Abstract
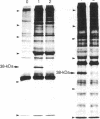
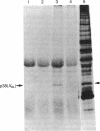
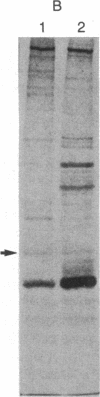
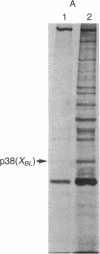
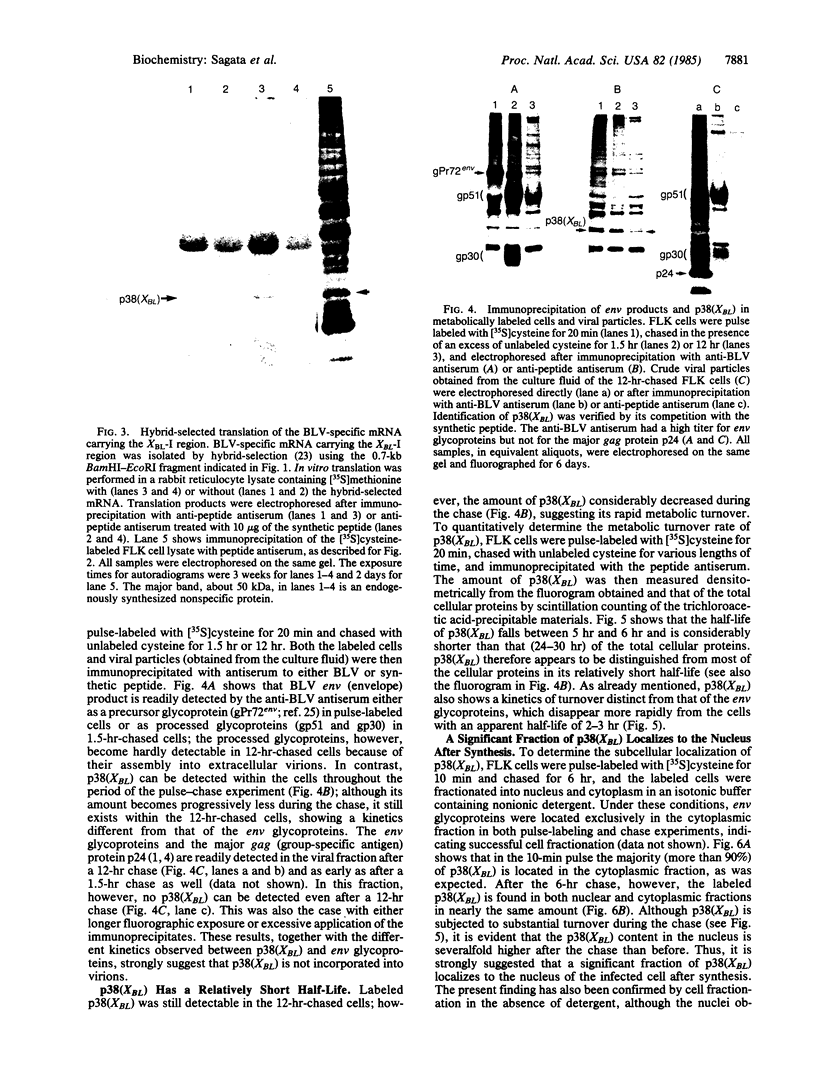
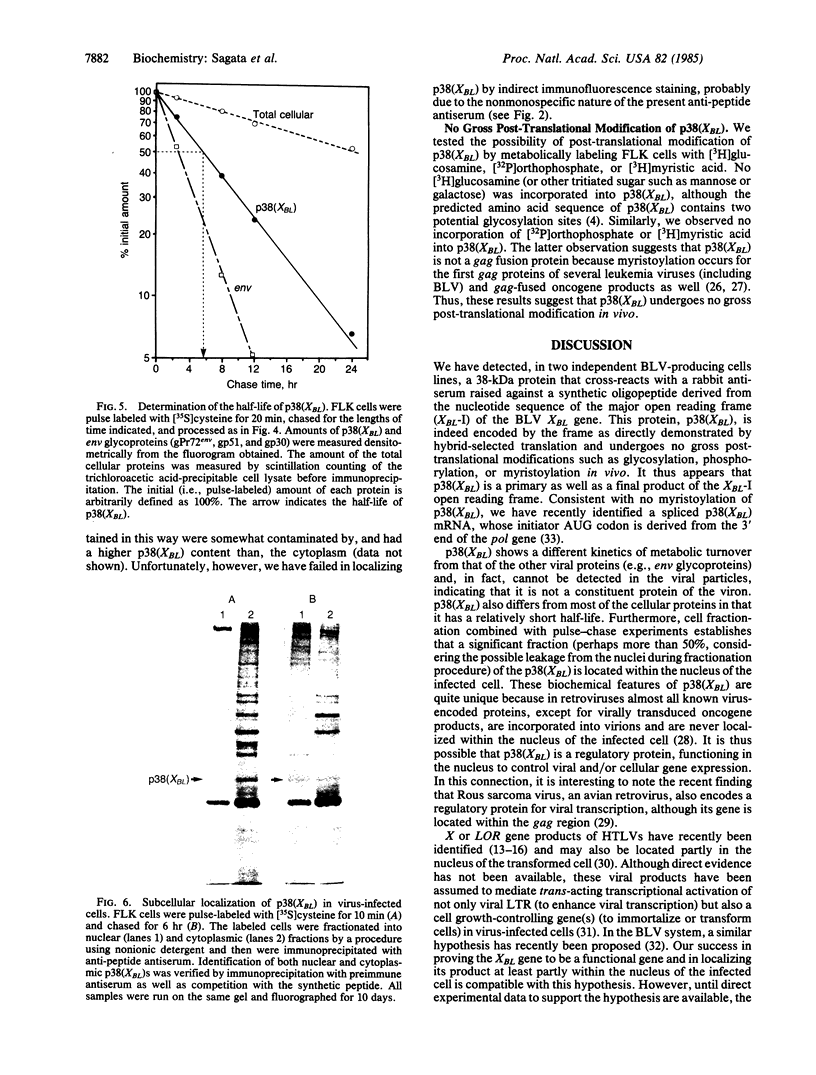
Using a rabbit antiserum directed against a synthetic oligopeptide whose sequence was deduced from the nucleotide sequence of the XBL gene of bovine leukemia virus, we detected a 38-kDa protein in virus-producing cell lines. In vitro translation of hybrid-selected RNA unequivocally demonstrates that this protein, designated p38(XBL), is indeed encoded by the XBL gene. Unlike the other virus-encoded proteins, however, p38(XBL) resides within the cells without being incorporated into virions. It undergoes no gross post-translational modifications and has a relatively short half-life (5-6 hr) in vivo. Furthermore, cell fractionation combined with pulse-chase experiment reveals that a significant fraction (more than half) of the p38(XBL) localizes to the nucleus of the infected cell after synthesis. We conclude that the XBL gene of bovine leukemia virus is a functional gene encoding a nonvirion protein p38(XBL), which possibly functions within the nucleus of the infected cell to regulate viral or cellular gene expression. p38(XBL) is presumably translated from a doubly spliced, bicistronic mRNA that has the capability to encode another small polypeptide in a different reading frame.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Rous sarcoma virus encodes a transcriptional activator. Cell. 1985 Mar;40(3):537–546. doi: 10.1016/0092-8674(85)90202-8. [DOI] [PubMed] [Google Scholar]
- Bruck C., Rensonnet N., Portetelle D., Cleuter Y., Mammerickx M., Burny A., Mamoun R., Guillemain B., van der Maaten M. J., Ghysdael J. Biologically active epitopes of bovine leukemia virus glycoprotein gp51: their dependence on protein glycosylation and genetic variability. Virology. 1984 Jul 15;136(1):20–31. doi: 10.1016/0042-6822(84)90244-7. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Kettmann R., Burny A. Experiments with cloned complete tumor-derived bovine leukemia virus information prove that the virus is totally exogenous to its target animal species. J Virol. 1981 Nov;40(2):605–609. doi: 10.1128/jvi.40.2.605-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Kettmann R., Burny A. Translation of bovine leukemia virus virion RNAs in heterologous protein-synthesizing systems. J Virol. 1979 Mar;29(3):1087–1098. doi: 10.1128/jvi.29.3.1087-1098.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W. C., Sodroski J., Rosen C., Essex M., Haseltine W. A. Subcellular localization of the product of the long open reading frame of human T-cell leukemia virus type I. Science. 1985 Mar 8;227(4691):1227–1228. doi: 10.1126/science.2983419. [DOI] [PubMed] [Google Scholar]
