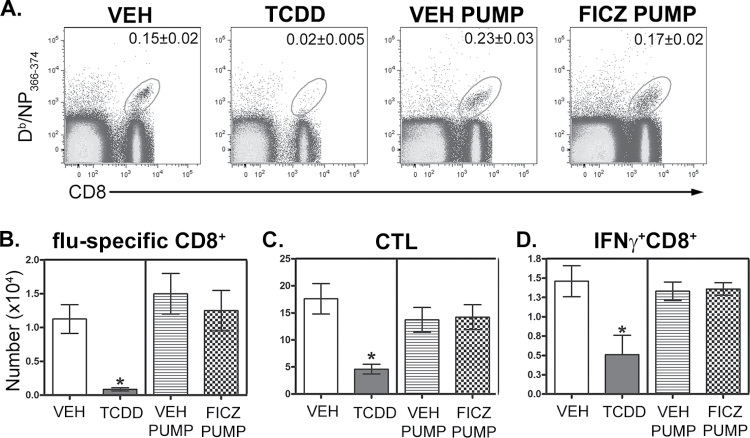
To directly compare the consequences of AhR activation by TCDD and FICZ, we administered the same dose of each ligand by oral gavage and examined pulmonary neutrophilia, iNOS levels in the lung, and CD8+ T-cell responses to influenza virus infection. The 7th day after infection is the point in time in which AhR-mediated increases in neutrophilia and iNOS are readily observed in the lung (Neff-LaFord et al., 2007; Wheeler et al., 2013). In contrast to TCDD, FICZ did not enhance the frequency of neutrophils or levels of iNOS in the infected lung (Fig. 1). Additionally, a one-time oral dose of FICZ administered 1 day prior to infection did not increase CYP1A1 protein levels in the lung 7 days after infection, which is in contrast to sustained CYP1A1 induction observed after a single dose of TCDD (Fig. 1B). Likewise, in contrast to the profoundly suppressive effect of TCDD, a single dose of FICZ did not alter influenza virus–specific CD8+ T-cell clonal expansion (Figs. 2A and 2B) and differentiation (Figs. 2C and 2D). We repeated these comparisons using 10-fold higher oral dose of FICZ (100 µg/kg) and again observed no effect on immune responses to influenza virus, as opposed to the immune modulation caused by treating infected mice with 10 µg/kg TCDD (data not shown).
FIG. 1.
AhR activation by TCDD and FICZ differentially affects immune responses to influenza virus infection in the lung. Female C57BL/6 mice (8 weeks of age) were gavaged with 10 µg/kg body weight TCDD (T), 10 µg/kg FICZ, or peanut oil anisole control (VEH). One day later, mice were infected intranasally with 120 hemagglutinating units of influenza virus (strain HKx31, H3N2). Mice were sacrificed 7 days postinfection. A, The frequency of neutrophils was determined by differential cell counts of cytospins of airway cells obtained by bronchoalveolar lavage. Bars depict the average percentage of neutrophils (± SEM) for each treatment group (n = 6–7 mice per treatment group). An * indicates a significant difference compared with vehicle control (p ≤ .05). B, Lung homogenates were subjected to SDS-PAGE and probed for iNOS and CYP1A1. β-Actin levels were assessed as a control. Blots show 2 representative samples from each treatment group (n = 6–7 mice per treatment group). Abbreviations: AhR, aryl hydrocarbon receptor; FICZ, 6-formylindolo[3,2-b]carbazole; iNOS, inducible nitric oxide synthase; SDS-PAGE, SDS-polyacrylamide gel electrophoresis; TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin; VEH, vehicle.
FIG. 2.
AhR activation by FICZ does not impair the CD8+ T-cell responses to influenza virus infection. Mice were treated and infected as described in Figure 1. On day 9 postinfection, MLN cells were collected, stained with MHC class I-restricted tetramers and antibodies to cell surface proteins, and analyzed by flow cytometry. A, Representative dot plots depict the frequency of Db/NP366–374-specific (flu-specific) CD8+ T cells. The number on each plot indicates the average percentage of total MLN cells for each treatment group (± SEM, n = 5 mice per treatment group). Bars represent the average number of (B) Db/NP366–374-specific CD8+ cells (flu-specific), (C) CD44hiCD62LloCD8+ cells (CTL), and (D) IFNγ+CD8+ cells in each treatment group (± SEM; n = 5 mice per treatment group). An * indicates a significant difference in cell number compared with vehicle control (p ≤ .05). Abbreviations: AhR, aryl hydrocarbon receptor; CTL, cytotoxic T lymphocyte; FICZ, 6-formylindolo[3,2-b]carbazole; IFN, interferon; MHC, major histocompatibility complex; MLN, mediastinal lymph node; NP, nucleoprotein; VEH, vehicle.
