Abstract
Background:
The use of virtual microscopy (VM) in clinical cytology has been limited due to the inability to focus through three dimensional (3D) cell clusters with a single focal plane (2D images). Limited information exists regarding the optimal scanning parameters for 3D scanning.
Aims:
The purpose of this study was to determine the optimal number of the focal plane levels and the optimal scanning interval to digitize gynecological (GYN) specimens prepared on SurePath™ glass slides while maintaining a manageable file size.
Subjects and Methods:
The iScanCoreo Au scanner (Ventana, AZ, USA) was used to digitize 192 SurePath™ glass slides at three focal plane levels at 1 μ interval. The digitized virtual images (VI) were annotated using BioImagene's Image Viewer. Five participants interpreted the VI and recorded the focal plane level at which they felt confident and later interpreted the corresponding glass slide specimens using light microscopy (LM). The participants completed a survey about their experiences. Inter-rater agreement and concordance between the VI and the glass slide specimens were evaluated.
Results:
This study determined an overall high intra-rater diagnostic concordance between glass and VI (89-97%), however, the inter-rater agreement for all cases was higher for LM (94%) compared with VM (82%). Survey results indicate participants found low grade dysplasia and koilocytes easy to diagnose using three focal plane levels, the image enhancement tool was useful and focusing through the cells helped with interpretation; however, the participants found VI with hyperchromatic crowded groups challenging to interpret. Participants reported they prefer using LM over VM. This study supports using three focal plane levels and 1 μ interval to expand the use of VM in GYN cytology.
Conclusion:
Future improvements in technology and appropriate training should make this format a more preferable and practical option in clinical cytology.
Keywords: Cytology, digital pathology, virtual microscopy, z-axis
INTRODUCTION
Virtual microscopy (VM) is a digital imaging technology which involves scanning specimens on glass slides at high resolution and converting them into virtual images (VI). VM has been implemented in various fields of research and education and its advantages have been affirmed,[1,2,3,4,5,6,7,8,9,10] however, the application of VM in clinical cytopathology is still limited.[11,12] The primary obstacle is the three-dimensional (3D) nature of cytology specimens compared with two-dimensional (2D) histology specimens. In contrast to surgical and histological specimens, which consist of a thin 2D sheet of tissue, cytology specimens particularly the ones prepared using a liquid based method, have 3D cell arrangements and groups.
It is often beneficial to analyze the morphology of the cell clusters from different cell layers in order to accurately interpret cytology cases. This cannot be achieved with 2D VI, which are scanned with a single focal plane.[13] In order to capture the 3D arrangement of cell clusters on a glass slide, 3D scanning or z-stack, also called z-axis scanning, is required.[14,15] The z-axis scanning is attained by having multiple scans of the same slide taken at various focal planes and stacked into a final composite image.[13,16]
The z-axis method has been found to be useful in focusing through the cell clusters,[17,18] however, this method has several disadvantages.[1,3,8,12,13,19] For instance, 3D VI requires an extended time to digitize it and results in large file size. Scanning a cytology specimen prepared on SurePath™ glass slide with z-stack (7 focal plane levels) requires approximately 40 minutes and results in 11 GB file size.[20] Additional focal plane levels cause longer scanning time and larger file size.[21] This results in extended download time as well as the need for a large server capacity to store the scanned images. In addition, the technology itself, and the skilled technical support are costly to acquire.
Therefore, even if the advantages of the technology outweigh its disadvantages, z-axis scanning faces significant barriers before it can be effectively implemented in the field of clinical cytopathology. This is due to the fact that specific scanning parameters have not yet been defined or established to scan cytology specimens;[22] hence, the study is needed to identify these parameters. The purpose of this study was to determine the optimal number of the focal plane levels and the optimal scanning interval necessary to digitize gynecological (GYN) specimens prepared on SurePath™ glass slides while maintaining the file size at a minimum.
