Summary
HPV oncogenes and ERα are required for cervical carcinogenesis. Transcriptional regulation by ERα is mediated by ERE-dependent and ERE-independent mechanism. Our current study demonstrates that ERE-dependent mechanism of ERα is required for cervical carcinogenesis in HPV transgenic mouse model.
Abstract
Cervical cancer is caused by human papillomavirus (HPV) in collaboration with other non-viral factors. The uterine cervix is hormone responsive and female hormones have been implicated in the pathogenesis of the disease. HPV transgenic mice expressing HPV16 oncogenes E6 (K14E6) and/or E7 (K14E7) have been employed to study a mechanism of estrogen and estrogen receptor α (ERα) in cervical carcinogenesis. A chronic exposure to physiological levels of exogenous estrogen leads to cervical cancer in the HPV transgenic mice, which depends on ERα. The receptor is composed of multiple functional domains including a DNA-binding domain (DBD), which mediates its binding to estrogen-responsive elements (EREs) on target genes. A transcriptional control of genes by ERα is mediated by either DBD-dependent (classical) or DBD-independent (non-classical) pathway. Although molecular mechanisms of ERα in cancer have been characterized extensively, studies investigating importance of each pathway for carcinogenesis are scarce. In this study, we employ knock-in mice expressing an ERα DBD mutant (E207A/G208A) that is defective specifically for ERE binding. We demonstrate that the ERα DBD mutant fails to support estrogen-induced epithelial cell proliferation and carcinogenesis in the cervix of K14E7 transgenic mice. We also demonstrate that cervical diseases are absent in K14E7 mice when one ERα DBD mutant allele and one wild-type allele are present. We conclude that the ERα classical pathway is required for cervical carcinogenesis in a mouse model.
Introduction
Although cervical cancer incidence has been decreasing in developed countries, the disease remains the second most prevalent malignancy and the second leading cause of death by cancer in women in developing countries (1). Most cervical malignancies arise in premenopausal women and are associated with high-risk human papillomavirus (HPV) (2). Regular Pap test and HPV vaccines are effective in preventing the cancer (3) but not readily available to women in developing countries or low-income population. In addition, the current therapies for cervical cancer (i.e. surgery, chemotherapy and radiation) are not effective in treating late stage or recurrent diseases (4). Precancer lesions [i.e. cervical intraepithelial neoplasia (CIN)] detected by Pap test can be easily removed by simple surgical procedures, thereby preventing the cancer. These surgeries, however, are associated with adverse outcomes in future pregnancy, including preterm birth and infant morbidity (5). A better understanding of cervical cancer pathogenesis is needed to develop a non-invasive method to manage the cancer and CIN more effectively.
A subset of >100 HPV types is causally associated with various human cancers including those in the uterine cervix (2). They are called high-risk HPVs and transmitted mainly by sexual contacts. Most commonly found in cancers is HPV16 followed by HPV18, both of which are commonly targeted by current prophylactic HPV vaccines (3). These two types account only for 70–80% of all cervical cancers and thus those vaccines have little impact on the remainder. High-risk HPVs code for E6 and E7 oncogenes and numerous studies have proved their potent oncogenic activities in vitro and in vivo (2,6). The tumorigenic potential of these viral oncoproteins depends on their ability to interact with various cellular proteins regulating cancer-associated cellular processes such as apoptosis and cell cycle (2). Most notably, E6 promotes degradation of a key apoptosis regulator p53 and E7 inactivates a cell cycle regulator pRb. E6 and E7 are required not only for the development of cervical cancer but also for its continued growth (7–9). Evidence also suggests that HPV is not sufficient to cause cervical cancer and other HPV-associated malignancies (6,10).
Several lines of epidemiological evidence implicate female hormones (e.g. estrogen) in HPV-associated cervical cancer (10,11). Consistently, estrogen is required for cervical cancer to develop in HPV transgenic mice (12,13). Cervical disease occurring in those mice is progressive and preferentially arises in the transformation zone, which mimics human cervical cancer (12). It also shows the expression pattern of biomarkers similar to that of the human cancer (14). Estrogen receptor α (ERα) is necessary for the development and continued growth of cervical cancer in the same mouse model but not for HPV oncogene functions (15,16). ERα is also crucial for estrogen-induced cell proliferation in the normal cervical epithelium (16).