AhR Activation Is More Transient With a Single Dose of FICZ Compared With a Single Dose TCDD
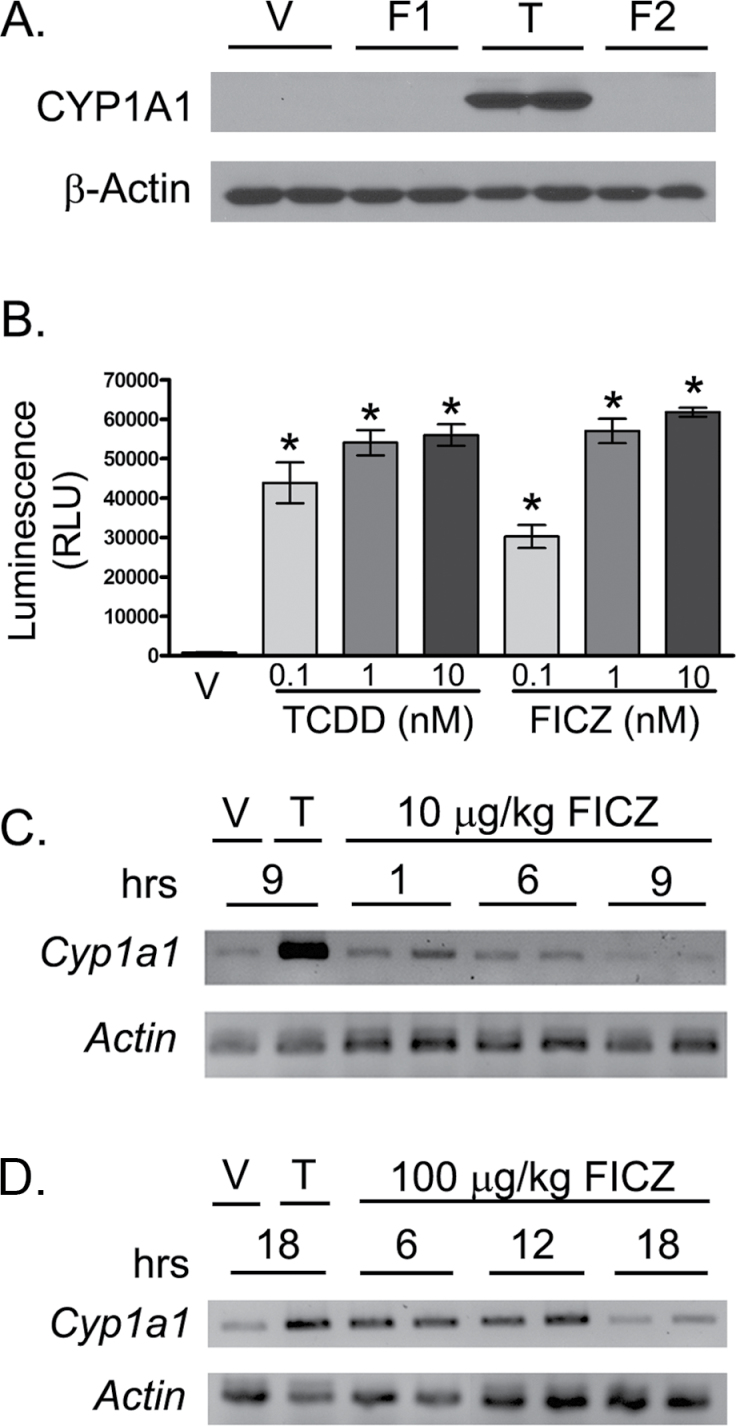
In C57BL/6 mice, the in vivo half-life of TCDD is about 11 days (Gasiewicz et al., 1983); however, the in vivo half-life of FICZ in mice remains to be formally determined. Like TCDD, FICZ is a potent inducer of Cyp1a1, 1a2, and 1b1 (Wei et al., 1998; Wincent et al., 2009). However, unlike TCDD, FICZ is readily metabolized, largely by CYP1A1 (Mukai and Tischkau, 2007), which suggests that the in vivo half-life of FICZ is likely shorter than TCDD’s. Using the induction of CYP1A1 as a measure of AhR activation, we compared a single oral dose (10 µg/kg) of either TCDD or FICZ administered 1 day prior to infection. In mice given TCDD, increased CYP1A1 protein levels were observable in the lung 7 days after infection (Fig. 3A). However, there was no difference in CYP1A1 levels in lungs of mice that received a single oral dose of FICZ (Fig. 3A, lanes labeled F1). Moreover, administering 10 µg/kg FICZ twice daily starting 1 day prior to, and continuing until 7 days after infection, did not result in detectable CYP1A1 levels in the lung (Fig. 3A, lanes labeled F2). In separate studies, we directly compared the ability of FICZ and TCDD to activate the AhR. Using a mouse hepatoma cell line that has been stably transfected with a luciferase gene under the control of the Cyp1a1 promoter, FICZ and TCDD elicit similar levels of AhR activation in an in vitro system (Fig. 3B). Collectively, this information suggests that FICZ results in transient AhR activation in vivo, whereas TCDD treatment with the same oral dose results in sustained receptor activation.
FIG. 3.
FICZ is a more transient activator of the AhR than TCDD. A, C57BL/6 mice were gavaged with 10 µg/kg TCDD, 10 µg/kg FICZ, or peanut oil vehicle 1 day prior to infection. Mice receiving FICZ were dosed either once a day (F1) or every 12h (F2) until day 7 postinfection. Lung homogenates were subjected to SDS-PAGE and probed for CYP1A1. β-Actin levels were assessed as a control. Blots show 2 representative samples from each treatment group (n = 6–7 mice per treatment group). B, Triplicate wells of H1L1.1c2 cells (2×105 cells/well) were cultured overnight in media containing 0.1% DMSO (V) or the indicated concentration of TCDD or FICZ. The graphs depict the mean (± SEM) luciferase activity in each treatment group. C and D, C57BL/6 mice (2–3 per group) were gavaged with vehicle control, 10 μg/kg TCDD (C), 10 µg/kg FICZ (C), or 100 µg/kg FICZ (D), and Cyp1a1 gene expression levels were determined examined at the indicated points in time (hours) relative to treatment using RT-PCR, as described in the Materials and Methods section. PCR products were run on an agarose gel and visualized by ethidium bromide staining. Representative data are shown, where T = TCDD and V = vehicle. An * indicates a significant difference in compared with vehicle control group (p ≤ .05). Abbreviations: AhR, aryl hydrocarbon receptor; DMSO, dimethysulfoxide; FICZ, 6-formylindolo[3,2-b]carbazole; RT-PCR, reverse transcription-PCR; SDS-PAGE, SDS-polyacrylamide gel electrophoresis; TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin.