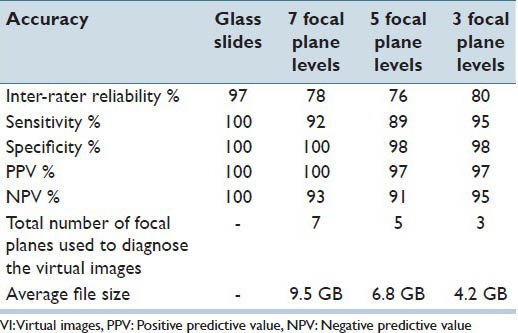
This study was conducted in three phases. Phase I, which aimed to determine the optimal scanning interval necessary to digitize GYN specimens prepared on SurePath™ glass slides, determined that VI scanned at 1 μ interval had the highest inter-rater reliability, sensitivity and negative predictive value (NPV) while using a lower number of focal plane level [Table 1]. In addition, the number of focal planes was narrowed down from 13 to 7 [Table 2].[23] Phase II, aimed to determine the number of focal planes necessary to digitize GYN specimens prepared on SurePath™ glass slides determined that, VI scanned with three focal plane levels at 1 μ interval had the highest inter-observer reliability, sensitivity, NPV and the lowest file size [Table 3].[24]
Table 1.
Inter-rater reliability, sensitivity, specificity, PPV, NPV and the total number of focal planes used to interpret the VI scanned using 13 focal plane levels at 1 μ, 0.8 μ and 0.5 μ interval levels
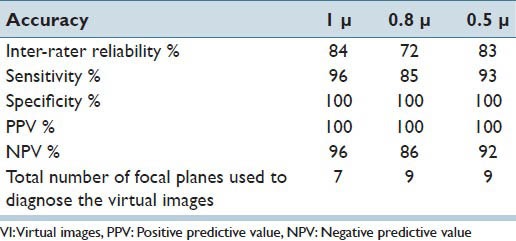
Table 2.
Number of focal planes used by the participants to interpret the VI scanned using 13 focal plane levels at 1 μ, 0.8 μ and 0.5 μ intervals
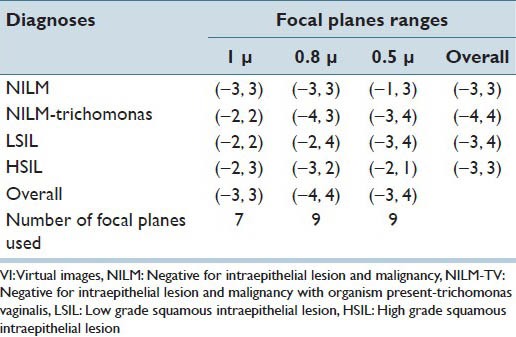
Table 3.
Inter-rater reliability, sensitivity, specificity, PPV, NPV and the total number of focal planes used to interpret the VI scanned at 7, 5, 3 focal plane levels at 1 μ interval
In this paper, the final phase (phase 3) of the study is described. This study aimed: (a) To determine concordance between diagnoses obtained using VI scanned using the determined number of the focal plane levels and scanning interval to glass slides specimens and (b) to evaluate pathologists, pathology residents, and cytotechnologist perception for VM as a diagnostic tool compared with light microscopy (LM) in diagnosing GYN cytology specimens. The hypotheses were that for the GYN specimens prepared on SurePath™ glass slides: (a) The diagnoses made on the VI scanned using three focal plane levels at 1 μ interval would be concordant with those of the glass slide specimens and (b) the study participants would equally accept the use of VM when compared with LM.