- Graves D. C., Ferrer J. F. In vitro transmission and propagation of the bovine leukemia virus in monolayer cell cultures. Cancer Res. 1976 Nov;36(11 Pt 1):4152–4159. [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Lucher L. A., Symington J. S., Kramer T. A. Human adenovirus 2 E1B-19K and E1B-53K tumor antigens: antipeptide antibodies targeted to the NH2 and COOH termini. J Virol. 1983 Dec;48(3):604–615. doi: 10.1128/jvi.48.3.604-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Couez D., Claustriaux J. J., Palm R., Burny A. Chromosome integration domain for bovine leukemia provirus in tumors. J Virol. 1983 Jul;47(1):146–150. doi: 10.1128/jvi.47.1.146-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa T., Seiki M., Imagawa K., Shimizu F., Yoshida M. Identification of a protein (p40x) encoded by a unique sequence pX of human T-cell leukemia virus type I. Gan. 1984 Sep;75(9):747–751. [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Sodroski J. G., Haseltine W. A., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Essex M. Antigens encoded by the 3'-terminal region of human T-cell leukemia virus: evidence for a functional gene. Science. 1984 Oct 5;226(4670):57–61. doi: 10.1126/science.6089350. [DOI] [PubMed] [Google Scholar]
- Miwa M., Shimotohno K., Hoshino H., Fujino M., Sugimura T. Detection of pX proteins in human T-cell leukemia virus (HTLV)-infected cells by using antibody against peptide deduced from sequences of X-IV DNA of HTLV-I and Xc DNA of HTLV-II proviruses. Gan. 1984 Sep;75(9):752–755. [PubMed] [Google Scholar]
- Onuma M., Sagata N., Okada K., Ogawa Y., Ikawa Y., Oshima K. Integration of bovine leukemia virus DNA in the genomes of bovine lymphosarcoma cells. Microbiol Immunol. 1982;26(9):813–820. doi: 10.1111/j.1348-0421.1982.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Onuma M., Watarai S., Suneya M., Mikami T., Izawa H. Induction of transformed phenotypes in sheep fibroblasts by culture fluids from cells persistently infected with bovine leukemia virus. Microbiol Immunol. 1981;25(5):445–454. doi: 10.1111/j.1348-0421.1981.