To test this idea further, we examined Cyp1a1 levels several hours after AhR ligand exposure (Fig. 3C). Compared with mice given the vehicle control, increased Cyp1a1 expression was discernible 1 and 6h after gavage with 10 µg/kg FICZ. However, by 9h, Cyp1a1 levels were not different from vehicle-treated mice. In contrast, Cyp1a1 expression is still clearly elevated 9h later in mice administered the same oral dose of TCDD (Fig. 3C). When treated with 10-fold more FICZ (100 μg/kg), Cyp1a1 levels were qualitatively higher and persisted longer than when mice were treated with the lower dose; however, 18h after treatment, Cyp1a1 levels were not different than vehicle-treated mice (Fig. 3D). Together, these data suggest that a single oral dose of TCDD results in AhR activation being sustained for the duration of the immune response to infection (ie, up to at least 9 days), whereas a single dose of FICZ transiently activates AhR, with the effect waning in less than 18h. This implies that sustained rather than transient activation of the AhR may be necessary to observe altered immune responses to influenza virus over the course of the infection.
Prolonged FICZ-Mediated AhR Activation During Infection Does Not Alter the Response to Infection That Are Modulated When AhR Is Activated Using TCDD
To test whether the duration of AhR activation influences immune responses to influenza virus, we sought to prolong FICZ-mediated AhR activation in vivo. To ensure the delivery of a consistent dose of FICZ during infection, micro-osmotic pumps were used to administer 10 μg FICZ/kg body weight/h throughout the course of infection. To monitor whether AhR activation was prolonged by administering FICZ in this manner, Cyp1a1 expression was measured during the course of infection. Mice implanted with FICZ-loaded pumps had higher expression of Cyp1a1 compared with mice that were implanted with pumps loaded with vehicle. Moreover, Cyp1a1 induction was sustained up to 9 days postinfection (Fig. 4A). Thus, the induction of Cyp1a1 was qualitatively similar in duration to that caused by a single oral dose of TCDD (Fig. 4B).
FIG. 4.
FICZ-mediated AhR activation is prolonged through micro-osmotic pump implantation. Female C57BL/6 mice were treated with vehicle (VEH) or TCDD as described in Figure 1 or surgically implanted with subcutaneous micro-osmotic pumps loaded with either vehicle control (45% HPβCD) or FICZ released at a concentration of 10 μg/kg body weight/h. Mice were infected as described in Figure 1, 1 day after implantation or gavage. On the indicated days postinfection, RNA was isolated from lung and qRT-PCR was performed on RNA isolated from lungs of (A) mice implanted with FICZ-loaded (FICZ PUMP) and vehicle-loaded (VEH PUMP) micro-osmotic pumps and (B) TCDD and vehicle-treated mice. The mean fold change (± SEM) in Cyp1a1 was determined using the 2−ΔΔCT method with L13 as the control gene (n = 2–9 mice per treatment group per day). An * indicates a significant difference in fold change Cyp1a1 expression compared with the vehicle control mice sacrificed on the same day of infection (p ≤ .05). Abbreviations: AhR, aryl hydrocarbon receptor; FICZ, 6-formylindolo[3,2-b]carbazole; HPβCD, hydroxypropyl-β-cyclodextrin; qRT-PCR, quantitative reverse transcription-PCR; TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin.
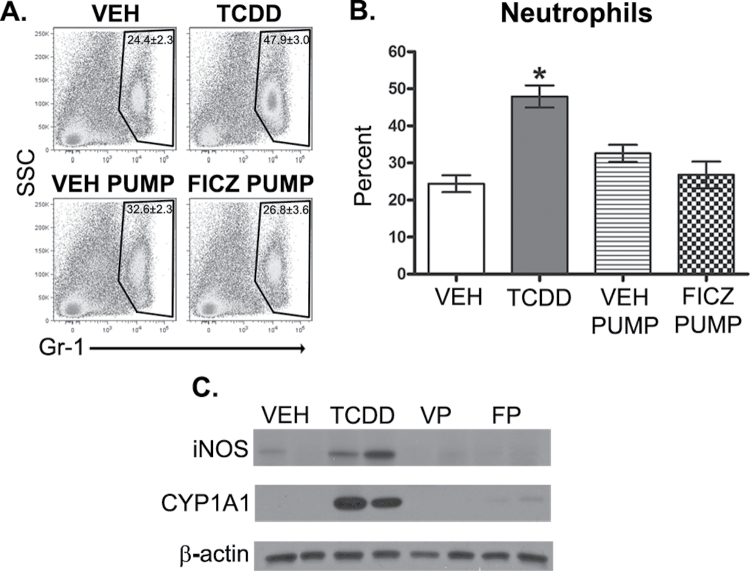
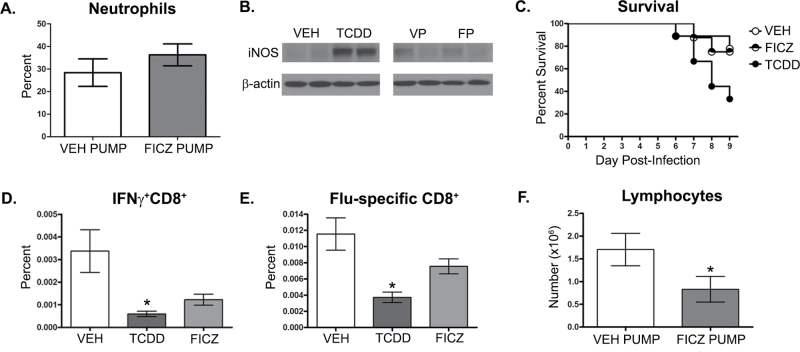
Using this approach, we investigated whether prolonged AhR activation with FICZ, implanted in micro-osmotic pumps, mimics the immunomodulatory effects of TCDD-mediated AhR activation. In contrast to a single dose of TCDD, continuous FICZ dosing did not cause a significant difference in neutrophil frequency (percent or number) in the lungs of infected mice compared with infected mice implanted with vehicle-loaded pumps (Fig. 5A). Similarly, iNOS levels in the lung were unchanged following prolonged AhR activation with FICZ during infection (Fig. 5B). The effect of prolonging AhR activation by FICZ on CD8+ T-cell responses was also evaluated. Both the percentage and number of influenza-specific CD8+ T cells were reduced by TCDD but were not altered in infected mice implanted with FICZ-loaded pumps (Figs. 6A and 6B). Additionally, the number of CTL and IFNγ+CD8+ T cells remained unchanged following FICZ-pump treatment (Figs. 6C and 6D). Thus, even with continuous FICZ exposure, these metrics of host response to influenza virus infection were unaffected.