SUBJECTS AND METHODS
After approval by the Institutional Review Board was granted, a total of 192 previously diagnosed and archived SurePath™ liquid based-cervical cytology glass slide specimens were retrieved from the cytology laboratory using CopathPlus computer application. The retrieved slides contained ink dots made by the cytotechnologists and the pathologists who originally interpreted or diagnosed the cases. The selected glass slides were scanned with the original ink dots placed by the cytotechnologists and the pathologists who interpreted the cases. However, these dotted glass slides resulted in blurry images and out of focus cells. It was believed that the scanner was focusing on the ink dots rather than the cells while scanning which resulted in blurriness of the images. The dotted slides were photocopied for future references, de-identified with new labels and the ink dots were removed prior to scanning to obtain optimal scans, per the advice of a BioImagene technologist. Based on the results of our Phase I and Phase II studies, these de-identified slides were scanned using the iScanCoreo Au Scanner (Ventana, Inc., Tuscon, AZ, USA) at ×40 magnification (Numerical Aperture = 0.75) at three focal plane levels and 1 μ interval. Ultimately, a total of 192 VI were produced with an average file size of 4.2 gigabytes. The output image files in bif (BioImagene Format), were saved in a password protected encrypted external hard drive.
After scanning the glass slides, original dots from the photocopies of the glass slides were placed back on the glass slides for the purpose of the study. These newly dotted glass slides were used as a reference base to annotate (virtually dot) the corresponding digitized VI using the software Image Viewer (version 3.0.0.0) (Ventana, Inc., Tucson, AZ, USA) a web-based application by BioImagene. Two cytopathologists, two pathology residents, and a cytotechnologist participated in this study and were given a brief training session on accessing the VI saved on the encrypted external hard drive and using the Image Viewer software to screen the given VI. After that, the participants were asked to look at the pre-annotated VI and provide their interpretations by selecting from the given options: Negative for intraepithelial lesion and malignancy (NILM), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL); and unable to diagnose. They were encouraged to review the cells in addition to the annotated cells in the VI if they felt it necessary and to use the image enhancement feature of the software, which helps to increase/decrease the brightness and contrast of the image. They were also asked to record the focal plane level at which they felt most confident to interpret the cases. In addition, they were encouraged to comment on the quality of the VI on a diagnosis sheet.
All participants independently interpreted the VI using their personal workstations and computer monitors. After a period of 2 weeks, the participants reviewed the corresponding glass slides using the conventional LM and rendered their interpretations. To interpret the glass slide specimens, the participants were asked to review the pre-dotted cells on the glass slides and give their interpretations by selecting from the following diagnostic categories: NILM, LSIL, HSIL and unable to diagnose. They were encouraged to review cells in addition to the annotated cells if they felt it necessary. In addition, they were encouraged to give comments on a glass slide specimens in the comment section of the diagnosis log sheet.
At the end of the study, all the participants were sent a link to an online survey website (www.surveymonkey.com) and requested to respond anonymously and voluntarily to a series of questions with responses ranging from (a) strongly agree, (b) agree, (c) neutral, (d) disagree, (e) strongly disagree. The survey consisted of 14 statements. The first five statements compared technical aspects of the VM and LM. The rest of the statements concerned the participants’ experiences in interpreting the GYN specimen using VM. The survey also had an open comment section for additional suggestions, to explain/justify their answers and to add any other comments not mentioned in the series of questions.
Statistical Analysis
In GYN cytology, LSIL and HSIL are two different grades of dysplasia. Although they have different prognostic implications, the patient care or immediate follow-up is the same, however, if a diagnosis of negative or normal is given the patient will not be given the same follow-up. For this reason, statistical analysis was also conducted by considering LSIL and HSIL as one single category.
Concordance
Concordance between the interpretations obtained using VI and the glass slides by all the participants were evaluated. Concordance between the glass slide and VM was attained when all five participants agreed on the diagnoses for the patient using both VM and glass slide. Concordance was summarized using a proportion and a 95% confidence interval. For each participant, concordance was defined as agreement between his/her interpretation of the glass slide and VM with reference diagnosis. Concordance for each participant was summarized using a proportion and a 95% confidence interval.
Inter-rater Reliability
Inter-rater reliability was determined for all cases in which the LGSIL and HGSIL was combined together (NILM, LSIL/HSIL) using kappa statistics.