ERα is a transcription factor and composed of multiple functional domains, activation function 1 domain, DNA-binding domain (DBD) and ligand-binding domain encompassing activation function 2 domain. In the classical pathway, the DBD is responsible for direct binding of ERα to estrogen-responsive elements (EREs) on its target genes, thereby regulating their transcription (17). In non-classical or tethering pathway, ERα indirectly binds DNA through interactions with other transcription factors such as AP1 and Sp1. Physiological importance of the ERα classical pathway in estrogen-responsive tissues such as uterus and bone has been demonstrated by studies using a knock-in mouse model, which expresses an ERα DBD mutant (E207A/G208A) defective for binding EREs (i.e. deficient for the classical pathway) (18–20). Using the same knock-in mice, we here report that the ERα DBD is required for estrogen-dependent cervical carcinogenesis in the K14E7 transgenic mouse model. Our results support that the ERα classical pathway is crucial for estrogen-dependent cervical cancer in vivo.
Materials and methods
Mice and treatments
Mice used in this study were described previously (18,21–24) and summarized in Supplementary Table SI, available at Carcinogenesis Online. All mice were genotyped by PCR. Some mice were ovariectomized at the age of 6–8 weeks and recovered for 2 weeks. They were then intraperitoneally injected with ethanol vehicle or 17β-estradiol (E2; 1 μg) for 7 days. For longer E2 treatments, slow-releasing E2 tablets (0.05mg/60 days) (Innovative Research of America, Sarasota, FL) were subcutaneously inserted under the dorsal skin every 2 months as described previously (25). Subsets of mice were intraperitoneally injected with bromo-deoxyuridine (BrdU) (3.75 mg per mouse) 1 h prior to killing to measure cell proliferation. All procedures were carried out according to animal protocols approved by the University of Houston Institutional Animal Care and Use Committee and the University of Wisconsin School of Medicine and Public Health Institutional Animal Care and Use Committee.
Tissue processing and histology
The female reproductive tracts were harvested, fixed in 4% paraformaldehyde and embedded in paraffin. The tissues were serially sectioned throughout cervices at 5 μm thickness. Every 10th slide was stained with hematoxylin and eosin and the worst disease was determined as described previously (13,14).
Immunohistochemistry
Antibodies were purchased from Santa Cruz Biotechnology [ERα (H184), progesterone receptor (PR) (H190) and p16], Calbiochem (BrdU), Neomarkers (MCM7), Thermo Scientific (Ki67) and Rockland Immunochemicals (biotinylated anti-rabbit/mouse IgG). For Ki67 immunohistochemistry, sections were sequentially incubated with anti-Ki67 antibody (1:200 in 5% goat serum in phosphate-buffered saline) and biotinylated anti-rabbit IgG, and then with ABC complex (Vector Laboratories) according to the manufacturer’s instructions. The immune complex was visualized by an incubation in 3,3′-diaminobenzidine (Sigma) dissolved in phosphate-buffered saline (0.5mg/ml). Immunohistochemistry for MCM7, PR, p16, ERα and BrdU was performed as described previously (14,26,27).
Statistical analyses
One-sided Fisher’s exact test and Wilcoxon rank-sum test were carried out with MSTAT software version 5.5, which may be downloaded at www.mcardle.wisc.edu/mstat. Fisher’s exact test was used for cancer incidence, and Wilcoxon rank-sum test for disease severity and percentage of Ki67+/BrdU+ cells.
Results
A functional ERα DBD is required for E2-induced cell proliferation in the cervical epithelium
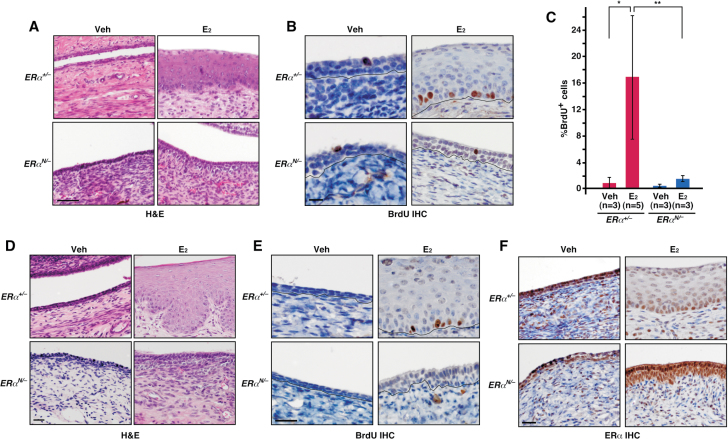
Epithelial cell proliferation in the mouse cervix depends on estrogen (E2) and ERα (16). To determine whether the ERα classical pathway is crucial for cervical epithelial cell proliferation, we employed the non-classical ER knock-in (NERKI) mice expressing the DBD mutant (E207A/G208A) (18). This mutant allele will be referred to as NERKI or N herein. Ovariectomized mice were treated with vehicle or E2, and cervical tissues were histologically evaluated and analyzed for BrdU incorporation to measure cells undergoing DNA synthesis. The epithelia of cervical tissues from vehicle-treated ERα +/− and ERα N/− mice were similarly hypoplastic (Figure 1A). The treatment with E2 resulted in physiologic hyperplasia in the cervical epithelium of ERα +/− but not ERα N/− mice (Figure 1A). Consistently, compared with vehicle control, number of BrdU-positive cervical epithelial cells was significantly increased upon E2 treatment in ERα +/− but not in ERα N/− mice (Figure 1B and C). We observed similar results in the vaginal epithelium (Figure 1D and E). ERα expression was predominant in the nuclei of cervical stromal and epithelial cells regardless of E2 treatment, which was similar in ERα +/− and ERα N/− mice (Figure 1F). These results demonstrate that the classical pathway of ERα (i.e. ERE binding) is required for E2-induced epithelial cell proliferation and thickening of epithelia in the lower reproductive tract of female mice.