FIG. 5.
Prolonged AhR activation with FICZ does not alter neutrophilia or iNOS levels in the infected lung. Mice were treated and infected as described in Figure 4 (n = 4–8 mice per treatment group). A, On day 7 postinfection, immune cells were isolated from collagenase-digested lung, stained with antibodies against Gr-1 and analyzed by flow cytometry. Representative dot plots depict the percentage of Gr-1+ cells in the lung ± SEM (upper right corner). B, The average percentage of neutrophils in the lung was also determined by performing differential cell counts on hematoxylin and eosin–stained cytospins of lung-derived immune cells (n = 4–8 mice per treatment group). C, Lung from mice treated orally with vehicle (VEH), TCDD, implanted with vehicle pump (VP), and FICZ pump (FP) were homogenized, proteins subjected to SDS-PAGE, and probed for iNOS and CYP1A1 by immunoblotting. β-Actin levels were assessed as a control. Blots show 2 representative samples from each treatment group (n = 4–8 mice per treatment group). An * indicates a significant difference compared with vehicle control (p ≤ .05). Abbreviations: AhR, aryl hydrocarbon receptor; FICZ, 6-formylindolo[3,2-b]carbazole; iNOS, inducible nitric oxide synthase; SDS-PAGE, SDS-polyacrylamide gel electrophoresis; TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin.
FIG. 6.
Prolonged AhR activation with FICZ does not suppress the CD8+ T-cell response to influenza virus infection. Mice were treated and infected as described in Figure 4. On day 9 postinfection, MLN cells were collected and stained with MHC class I tetramers and antibodies as described in the Materials and Methods section. A, Representative dot plots depict the frequency of Db/NP366–374-specific (virus-specific) CD8+ T cells obtained from mice treated orally with vehicle (VEH), TCDD, implanted with vehicle pump (VEH PUMP), and FICZ-loaded pump (FICZ PUMP). The number on each plot indicates the percentage of total MLN cells (± SEM). Bar graphs show the average number of (B) virus NP-specific CD8+ T cells, (C) CD44hiCD62LloCD8+ (CTL), and (D) IFNγ+CD8+ T cells in each treatment group (± SEM) (n = 8–10 mice per treatment group). An * indicates a significant difference compared with vehicle control (p ≤ .05). Abbreviations: AhR, aryl hydrocarbon receptor; FICZ, 6-formylindolo[3,2-b]carbazole; IFN, interferon; MHC, major histocompatibility complex; MLN, mediastinal lymph node; NP, nucleoprotein; TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin.
Prolonged FICZ Dosing in Influenza Virus–Infected Cyp1a1-Deficient Mice Does Not Enhance Pulmonary Inflammation But Causes Slight Reductions in CD8+ T-Cell Responses
One explanation for these findings is that although the pumps prolong the presence of FICZ in vivo, FICZ is still readily metabolized by CYP1A1 (Wei et al., 1998, 2000). If so, then a comparison of immune modulation when AhR is activated in vivo by these 2 distinct ligands may be skewed by the rapid metabolism of FICZ versus the poor metabolism of TCDD. In order to evaluate the influence of FICZ metabolism, we used Cyp1a1-deficient mice (Cyp1a1 −/− mice). Specifically, Cyp1a1 −/− mice were implanted with either FICZ or vehicle-loaded pumps 1 day before infection. When pulmonary inflammation was evaluated at day 7 postinfection, no difference in neutrophil frequency or iNOS levels were observed in the lungs of Cyp1a1 −/− mice that were implanted with FICZ pumps compared with vehicle controls (Figs. 7A and 7B). In contrast, Cyp1a1 −/− mice treated with TCDD had significantly enhanced pulmonary neutrophilia and elevated iNOS levels, similar to TCDD-treated wild-type mice (data not shown). Additionally, mice treated with FICZ-loaded pumps exhibited infection-associated morbidity and mortality that were more similar to vehicle-treated controls, whereas enhanced mortality was observed in the TCDD-treated group (Fig. 7C).
FIG. 7.