For interpretation of kappa statistics, a kappa statistic below 0.00 was considered as “poor agreement”; 0.00-0.20 was considered as “slight agreement,” 0.21-0.40 was considered as “fair agreement,” 0.41-0.60 was considered as “moderate agreement”; 0.61-0.80 was considered as “substantial agreement” and 0.81-1.00 was considered as “almost perfect agreement.”[25]
Analyses were conducted using SAS/STAT® software for Windows version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Case Details
The set of 192 cases consisted of 85 NILM, 91 LGSIL and 16 HGSIL. The 192 cases were interpreted by the five participants using glass slides/LM and VI/VM scanned using three focal plane levels at 1 μ interval.
Concordance
Concordance for Interpretations among All the Participants
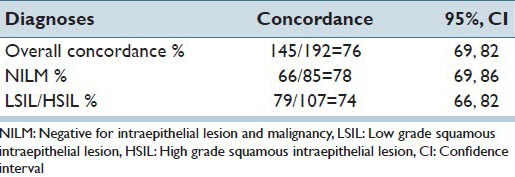
With LSIL and HSIL combined as one category, the overall concordance for NILM, LSIL/HSIL and unable to diagnose, the five raters correctly identified and showed complete agreement on the glass and VM slides 76% of the time (95% CI: 0.69, 0.82). For LSIL/HSIL cases only, the five participants correctly identified and showed complete agreement on the glass and VM slides for 74% of the time [Table 4].
Table 4.
Concordance for diagnosis: NILM, LSIL/HSIL or unable to diagnose
Intra-rater Diagnostic Concordance
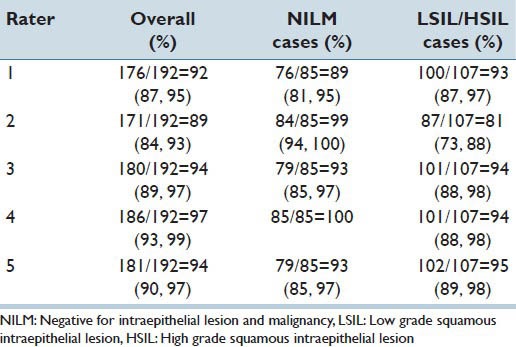
The overall concordance between the glass slides and VI for the cases, NILM, LSIL/HSIL, and unable to diagnose was 92% for participant one, 89% for participant two, 94% for participant three, 97% for participant four and 94% for participant five [Table 5].
Table 5.
Concordance for diagnosis: NILM, LSIL/HSIL or unable to diagnose by participant (intra-rater diagnostic concordance)
Inter-rater Reliability
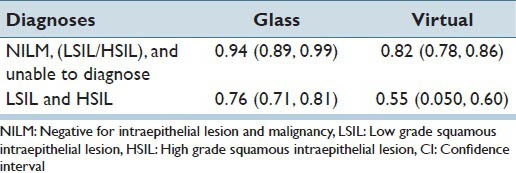
For the interpretations: NILM, LSIL/HSIL and unable to diagnose, the overall kappa among all participants using glass slides was 94% (almost perfect agreement). For the interpretations: NILM, LSIL/HSIL and unable to diagnose, the overall kappa among all participants using VI was 82% (almost perfect agreement). For exclusive positive cases (LGSIL, HGSIL), the overall inter-rater reliability was 76% (substantial agreement) for the glass slides and 55% (moderate agreement) for the VI [Table 6].
Table 6.
Inter-rater reliability (kappa and 95% CI)
Survey
All five participants voluntarily and anonymously participated in the online survey. The participants’ responses to the survey statements are summarized in Tables 7 and 8.
Table 7.
Participants’ responses to survey statements regarding Light microscopy compared to Virtual microscopy in diagnosing the gynecological cases.