Fig. 1.
Female lower reproductive tracts of ERα N/− mice lack physiologic responses to E2. (A) ERα DBD mutant fails to support E2-induced hyperplasia in the cervical epithelia. Ovariectomized mice of indicated genotype were treated with ethanol vehicle (Veh) or E2 for 7 days. Representative images of hematoxylin and eosin (H&E)-stained cervical sections are shown. Scale bar, 50 μm. (B) ERα DBD mutant fails to support E2-induced cell proliferation in the cervical epithelia. Cervical sections shown in A are analyzed by BrdU immunohistochemistry (IHC) and representative images are shown. Black lines separate epithelia from stroma. Scale bar, 10 μm. (C) Results shown in B are quantified and shown as mean ± standard error of the mean. Group size (n) is indicated. *P = 0.05, **P = 0.01. (D and E) ERα DBD mutant does not support physiologic responses to E2 in the vaginal epithelia. Vaginal tissue sections from mice described in A were stained with H&E (D) or for BrdU (E). Scale bars, 25 μm. (F) ERα expression in ERα N/− is similar to ERα +/− cervix. Cervical sections were stained for ERα and nuclei were counterstained with hematoxylin. Scale bar, 25 μm.
A functional ERα DBD is required for early stage of cervical carcinogenesis in K14E7 mice
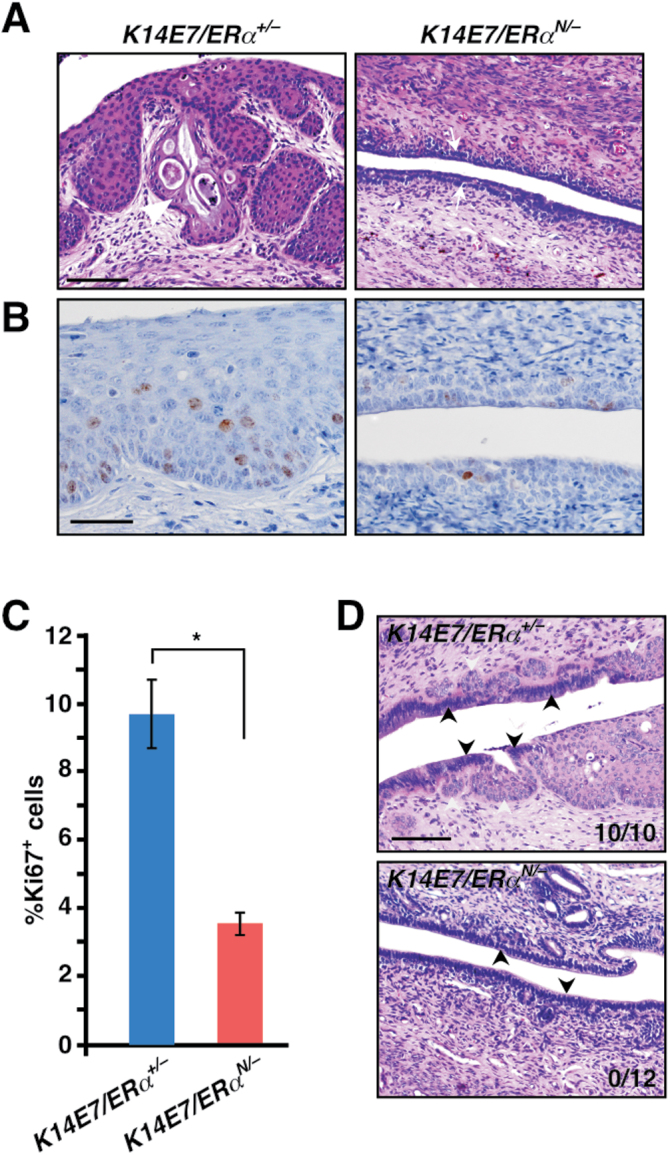
The phenotypes in ERα N/− mice described above were reminiscent of those observed in ERα −/− mice (16). We therefore hypothesized that cervical neoplasia would not arise in K14E7/ERα N/− mice similar to K14E7/ERα −/− mice (16). To test this hypothesis, we generated K14E7/ERα N/− and K14E7/ERα +/− mice and treated them for 6 months with E2. Three of 10 (30%) K14E7/ERα +/− mice had cervical cancer and the remainder had high-grade dysplasia, CIN2 or CIN3 (Table I). Cervical disease burden in this genotype was comparable with that in K14E7/ERα +/+ mice on a mixed genetic background (25), indicating that ERα is haplosufficient for cervical carcinogenesis. In contrast, none of 12 K14E7/ERα N/− mice had cervical cancer or CIN (Table I). Although difference in cancer incidence did not reach statistical significance (P = 0.08), overall cervical disease severity was significantly worse in K14E7/ERα +/− mice compared with K14E7/ERα N/− mice (P = 6.91×10−6). It was also evident that the cervical epithelia of K14E7/ERα N/− mice were hypoplastic as opposed to the hyperplastic epithelia in K14E7/ER +/− control mice (Figure 2A). Consistently, a fraction of Ki67-positive cells (i.e. proliferating cells) was dramatically diminished in K14E7/ERα N/− mice compared with K14E7/ERα +/− control mice (Figure 2B and C). Atypical squamous metaplasia (ASM) in the cervical transformation zone is believed to be the first step in cervical carcinogenesis and ERα is required for its development (16). We therefore investigated whether