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Sarngadharan M. G., Copeland T. D., Kalyanaraman V. S., Gilden R. V., Gallo R. C. Primary structure analysis of the major internal protein p24 of human type C T-cell leukemia virus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1291–1294. doi: 10.1073/pnas.79.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Kraus M., Arnstein P. Neoplastic transformation of fetal lamb kidney cells by bovine leukemia virus. Int J Cancer. 1983 Jun 15;31(6):791–795. doi: 10.1002/ijc.2910310620. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Couez D., Deschamps J., Kettmann R., Burny A., Gilden R. V. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology. 1984 Oct 15;138(1):82–93. doi: 10.1016/0042-6822(84)90149-1. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Burny A., Haseltine W. A. Trans activation of the bovine leukemia virus long terminal repeat in BLV-infected cells. Science. 1985 Jan 18;227(4684):320–322. doi: 10.1126/science.2981432. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ikawa Y. Identification of a potential protease-coding gene in the genomes of bovine leukemia and human T-cell leukemia viruses. FEBS Lett. 1984 Dec 3;178(1):79–82. doi: 10.1016/0014-5793(84)81244-2. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ogawa Y., Tsuzuku-Kawamura J., Ikawa Y. Bovine leukemia virus: unique structural features of its long terminal repeats and its evolutionary relationship to human T-cell leukemia virus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4741–4745. doi: 10.1073/pnas.81.15.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ohishi K., Tsuzuku-Kawamura J., Onuma M., Ikawa Y. Comparison of the entire genomes of bovine leukemia virus and human T-cell leukemia virus and characterization of their unidentified open reading frames. EMBO J. 1984 Dec 20;3(13):3231–3237. doi: 10.1002/j.1460-2075.1984.tb02283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Press M. F., Souza L. M., Murdock D. C., Cline M. J., Golde D. W., Gasson J. C., Chen I. S. Studies of the putative transforming protein of the type I human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1427–1430. doi: 10.1126/science.2990027. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Shimotohno K., Cline M. J., Golde D. W., Chen I. S. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science. 1984 Oct 5;226(4670):61–65. doi: 10.1126/science.6089351. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]