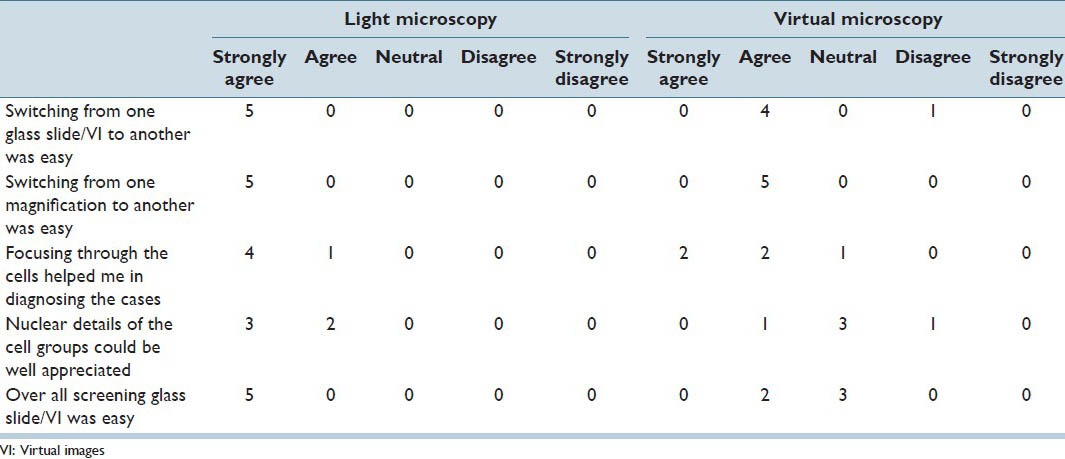
Table 8.
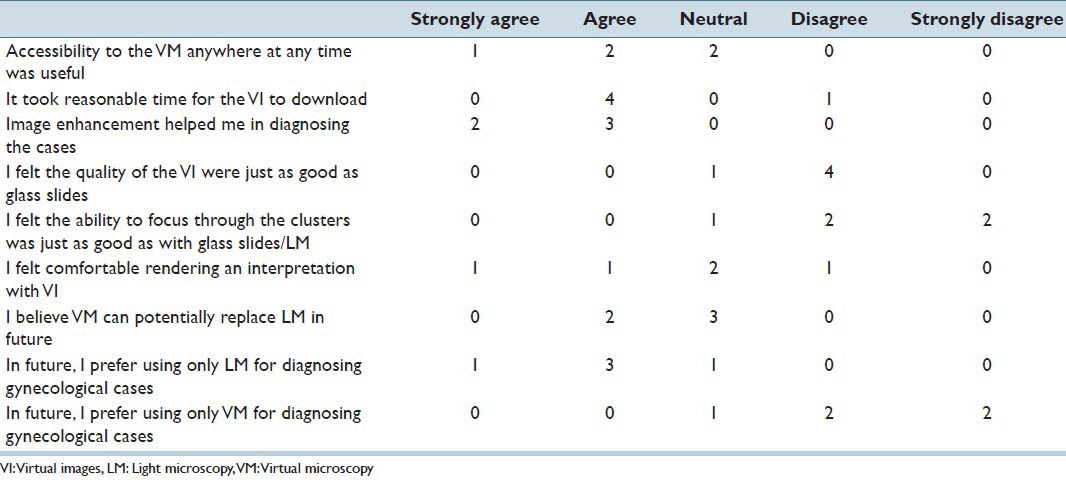
Participants’ responses to survey statements regarding virtual microscopy in diagnosing gynecological cases
Glass Slides
All participants rated glass slides and LM positively for all the statements. Switching from one glass to another and switching the magnification while screening the glass slide specimens were considered easy. Focusing through the cells in the glass slide specimens helped the participants to diagnose the cases. The participants could appreciate the nuclear detail of the cell groups in the glass slide specimens. Overall screening the glass slide specimens was considered to be easy.
Virtual Images
All participants stated that switching from one magnification in the VI to another was easy. Focusing through the cells helped four out of five participants in diagnosing the cases in VM. Switching from one image to another was easy for four out of five participants. Three participants were undecided on appreciation of nuclear details in the VI and ease of the VI screening; however, two out of five participants rated VI screening to be easy.