K14E7/ERα N/− mice lacked ASM. Identical to K14E7/ERα −/− mice (16), ASM was absent in all K14E7/ERα N/− mice, whereas it was present in all K14E7/ERα +/− control mice (Figure 2D). K14E7/ERα +/+ mice are also susceptible to vaginal neoplasia upon E2 treatment (13,16). Although all K14E7/ERα +/− control mice had varying severity of vaginal neoplastic disease including cancer, none of the K14E7/ERα N/− mice displayed such disease (Table I). These results indicate that the ERα classical pathway (i.e. ERα DBD function) is necessary for initiation of cervical/vaginal neoplasia in K14E7 transgenic mice.
Table I.
Summary of lower reproductive tract disease statesa
| Genotypes | Group size, n | No disease | Dysplasia only | Cancer and dysplasia | Cancer incidence, % | ||
|---|---|---|---|---|---|---|---|
| Cervix (vagina) | CIN1 (VIN1) | CIN2 (VIN2) | CIN3 (VIN3) | Cervix (vagina) | Cervix (vagina) | ||
| K14E7/ERα +/− | 10 | 0 (0) | 0 (2) | 4 (3) | 3 (3) | 3 (2) | 30 (20) |
| K14E7/ERα N/− | 12 | 12 (12) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| b K14E7/ERα +/f | 37 | 0 (3) | 0 (16) | 13 (16) | 18 (1) | 6 (1) | 16.2 (2.7) |
| c K14E7/ERα N/f | 22 | 22 (22) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
VIN, vaginal intraepithelial neoplasia.
aMice were scored histopathologically for the worst disease present in the cervix or, in parentheses, the vagina of each mouse.
b K14E7/ERα +/f (n = 20) and CMVCreER/K14E7/ERα +/f (n = 17) were pooled; the two genotypes showed similar neoplastic diseases (data not shown). No mice were treated with tamoxifen.
c K14E7/ERα N/f (n = 11) and CMVCreER/K14E7/ERα N/f (n = 11) were pooled; the two genotypes showed similar neoplastic diseases (data not shown). No mice were treated with tamoxifen.
Fig. 2.
Cervical diseases are absent in K14E7/ERα N/− mice. (A) Mice of indicated genotypes were treated with E2 for 6 months. Representative images of hematoxylin and eosin-stained cervical sections are shown. White arrowhead indicates an invading carcinoma and arrows point to epithelia. Scale bar, 100 μm. (B) Cervical epithelial cell proliferation is greatly reduced in K14E7/ERα N/− mice. Cervical sections were stained for Ki67 (brown nuclei staining) and nuclei were counterstained with hematoxylin. Scale bar, 50 μm. (C) Results shown in B are quantified and displayed as mean ± standard error of the mean (n = 3), *P = 0.02. (D) ASM is absent in K14E7/ERα N/− mice. Representative images of hematoxylin and eosin-stained cervical transformation zone are shown. White and black arrowheads point to squamous and columnar epithelium, respectively. Note that squamous epithelia are embedded in columnar epithelium in K14E7/ERα +/− mice but not in K14E7/ERα N/− mice. ASM incidence is indicated in the lower right corner of each image. Scale bar, 100 μm.