Cyp1a1 −/− mice have slight reductions in CD8+ T-cell responses to influenza virus infection, but not in lung neutrophil or iNOS levels, following prolonged AhR activation with FICZ. Cyp1a1−/− mice were treated and infected as described in Figure 4. A, On day 7 postinfection, mice implanted with either vehicle (VEH PUMP) or FICZ-loaded pumps (FICZ PUMP) were sacrificed and immune cells were isolated from collagenase-digested lung and the average percentage of neutrophils were determined by performing differential cell counts on hematoxylin and eosin–stained cytospins of lung-derived immune cells (n = 4–8 mice per treatment group). B, On day 7 postinfection, lungs from mice implanted with vehicle pump (VP) or FICZ pump (FP) were homogenized, proteins subjected to SDS-PAGE, and probed for iNOS. β-Actin levels were assessed as a control. Representative samples from orally treated VEH and TCDD mice were used at negative and positive controls, respectively. Blots show 2 representative samples from each treatment group (n = 4–8 mice per treatment group). C, Survival was monitored up to 9 days postinfection in orally treated VEH, TCDD, or mice implanted with pumps loaded with FICZ (FICZ PUMP) (D and E). MLN were isolated from mice on day 9 postinfection and stained with antibodies for flow cytometry as described in Figure 2. Bar graphs show the average percentage (± SEM) of (D) IFNγ+CD8+ T cells and (E) influenza virus NP-specific CD8+ T cells in each treatment group (n = 8–10 mice per treatment group). F, On day 7 postinfection, immune cells were isolated from collagenase-digested lung and the average percentage of lymphocytes were determined by performing differential cell counts on hematoxylin and eosin–stained cytospins of lung-derived immune cells (n = 4–8 mice per treatment group). An * indicates a significant difference compared with vehicle control (p ≤ .05). Abbreviations: AhR, aryl hydrocarbon receptor; FICZ, 6-formylindolo[3,2-b]carbazole; IFN, interferon; iNOS, inducible nitric oxide synthase; MLN, mediastinal lymph node; NP, nucleoprotein; SDS-PAGE, SDS-polyacrylamide gel electrophoresis; TCDD, 2,3,7,8 tetrachlorodibenzo-p-dioxin.
CD8+ T-cell responses in infected Cyp1a1 −/− mice were also examined. In the absence of CYP1A1-mediated metabolism, prolonged FICZ treatment slightly diminished the magnitude of the CD8+ T-cell response to infection. Specifically, the percentage of IFNγ+CD8+ T cells were reduced in FICZ-treated Cyp1a1 −/− mice compared with vehicle controls, although this did not reach statistical significance (p = .07, Fig. 7D). Slight reductions in the frequency of NP-specific CD8+ T cells were also observed in FICZ-treated mice (p = .06, Fig. 7E). Consistent with these observations in the lymph nodes, infected FICZ-treated Cyp1a1 −/− mice had significantly fewer lymphocytes in their lungs compared with vehicle-treated Cyp1a1 −/− mice (Fig. 7F). However, the diminution of these responses in Cyp1a1 −/− mice was not as substantial as the change caused by a single dose of TCDD 1 day prior to infection (data not shown). These findings suggest that although innate immune responses may be unaffected by reduced metabolism of and prolonged exposure to FICZ, decreasing FICZ metabolism may attenuate the CD8+ T-cell response to influenza virus infection.
DISCUSSION
The AhR is a fascinating molecule, as it is a ligand-activated transcription factor triggered by a broad spectrum of structurally diverse ligands (Nguyen and Bradfield, 2008). Evidence from numerous research groups demonstrates that it plays a role in the development and function of the immune system. However, it is not completely understood how different AhR ligands modulate immune function. In the work presented here, we compared the consequences of AhR activation by TCDD and FICZ on the 3 distinct components of the response to infection with a common respiratory virus. Rather than skew responses to infection in a manner that is opposite of TCDD, FICZ had no effect whatsoever. Specifically, whether FICZ was administered orally in a single bolus, given twice daily, or released continuously over the course of infection, pulmonary neutrophilia, iNOS levels in the infected lung, and the clonal expansion and differentiation of CD8+ T cells were not altered. One interpretation of these findings is that AhR-dependent pathways in certain immune cell populations, such as CD4+ T cells, could be more sensitive to differential AhR ligation than others. In the EAE model, CD4+ T cells are one of the critically important cell types involved in progression of the disease (Chen and Shannon, 2013). However, CD4+ T cells are not required for host resistance to most primary influenza virus infections (Belz et al., 2001). Instead, viral clearance and host resistance are heavily dependent on the response of CD8+ T cells and neutrophils (Tate et al., 2009; Topham et al., 1997). Moreover, although an AhR-mediated reduction in the CD8+ T-cell response to infection requires AhR in hematopoietic cells, increased neutrophilia and iNOS levels in the infected lung are due to AhR activation in nonhematopoietic cell lineages (Lawrence et al., 2006; Neff-LaFord et al., 2007; Teske et al., 2008; Wheeler et al., 2013). Therefore, it is possible that differential AhR activation could have varying effects dependent upon the specific cell type in which the receptor is activated. Thus, although the FICZ-mediated immunomodulation, such as that observed in the EAE model, may be dependent on AhR activation within CD4+ T cells, the immune perturbation caused by AhR activation during influenza virus infection may be dependent on activation of the AhR in non-CD4+ cell types.
Other discoveries support the idea that different AhR ligands may have varying effects depending on the model system. For example, during occular herpes simplex virus (HSV) infection, inflammatory lesions and total CD4+ T-cell number were reduced in TCDD-treated mice compared with infected controls; yet, TCDD skewed differentiation of CD4+ T cells toward Tregs (Veiga-Parga et al., 2011). However, treatment with FICZ failed to cause any change in the immune response to HSV. In another study, FICZ had no effect on peanut allergen sensitization, whereas TCDD significantly modulated multiple parameters of the allergic response, including antibody and cytokine production, and the frequency of CD4+CD25+Foxp3+ cells (Schulz et al., 2012). In contrast to these reports of null effects, FICZ reduced pulmonary eosinophilia and Th2 cytokines in the lungs of mice in an ovalbumin (OVA) model of allergic asthma (Jeong et al., 2012) in a manner quite similar to reported consequences of TCDD-mediated AhR activation in OVA and house dust mite allergy models (Luebke et al., 2001; Nohara et al., 2002). Collectively, these findings suggest that immune responses are sensitive to differential AhR activation, but differences in the consequences may be dependent on not only key cell types involved but also site of antigen challenge and type of infection or insult.