Participants’ Perception on VM Screening Experience
The image enhancement tool of the software helped all participants in diagnosing the GYN cases. Four out of five participants felt that the VI took a reasonable time to download. Accessibility of VI from anywhere at any time was considered useful for three out of five participants. Four out of five participants did not feel the quality of the VI and focusing through the cell clusters in the VI was similar to that of glass slide specimens and LM. Only two participants felt comfortable rendering an interpretation using VI while, one participant disagreed and two participants had neutral opinions. Four out of five participants preferred to use only LM for diagnosing GYN cases. Four out of five participants did not prefer to use VM alone for diagnosing GYN cases in the future. Even though three participants indicated neutral opinions on VM potentially replacing LM in the future, two participants believed in the replacement by VM.
DISCUSSION
The overall purpose of this study was to determine the optimal number of focal plane levels and the optimal scanning interval necessary to digitize GYN specimens prepared on SurePath™ glass slides while maintaining the file size at a minimum.
In spite of its success in surgical pathology specimens, VM has not been widely utilized in cytology. The primary reason for this is the need for z-axis scanning to focus through the 3D cell clusters of the cytological specimens. Several studies have been conducted to investigate the currently available z-axis scanning method. When VM was evaluated in terms of diagnostic performances, it was found that the VI scanned using 21 focal plane levels at a 1.5 μ interval provided the best images with good depth of focus when compared with the VI scanned using five focal plane levels at a 1 μ interval.[18] Another study which determined the accuracy and efficiency of VI (scanned at ×20, ×40, and ×40 with a seven layer z-stack) as compared with traditional glass slides, reported that, among the VI, the ones scanned using ×40 or ×40 z-stack had the highest diagnostic accuracy. Even the VI scanned using seven focal plane levels were found to have a large file size (11 GB), though, thereby limiting the use of VM for daily diagnostic cytology/screening.[20] Our current study, therefore, was very important in determining optimal scanning parameters (the optimal number of focal planes and the optimal interval) necessary to digitize GYN cytology specimens prepared on SurePath™ glass slides while maintaining the file size to a minimum.
As the results of this study indicated, there was an excellent intra-rater concordance between the glass slides/LM and the VI scanned using three focal plane levels at 1 μ interval. While inter-rater agreement was higher for LM, it was still very good using the VI scanned three focal plane levels at 1 μ interval. Therefore, clinical application of VM using these scanning parameters was shown to be feasible in cytology, though, lower diagnostic concordance for the HSIL cases was observed in this study. It is important to mention here that this study had only 16 HSIL cases out of the 192 cases. There is a possibility that the concordance level for HSIL would be higher if there were more HSIL cases. The investigators re-reviewed the glass slides and VI for all HSIL cases. They determined the quality of the VI were not as good with these cases. There were many hyperchromatic crowded groups and very few single cells. For this type of specimen, more levels may be necessary. Just as we re-process specimens in the laboratory, we may have to re-scan VI in order to achieve the best image possible for diagnoses.
Participants in this study indicated that accurate interpretation can be given in most of the virtual cases, however the screening time compared with LM may be prohibitive. The screening time was not recorded in this study, but a longer screening time for VI compared with glass slides has been reported in previous studies.[18,20] This longer screening time on VM could be in part due to lack of familiarity and/or lack of training on the new technology. The reviewers of our previous studies,[23,24] as well as the current study, were pathologists, cytotechnologists and pathology residents. These reviewers had years of experience in screening glass slide specimens using LM during their clinical practice. Their experience with glass slide and LM was several-fold more than their experience with VM. It is hypothesized that appropriate training would decrease the time to screen the VI in the same manner as the screening time with glass slides (LM) decreases with training.
A decreasing trend in misinterpretation in the later set of VI cases in comparison to the earlier set was observed. This may be interpreted as an improvement in VM abilities with training. Future studies need to determine the effect of training on the accuracy of interpretation in VM. In this study, a participant who was younger and less experienced with LM compared to the other participants, interpreted more cases (5 NILM, 4 LSIL and 4 HSIL) correctly using VM than using LM. This could be due to younger individuals-who are more adaptable to the new technology of VM or have less bias towards LM since they have not used it as long as the experienced participants. Further studies, however, which statistically compare the accuracy of diagnosis between a younger and less experienced LM group and an older and more experienced LM group, are needed to prove this hypothesis.