HPV16 E7 and the ERα classical pathway are functional in K14E7/ERα N/f mice treated with E2 for 4 weeks
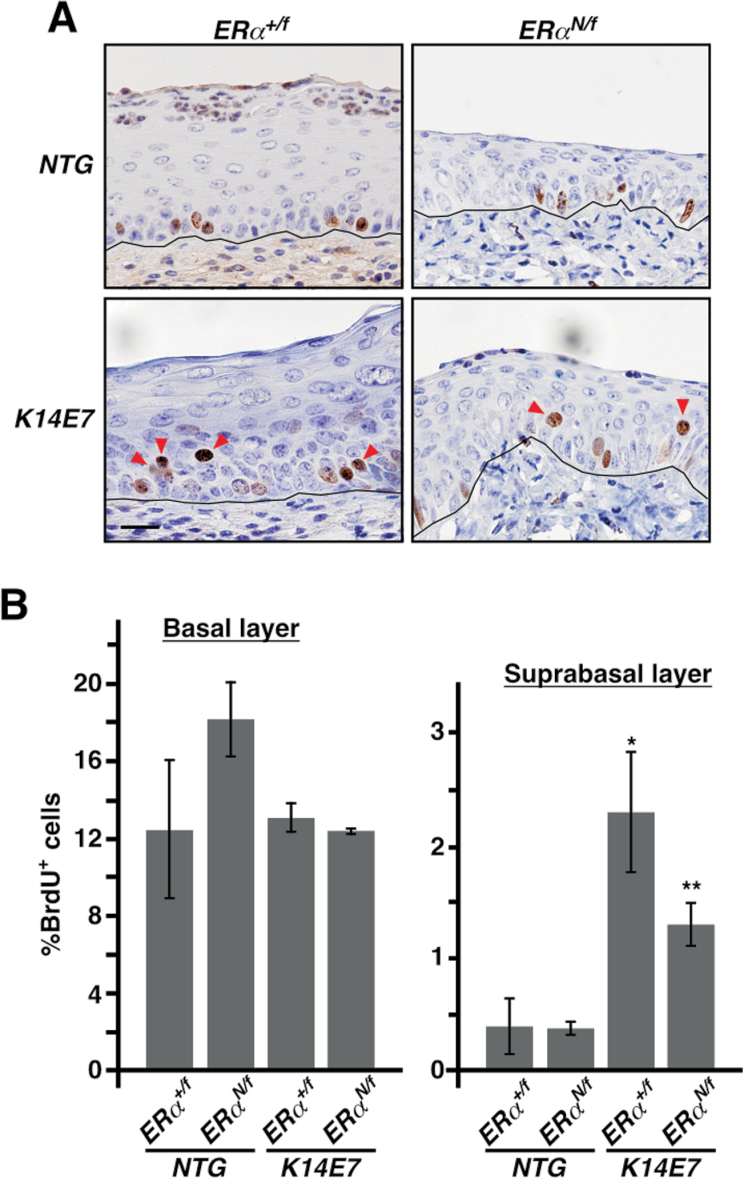
We next sought to determine whether the ERα classical pathway is dispensable for progression and/or persistence of cervical diseases. We reasoned that it could be addressed using CMVCreER/K14E7/ERα N/f mice, which allow temporal deletion of floxed ERα allele [expressing wild-type (wt) ERα] after the disease is developed. The ERα mutant (E207A/G208A) does not interfere with ERE binding of wt ERα in an in vitro reporter assay (18). To verify this in the mouse cervix, we generated non-transgenic (NTG)/ERα +/f, NTG/ERα N/f, K14E7/ERα +/f and K14E7/ERα N/f mice and treated them for 4 weeks with same dose of E2 used in the cancer study shown in Table I. Cervical tissue sections were evaluated for BrdU incorporation. Basal and suprabasal cell proliferations in the cervical epithelia of NTG/ERα +/f and NTG/ERα N/f were not significantly different (Figure 3A and B), indicating that the classical pathway of wt ERα was not inhibited in those heterozygotic mice. Induction of suprabasal cell proliferation is one of the hallmarks for E7 function in the squamous epithelium (25). Suprabasal cell proliferation was dramatically increased in K14E7/ERα +/f mice compared with NTG/ERα +/f mice (2.3±0.5 versus 0.4±0.3%, P = 0.02); it was similarly increased in K14E7/ERα N/f mice compared with NTG/ERα N/f mice (1.3±0.2 versus 0.4±0.1%, P = 0.02) (Figure 3A and B). Suprabasal cell proliferation in K14E7/ERα N/f mice was not different from that in K14E7/ERα +/f mice (P = 0.14). These results indicate that E7 is functional on ERα N/f background. Basal cell proliferation in K14E7/ERα +/f and K14E7/ERα N/f mice was similar to that in NTG/ERα +/f and NTG/ERα N/f mice (P = 0.22), which further supports that the ERα classical pathway is functional in ERα N/f mice.