Another potential explanation for dissimilarities in modulation of the response to influenza virus by FICZ versus TCDD is the duration of AhR activation. We addressed this possibility by simultaneously prolonging FICZ’s presence using micro-osmotic pumps and reducing the metabolism of FICZ using Cyp1a1 −/− mice. In the absence of CYP1A1, TCDD still altered immune responses to influenza virus, suggesting that the induction of CYP enzymes and changes in immune function occur by two independent, AhR-mediated pathways. This is consistent with earlier reports, in which thymic atrophy and dysregulated leukocyte distribution were observed, but hepatotoxicity was attenuated, in Cyp1a1 −/− mice given a very high dose of TCDD (Uno et al., 2004). Yet, our data suggest that duration of activation alone does not account for immunomodulatory differences between these two AhR ligands, given that FICZ still had no effect on neutrophil frequency or lung iNOS levels in Cyp1a1 −/− mice. However, there was a modest reduction in the response of CD8+ T cells in Cyp1a1 −/− mice treated with FICZ, suggesting that TCDD’s longer lasting effects on AhR activation can be mimicked by continual FICZ treatment if its metabolism is reduced. This idea is consistent with prior reports that have shown differential sensitivities among immune cell subsets to AhR activation. For example, during infection with influenza virus, enhanced pulmonary neutrophilia waned faster than the suppressed lymphocyte responses with decreasing doses of TCDD (Vorderstrasse et al., 2003). In other words, suppression of lymphocyte responses may be slightly more sensitive to AhR modulation than enhanced lung inflammation. Thus, when clearance of FICZ was reduced in Cyp1a1 −/− mice, the effects on T cells were evident even though, at the dose of FICZ administered, pulmonary inflammation was not altered.
Another observation reported herein is that, although the pattern of increased Cyp1a1 induction by FICZ when implanted in pumps was qualitatively similar in duration to that induced by a single oral dose of TCDD, the overall level of Cyp1a1 expression was approximately 10-fold lower. FICZ and TCDD have high affinities for the AhR (FICZ K d = 0.07nM, TCDD K d = 0.48nM) (Rannug et al., 1987); thus, binding affinity per se is unlikely to explain this difference. Our data are similar to findings from Shulz et al. (2012), in which TCDD induced Cyp1a1, 1a2, 1b1 mRNA, and EROD activity at levels much greater than FICZ. There are several reasons why FICZ, while having a slightly higher affinity for AhR than TCDD, may not induce AhR target genes at levels similar to TCDD. It has been posited that the intrinsic properties of structurally distinct ligands influence downstream consequences of AhR activation (Denison et al., 2011). This phenomenon has been reported for other ligand-activated nuclear receptors, in which distinct ligands result in differences in receptor conformation, which are thought to influence the “quality” of activation through recruitment of various coactivators and transcription proteins (Jin and Li, 2010). Therefore, it is plausible that activation of the AhR with structurally distinct ligands induces different conformational states. Although a crystal structure has not yet been elucidated for the AhR, this idea is supported by some studies using homology modeling (Pandini et al., 2007). Also, variation in coactivator recruitment and histone modifications at AhR target genes have been reported for different AhR ligands (Hestermann and Brown, 2003; Ovesen et al., 2011; Pansoy et al., 2010). It has also been shown that compounds with different toxic equivalency factors have strikingly similar histone marking profiles on genes downstream of AhR activation (Ovesen et al., 2011). Additionally, mouse fibroblasts treated with another AhR ligand, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), had qualitatively similar patterns of gene induction compared with TCDD (Henry et al., 2010). However, although the same genes were induced by ITE and TCDD in this study, differences in the kinetics of the response were observed. Related to this concept is the fact that different ligands are metabolized at different rates. Thus, disparities in gene expression and downstream functional consequences of AhR activation by FICZ versus TCDD could be due to the rapid metabolism of FICZ. Although in vitro studies reveal that CYP1A1 is the primary enzyme responsible for FICZ metabolism, CYP1B1, CYP1A2, and possibly other enzymes also affect its metabolism (Mukai and Tischkau, 2007). Indeed, it is important to realize that complete in vivo pharmacokinetic data on FICZ remain to be fully developed. Hence, complete inhibition of FICZ metabolism in vivo may be difficult to achieve without an improved understanding of the in vivo metabolism of this tryptophan photodegradation product. Our findings add to the body of knowledge about in vivo metabolism and biological effects of FICZ. When combined with reports from others (Wei et al., 1998; Wincent et al., 2009, 2012), this information collectively demonstrates that differences in the nature of AhR activation and ligand metabolism could affect the manner in which different ligands modulate pathophysiology in vivo.
As we consider the idea of targeting AhR signaling in connection with treating human disease, these studies emphasize that one must be cautious about assuming that an AhR ligand’s actions at the cellular level will necessarily translate to that compound’s ability to modulate complex pathophysiological processes in vivo. Moreover, even when there is compelling evidence that a particular AhR ligand can alter disease course in vivo, it is critically important to realize that the immunomodulatory efficacy of this ligand may depend upon, and indeed vary among, specific in vivo contexts. A better understanding of how different AhR ligands alter immune function in multiple model systems will shed light on not only mechanisms through which the AhR could be manipulated as a therapy for disease but will also inform public health risks of environmental ligands and help to reveal the endogenous functions of this enigmatic receptor.
FUNDING
National Institutes of Health (R01-ES017250, RC2-ES018750, R01-HL097141, T32-ES07026, P30-ES01247); University of Rochester; National Institues of Health Career Development Award (K02-ES012409 to B.P.L.); Bristol-Myers Squibb Drug Safety Evaluation/Toxicology Pilot Research Award (to J.L.H.W.).
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank Dr Guangbi Jin, Amanda Moore, and Bethany Winans for technical support on experiments, and Dr Jacob Finklestein and members of his laboratory for assistance with the luciferase assay system. We are also deeply grateful to the generosity of others, in particular Dr Daniel Nebert (University of Cincinnati) for providing the original breeding stock of the Cyp1a1 −/− mice, and Dr Michael Denison (University of California at Davis) for the H1L1.1c2 cells.