Although the VI scanned using three focal plane levels and 1 μ interval had some dark clusters making it difficult to focus through the cells and the nuclear material, the participants had the following observations for these VI: (a) The overall quality of images was good; (b) LGSIL cases and koilocytes were very easy to diagnose using the three focal plane levels; (c) It was quicker to move between annotated spots on the virtual slides versus glass slides; (d) the image enhancement tool was found to be useful in many cases; (e) switching from one slide to another was easy; (f) switching from one magnification to another was easy; and, (g) focusing through the cells helped in interpreting the cases. This indicates that the technological advancements in the z-axis scanning methods had helped in improving the perception of the participants for VM.
It is believed that using the scanning parameters of three focal plane levels and 1 μ interval will enable VM to become a more viable option in GYN cytology. In addition, with these scanning parameters, the file size (averaging 4.2 GB), and therefore the required storage space is less.
CONCLUSIONS
This study concludes that, while considering the file size and intra-rater diagnostic concordance, the optimal scanning parameters for SurePath™ prepared GYN cytology specimens are three focal plane levels at a 1 μ interval. Although the VI produced using these scanning parameters were sometimes challenging for the participants especially when hyperchromatic crowded groups were present, future improvements in the technology and appropriate training should enhance the ease of use and make this format practical in clinical cytology.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2013/4/1/38/124015
REFERENCES
- 1.Steinberg DM, Ali SZ. Application of virtual microscopy in clinical cytopathology. Diagn Cytopathol. 2001;25:389–96. doi: 10.1002/dc.10021. [DOI] [PubMed] [Google Scholar]
- 2.Kalinski T, Zwönitzer R, Sel S, Evert M, Guenther T, Hofmann H, et al. Virtual 3D microscopy using multiplane whole slide images in diagnostic pathology. Am J Clin Pathol. 2008;130:259–64. doi: 10.1309/QAM22Y85QCV5JM47. [DOI] [PubMed] [Google Scholar]
- 3.Mori I, Nunobiki O, Ozaki T, Taniguchi E, Kakudo K. Issues for application of virtual microscopy to cytoscreening, perspectives based on questionnaire to Japanese cytotechnologists. Diagn Pathol. 2008;3(Suppl 1):S15. doi: 10.1186/1746-1596-3-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen PS, Lindebjerg J, Rasmussen J, Starklint H, Waldstrøm M, Nielsen B. Virtual microscopy: An evaluation of its validity and diagnostic performance in routine histologic diagnosis of skin tumors. Hum Pathol. 2010;41:1770–6. doi: 10.1016/j.humpath.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Neel JA, Grindem CB, Bristol DG. Introduction and evaluation of virtual microscopy in teaching veterinary cytopathology. J Vet Med Educ. 2007;34:437–44. doi: 10.3138/jvme.34.4.437. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg HR, Dintzis R. The positive impact of team-based virtual microscopy on student learning in physiology and histology. Adv Physiol Educ. 2007;31:261–5. doi: 10.1152/advan.00125.2006. [DOI] [PubMed] [Google Scholar]