Fig. 3.
ERα DBD mutant does not inhibit cervical epithelial cell proliferation when treated with E2 for short term. (A) Mice with indicated genotypes were treated with E2 for 4 weeks starting at 4–6 weeks of age and cervical sections were stained for BrdU. Nuclei were counterstained with hematoxylin. Representative images are shown. Red arrowheads point to suprabasal cells stained positive for BrdU. Black lines separate epithelium from stroma. Scale bar, 25 μm. (B) Results shown in A are quantified and displayed as mean ± standard error of the mean (n = 3). *P = 0.02 compared with NGT/ERα +/f, **P = 0.02 compared with NGT/ERα N/f.
Cervical disease is absent in K14E7/ERα N/f mice
To determine if the ERα classical pathway is also crucial for later stages of cervical carcinogenesis, we generated four genotypes of mice: K14E7/ERα N/f, K14E7/ERα +/f, CMVCreER/K14E7/ERα N/f and CMVCreER/K14E7/ERα +/f. We treated them with E2 for 6 months and anticipated that all genotypes would similarly develop cervical diseases based on results shown in Figure 3 as well as previously published results (26). If it were the case, we were to temporally activate CreER fusion protein by tamoxifen to delete floxed ERα allele (f), continue E2 treatment for additional months and compare disease severity in CMVCreER/K14E7/ERα N/f and CMVCreER/K14E7/ERα +/f mice at the endpoint. Surprisingly, however, cervical disease was absent on ERα N/f background after 6-month E2 treatment (Table I, see K14E7/ERα N/f). In contrast, 6 of 37 (16.2%) K14E7/ERα +/f control mice developed invasive cancer and the remainder had CIN2 or CIN3 (Table I). Both cancer incidence (P = 0.05) and overall disease severity (P = 1.09×10−11) were significantly greater in K14E7/ERα +/f mice compared with K14E7/ERα N/f mice. In addition, the cervical epithelia of K14E7/ERα N/f mice were hypoplastic, whereas those of K14E7/ERα +/f mice were hyperplastic (Supplementary Figure S1, available at Carcinogenesis Online). Consequently, we were unable to evaluate whether the ERα classical pathway is required for later stages of cervical carcinogenesis. It should be mentioned that genotypes were pooled based on ERα status regardless of CMVCreER status because CMVCreER transgene did not affect disease severity in this study (data not shown) similarly to previously published results (26) and none of the mice were treated with tamoxifen. Cervical cancer incidence in K14E7/ERα +/f mice was similar to K14E7/ERα +/− mice (P = 0.29), confirming haplosufficiency of ERα. Vaginal diseases were also absent in K14E7/ERα N/f mice, which was significantly different from K14E7/ERα +/f mice (P = 2.28×10−10; Table I).