REFERENCES
- Abel J., Haarmann-Stemmann T. (2010). An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 391, 1235–1248 [DOI] [PubMed] [Google Scholar]
- Akaike T., Noguchi Y., Ijiri S., Setoguchi K., Suga M., Zheng Y. M., Dietzschold B., Maeda H. (1996). Pathogenesis of influenza virus induced pneumonia: Involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. U.S.A. 93, 2448–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz G. T., Xie W., Doherty P. C. (2001). Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J. Immunol. 166, 4627–4633 [DOI] [PubMed] [Google Scholar]
- Bohonowych J. E., Denison M. S. (2007). Persistent binding of ligands to the aryl hydrocarbon receptor. Toxicol. Sci. 98, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchiel S. W., Luster M. I. (2001). Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 98, 2–10 [DOI] [PubMed] [Google Scholar]
- Chen G., Shannon M. (2013). Transcription factors and th17 cell development in experimental autoimmune encephalomyelitis. Crit. Rev. Immunol. 33, 165–182 [DOI] [PubMed] [Google Scholar]
- Dallaire F., Dewailly E., Vezina C., Muckle G., Weber J. P., Bruneau S., Ayotte P. (2006). Effect of prenatal exposure to polychlorinated biphenyls on incidence of acute respiratory infections in preschool Inuit children. Environ. Health Perspect. 114, 1301–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T. P., Dieter M. Z., Matlib R. S., Childs N. L., Shertzer H. G., Genter M. B., Nebert D. W. (2000). Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem. Biophys. Res. Commun. 267, 184–189 [DOI] [PubMed] [Google Scholar]
- Denison M. S., Soshilov A. A., He G., DeGroot D. E., Zhao B. (2011). Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 124, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn K. J., Belz G. T., Altman J. D., Ahmed R., Woodland D. L., Doherty P. C. (1998). Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8, 683–691 [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Mimura J. (2005). Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem. Biophys. Res. Commun. 338, 311–317 [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Geiger L. E., Rucci G., Neal R. A. (1983). Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug. Metab. Dispos. 11, 397–403 [PubMed] [Google Scholar]
- Gasiewicz T. A., Henry E. C., Collins L. L. (2008). Expression and activity of aryl hydrocarbon receptors in development and cancer. Crit. Rev. Eukaryot. Gene. Expr. 18, 279–321 [DOI] [PubMed] [Google Scholar]
- Henry E. C., Welle S. L., Gasiewicz T. A. (2010). TCDD and a putative endogenous AhR ligand, ITE, elicit the same immediate changes in gene expression in mouse lung fibroblasts. Toxicol. Appl. Pharmacol. 114, 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestermann E. V., Brown M. (2003). Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol. Cell. Biol. 23, 7920–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong K. T., Hwang S. J., Oh G. S., Park J. H. (2012). FICZ, a tryptophan photoproduct, suppresses pulmonary eosinophilia and Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma. Int. Immunopharmacol. 13, 377–385 [DOI] [PubMed] [Google Scholar]
- Jin L., Li Y. (2010). Structural and functional insights into nuclear receptor signaling. Adv. Drug Deliv. Rev. 62, 1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence B. P., Denison M. S., Novak H., Vorderstrasse B. A., Harrer N., Neruda W., Reichel C., Woisetschlager M. (2008). Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low molecular weight compound. Blood 112, 1158–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence B. P., Kerkvliet N. I. (2006). Immune modulation by TCDD and related polyhalogenated aromatic hydrocarbons. In Immunotoxicology and Immunopharmacology (Luebke R., House R., Kimber I., Eds.), 3 ed., pp. 239–258 CRC Press, Taylor & Francis Group, Boca Raton, FL [Google Scholar]
- Lawrence B. P., Roberts A. D., Neumiller J. J., Cundiff J. A., Woodland D. L. (2006). Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung. J. Immunol. 177, 5819–5828 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Luebke R. W., Copeland C. B., Daniels M., Lambert A. L., Gilmour M. I. (2001). Suppression of allergic immune responses to house dust mite (HDM) in rats exposed to 2,3,7,8-TCDD. Toxicol. Sci. 62, 71–79 [DOI] [PubMed] [Google Scholar]
- Mimura J., Yamashita K., Nakamura K., Morita M., Takagi T. N., Nakao K., Ema M., Sogawa K., Yasuda M., Katsuki M., et al. (1997). Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 2, 645–654 [DOI] [PubMed] [Google Scholar]
- Mukai M., Tischkau S. A. (2007). Effects of tryptophan photoproducts in the circadian timing system: Searching for a physiological role for aryl hydrocarbon receptor. Toxicol. Sci. 95, 172–181 [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Dalton T. P., Okey A. B., Gonzalez F. J. (2004). Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 279, 23847–23850 [DOI] [PubMed] [Google Scholar]
- Neff-LaFord H., Teske S., Bushnell T. P., Lawrence B. P. (2007). Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. J. Immunol. 179, 247–255 [DOI] [PubMed] [Google Scholar]
- Nguyen L., Bradfield C. (2008). The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 21, 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K., Izumi H., Tamura S., Nagata R., Tohyama C. (2002). Effect of low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on influenza A virus-induced mortality in mice. Toxicology 170, 131–138 [DOI] [PubMed] [Google Scholar]
- Ovesen J. L., Schnekenburger M., Puga A. (2011). Aryl hydrocarbon receptor ligands of widely different toxic equivalency factors induce similar histone marks in target gene chromatin. Toxicol. Sci. 121, 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini A., Denison M. S., Song Y., Soshilov A. A., Bonati L. (2007). Structural and functional characterization of the aryl hydrocarbon receptor ligand binding domain by homology modeling and mutational analysis. Biochemistry 46, 696–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansoy A., Ahmed S., Valen E., Sandelin A., Matthews J. (2010). 3-methylcholanthrene induces differential recruitment of aryl hydrocarbon receptor to human promoters. Toxicol. Sci. 117, 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., Weiner H. L. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 [DOI] [PubMed] [Google Scholar]