- 7.Chen YK, Hsue SS, Lin DC, Wang WC, Chen JY, Lin CC, et al. An application of virtual microscopy in the teaching of an oral and maxillofacial pathology laboratory course. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:342–7. doi: 10.1016/j.tripleo.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Dee FR. Virtual microscopy in pathology education. Hum Pathol. 2009;40:1112–21. doi: 10.1016/j.humpath.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Merk M, Knuechel R, Perez-Bouza A. Web-based virtual microscopy at the RWTH Aachen University: Didactic concept, methods and analysis of acceptance by the students. Ann Anat. 2010;192:383–7. doi: 10.1016/j.aanat.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Szymas J, Lundin M. Five years of experience teaching pathology to dental students using the WebMicroscope. Diagn Pathol. 2011;6(Suppl 1):S13. doi: 10.1186/1746-1596-6-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantanowitz L. Digital images and the future of digital pathology. J Pathol Inform. 2010;1:15. doi: 10.4103/2153-3539.68332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilbur DC. Digital cytology: Current state of the art and prospects for the future. Acta Cytol. 2011;55:227–38. doi: 10.1159/000324734. [DOI] [PubMed] [Google Scholar]
- 13.Khalbuss WE, Pantanowitz L, Parwani AV. Digital imaging in cytopathology. Patholog Res Int. 2011;2011:264683. doi: 10.4061/2011/264683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilbur DC, Madi K, Colvin RB, Duncan LM, Faquin WC, Ferry JA, et al. Whole-slide imaging digital pathology as a platform for teleconsultation: A pilot study using paired subspecialist correlations. Arch Pathol Lab Med. 2009;133:1949–53. doi: 10.1043/1543-2165-133.12.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashiro K, Taira K, Matsubayashi S, Azuma M, Okuyama D, Nakajima M, et al. Comparison between a traditional single still image and a multiframe video image along the z-axis of the same microscopic field of interest in cytology: Which does contribute to telecytology? Diagn Cytopathol. 2009;37:727–31. doi: 10.1002/dc.21078. [DOI] [PubMed] [Google Scholar]
- 16.Giansanti D, Grigioni M, D’Avenio G, Morelli S, Maccioni G, Bondi A, et al. Virtual microscopy and digital cytology: State of the art. Ann Ist Super Sanita. 2010;46:115–22. doi: 10.4415/ANN_10_02_03. [DOI] [PubMed] [Google Scholar]
- 17.Dee FR, Donnelly A, Radio S, Leaven T, Zaleski MS, Kreiter C. Utility of 2-D and 3-D virtual microscopy in cervical cytology education and testing. Acta Cytol. 2007;51:523–9. doi: 10.1159/000325788. [DOI] [PubMed] [Google Scholar]
- 18.Evered A, Dudding N. Accuracy and perceptions of virtual microscopy compared with glass slide microscopy in cervical cytology. Cytopathology. 2011;22:82–7. doi: 10.1111/j.1365-2303.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 19.Stewart J, 3rd, Miyazaki K, Bevans-Wilkins K, Ye C, Kurtycz DF, Selvaggi SM. Virtual microscopy for cytology proficiency testing: Are we there yet? Cancer. 2007;111:203–9. doi: 10.1002/cncr.22766. [DOI] [PubMed] [Google Scholar]
- 20.Wright AM, Smith D, Dhurandhar B, Fairley T, Scheiber-Pacht M, Chakraborty S, et al. Digital slide imaging in cervicovaginal cytology: A pilot study. Arch Pathol Lab Med. 2013;137:618–24. doi: 10.5858/arpa.2012-0430-OA. [DOI] [PubMed] [Google Scholar]
- 21.Huisman A, Looijen A, van den Brink SM, van Diest PJ. Creation of a fully digital pathology slide archive by high-volume tissue slide scanning. Hum Pathol. 2010;41:751–7. doi: 10.1016/j.humpath.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Lee RE, McClintock DS, Laver NM, Yagi Y. Evaluation and optimization for liquid-based preparation cytology in whole slide imaging. J Pathol Inform. 2011;2:46. doi: 10.4103/2153-3539.86285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radio S, Mukherjee M, Wright N, Meza J, Donnelly A. The optimal z-axis interval and focal planes to digitize 3-D gynecological SurePath® glass slides: Initial findings. Cytojournal. 2011;8:16a. [Google Scholar]
- 24.Horn A, Radio S, Mukherjee M, Lele S, Wright N, Meza J, et al. Establishing optimal digital scanning parameters of 3-D gynecological virtual images: Follow up study. Mod Pathol. 2012;25:91A. [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]