Long-term but not short-term E2 treatment results in defects in cervical epithelial cell proliferation in K14E7/ERα N/f mice
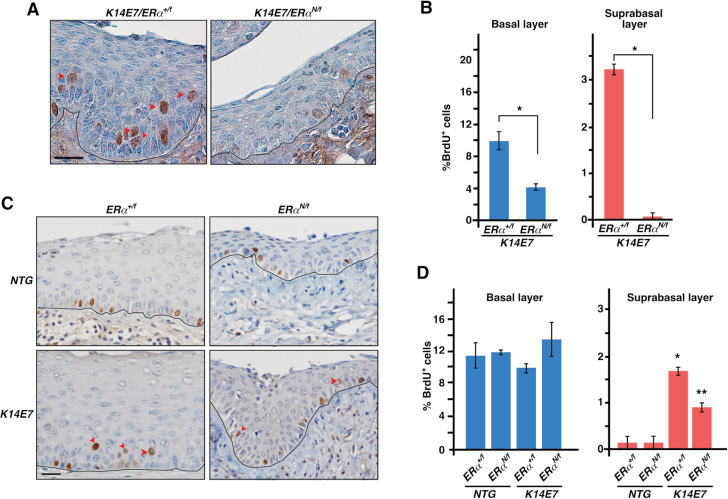
We compared cervical epithelial cell proliferation in K14E7/ERα N/f and K14E7/ERα +/f mice treated for 6 months with E2. Both basal and suprabasal cell proliferations were significantly reduced in K14E7/ERα N/f mice compared with K14E7/ERα +/f control mice (Figure 4A and B). This was surprising because we did not observe such defects in the same mice treated with E2 for 4 weeks (see Figure 3). Ages of mice at the endpoints were 2.5 months for those treated for 4 weeks in Figure 3 and 7.5 months for those treated for 6 months. To determine if the difference in phenotypes was due to age of mice, we generated NTG/ERα +/f, NTG/ERα N/f, K14E7/ERα +/f and K14E7/ERα N/f mice and treated them for 4 weeks with E2 at 6.5 months of age (7.5-month-old at killing). Basal cell proliferation was similar in all genotypes (Figure 4C and D). Suprabasal cell proliferation was significantly increased in K14E7/ERα +/f and K14E7/ERα N/f mice compared with NTG/ERα +/f and NTG/ERα N/f mice, respectively (Figure 4C and D). These results were similar to those shown in Figure 3, indicating that K14E7/ERα N/f mice respond similarly to 4-week long E2 treatment regardless of their age. Basal and suprabasal cell proliferations were significantly decreased in K14E7/ERα N/f mice treated for 6 months compared with age-matched mice treated for 4 weeks (P < 0.05) (Figure 4B and D). We conclude that K14E7/ERα N/f mice have altered response to long-term (e.g. 6 months) treatment with E2.
Fig. 4.
ERα DBD mutant inhibits cervical epithelial cell proliferation when treated with E2 for long term. (A) Mice with indicated genotypes were treated with E2 for 6 months starting at 4–6 weeks of age and cervical sections were stained for BrdU. Nuclei were counterstained with hematoxylin. Representative images are shown. Red arrowheads point to suprabasal cells stained positive for BrdU. Black lines separate epithelium from stroma. Scale bar, 50 μm. (B) Results shown in A are quantified and displayed as mean ± standard error of the mean (n = 3), *P = 0.05. (C) Mice with indicated genotypes were treated with E2 for 4 weeks starting at 6–6.5 months of age. Cervical sections were stained for BrdU and nuclei were counterstained with hematoxylin. Representative images are shown. Black lines separate epithelium from stroma. Scale bar, 50 μm. (D) Results shown in C are quantified and displayed as mean ± standard error of the mean (n = 3). *P = 0.02 compared with NGT/ERα +/f, **P = 0.02 compared with NGT/ERα N/f.
HPV oncogenes and ERα classical pathway are active in cervices of K14E7/ERα N/f mice treated with E2 for 6 months
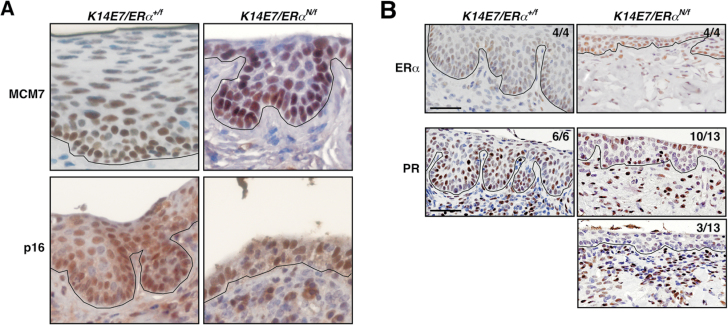
One possibility for the lack of cervical disease in K14E7/ERα N/f mice was that a function of E7 was inhibited. We, however, found that MCM7 and p16, surrogate markers for E7 function (28), were highly expressed in the cervical epithelium of K14E7/ERα N/f mice similar to K14E7/ERα +/f control mice (Figure 5A). These results are consistent with the fact that E7 expression and MCM7 upregulation are independent of ERα status (16). Another possibility was that the mutant ERα blocked ERE binding of wt ERα when treated with E2 for 6 months but not for 4 weeks (see Figure 3). We used PR as a marker for ERα classical pathway because PR was not expressed in ERα N/− cervix treated with E2 (Supplementary Figure S2, available at Carcinogenesis Online). PR expression was evident in cervical epithelia and stroma of all K14E7/ERα +/f control mice and 10 out of 13 K14E7/ERα N/f mice (Figure 5B). Interestingly, 3 of 13 cervical tissues showed strong PR staining in the stroma but not in the epithelia (Figure 5B). Subcellular localization of ERα was not altered in K14E7/ERα N/f cervices; the majority of ERα was present in the nucleus regardless of ERα genotype (Figure 5B). These results indicate that the ERα mutant did not grossly inhibit wt ERα in K14E7/ERα N/f cervices when treated for 6 months with E2.