- Rannug A., Rannug U., Rosenkranz H. S., Winqvist L., Westerholm R., Agurell E., Grafstrom A. K. (1987). Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J. Biol. Chem. 262, 15422–15427 [PubMed] [Google Scholar]
- Safe S. (1990). Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit. Rev. Toxicol. 21, 51–88 [DOI] [PubMed] [Google Scholar]
- Schrenk D. (1998). Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo- and xenobiotics. Biochem. Pharmacol. 55, 1155–1162 [DOI] [PubMed] [Google Scholar]
- Schulz V. J., Smit J. J., Huijgen V., Bol-Schoenmakers M., van Roest M., Kruijssen L. J., Fiechter D., Hassing I., Bleumink R., Safe S., et al. (2012). Non-dioxin-like AhR ligands in a mouse peanut allergy model. Toxicol. Sci. 128, 92–102 [DOI] [PubMed] [Google Scholar]
- Stevens E. A., Mezrich J. D., Bradfield C. A. (2009). The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology 127, 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolevik S. B., Nygaard U. C., Namork E., Haugen M., Kvalem H. E., Meltzer H. M., Alexander J., van Delft J. H., Loveren H., Lovik M., et al. (2011). Prenatal exposure to polychlorinated biphenyls and dioxins is associated with increased risk of wheeze and infections in infants. Food Chem. Toxicol. 49, 1843–1848 [DOI] [PubMed] [Google Scholar]
- Tate M. D., Deng Y. M., Jones J. E., Anderson G. P., Brooks A. G., Reading P. C. (2009). Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J. Immunol. 183, 7441–7450 [DOI] [PubMed] [Google Scholar]
- Tate M. D., Ioannidis L. J., Croker B., Brown L. E., Brooks A. G., Reading P. C. (2011). The role of neutrophils during mild and severe influenza virus infections of mice. PloS One 6, e17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske S., Bohn A. A., Hogaboam J. P., Lawrence B. P. (2008). Aryl hydrocarbon receptor targets pathways extrinsic to bone marrow cells to enhance neutrophil recruitment during influenza virus infection. Toxicol. Sci. 102, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske S., Bohn A. A., Regal J. F., Neumiller J. J., Lawrence B. P. (2005). Exploring mechanisms that underlie aryl hydrocarbon receptor-mediated increases in pulmonary neutrophilia and diminished host resistance to influenza A virus. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, 111–124 [DOI] [PubMed] [Google Scholar]
- Tijet N., Boutros P. C., Moffat I. D., Okey A. B., Tuomisto J., Pohjanvirta R. (2006). Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol. Pharmacol. 69, 140–153 [DOI] [PubMed] [Google Scholar]
- Topham D. J., Tripp R. A., Doherty P. C. (1997). CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159, 5197–5200 [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. (1986). The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44, 959–968 [DOI] [PubMed] [Google Scholar]
- Uno S., Dalton T. P., Sinclair P. R., Gorman N., Wang B., Smith A. G., Miller M. L., Shertzer H. G., Nebert D. W. (2004). Cyp1a1(-/-) male mice: Protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol. Appl. Pharmacol. 196, 410–421 [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel R. L., Koppen G., Staessen J. A., Hond E. D., Verheyen G., Nawrot T. S., Roels H. A., Vlietinck R., Schoeters G. E. (2002). Immunologic biomarkers in relation to exposure markers of PCBs and dioxins in Flemish adolescents (Belgium). Environ. Health Perspect. 110, 595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel J. P., Clark G. C., Thompson C. L., McCoy Z., Miller C. R., Lucier G. W., Bell D. A. (1993). CYP1A1 mRNA levels as a human exposure biomarker: Use of quantitative polymerase chain reaction to measure CYP1A1 expression in human peripheral blood lymphocytes. Carcinogenesis 14, 2003–2006 [DOI] [PubMed] [Google Scholar]
- Veiga-Parga T., Suryawanshi A., Rouse B. T. (2011). Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathogens 7, e1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A. M., Buer J., Dumoutier L., Renauld J. C., Stockinger B. (2008). The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 [DOI] [PubMed] [Google Scholar]
- Vorderstrasse B. A., Bohn A. A., Lawrence B. P. (2003). Examining the relationship between impaired host resistance and altered immune function in mice treated with TCDD. Toxicology 188, 15–28 [DOI] [PubMed] [Google Scholar]
- Wei Y. D., Bergander L., Rannug U., Rannug A. (2000). Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole. Arch. Biochem. Biophys. 383, 99–107 [DOI] [PubMed] [Google Scholar]
- Wei Y. D., Helleberg H., Rannug U., Rannug A. (1998). Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole. Chem. Biol. Interact. 110, 39–55 [DOI] [PubMed] [Google Scholar]
- Wheeler J. L., Martin K. C., Lawrence B. P. (2013). Novel cellular targets of AhR underlie alterations in neutrophilic inflammation and inducible nitric oxide synthase expression during influenza virus infection. J. Immunol. 190, 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincent E., Amini N., Luecke S., Glatt H., Bergman J., Crescenzi C., Rannug A., Rannug U. (2009). The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J. Biol. Chem. 284, 2690–2696 [DOI] [PubMed] [Google Scholar]
- Wincent E., Bengtsson J., Mohammadi Bardbori A., Alsberg T., Luecke S., Rannug U., Rannug A. (2012). Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U.S.A. 109, 4479–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziccardi M. H., Gardner I. A., Denison M. S. (2000). Development and modification of a recombinant cell bioassay to directly detect halogenated and polycyclic aromatic hydrocarbons in serum. Toxicol. Sci. 54, 183–193 [DOI] [PubMed] [Google Scholar]