Fig. 5.
E7 is expressed and the ERα classical pathway is active in K14E7/ERα N/f mice. (A) E7 is functional in K14E7/ERα N/f mice. Mice were treated with E2 for 6 months and cervical tissue sections were stained for MCM7 (upper panel) or p16 (lower panel). Nuclei were counterstained with hematoxylin. Black lines separate epithelium and stroma. (B) The ERα classical pathway is active in K14E7/ERα N/f cervix. Cervical tissue sections prepared from mice shown in A were subjected to immunohistochemistry for ERα (upper panel) and PR (middle and lower panels). Nuclei were counterstained with hematoxylin. Number of mice with the shown staining pattern and total number of mice analyzed are indicated at the upper right corner. Black lines separate epithelium and stroma. Scale bar, 50 μm.
Discussion
Molecular mechanisms by which ERα promotes estrogen-dependent cancers such as breast cancer have been extensively studied. Although many studies have focused on how ERα binds to DNA and how coregulators modulate transcription of ERα target genes (29), only few studies have evaluated whether ERE binding is crucial for growth of breast cancer cells (30,31). Our present study demonstrated that K14E7/ERα N/− mice were resistant to the development of ASM, the earliest stage of cervical neoplasia (Figure 2D), indicating that the ERα classical pathway is necessary for initiation of cervical cancer. We speculate that ERE binding may be also crucial for progression and/or persistence of cervical cancer because the ERα classical pathway is associated with proliferation of breast cancer cells (30,31).
Potential ERα target genes that are crucial for cervical carcinogenesis and regulated by the classical pathway
A plethora of genes are either upregulated or downregulated by ERα, many of which are involved in regulation of cell cycle, apoptosis and migration (32). ERα expression inhibits the invasive potential of human cervical cancer cells in vitro (33). ERα is often lost in human cervical cancer but expressed in surrounding stroma (34). Deletion of stromal ERα ablates cervical neoplasia in the K14E7 transgenic mice (26). These observations put forth the hypothesis that stromal ERα promotes but epithelial ERα suppresses cervical cancer. It is conceivable that ERα-dependent paracrine factor(s) secreted by stromal cells may promote cervical carcinogenesis. If these were correct, regulation of paracrine factors by ERα is likely independent of HPV oncogenes because cervical stromal cells do not express them in these mice (13). Estrogen upregulates genes coding for such factors including insulin-like growth factor 1 (IGF1), fibroblast growth factors (FGFs) and Wnt ligands. Transcriptional regulation of Igf1 by estrogen requires direct binding of ERα to EREs (35). IGF1 promotes proliferation of cervical cancer cells and IGF1 receptor is required for their growth in soft agar (36). Fgf2 and Fgf9 are upregulated by estrogen and contain consensus EREs upstream of transcription start site (37). Fgf10 also contains an ERE, of which location and sequence are conserved in human and mouse (37). Activation of FGFR2 IIIb (receptor for FGF10) or FGFR2 IIIc (receptor for FGF2 and FGF9) promotes proliferation of human cervical cancer cells (38,39). Genes coding for several Wnt ligands (e.g. WNT1, WNT2B, WNT4, WNT10B) also contain consensus EREs (37). Overexpression of constitutively active β-catenin (downstream of Wnt signaling) augments cervical carcinogenesis in K14E7 mice (40).
Potential mechanisms by which the ERα DBD mutant inhibits cervical carcinogenesis
It was surprising that cervical disease did not arise in K14E7/ERα N/f mice because, in the absence of Cre activity, they possessed an allele that expresses wt ERα. It is intriguing to note that enhanced eosin staining (presumably representing collagens and other acidic extracellular matrix proteins) in cervical stroma of K14E7/ERα N/f mice (Supplementary Figure S1, available at Carcinogenesis Online) was also observed in mice treated with fulvestrant or raloxifene (15). These selective ER modulators are efficient in treating cervical cancer in HPV transgenic mice (15). One possibility for the lack of cervical disease in K14E7/ERα N/f mice is that the mutant ERα predominantly forms heterodimer with wt ERα and thus inhibits its binding to EREs. Such a dominant-negative effect of the mutant, however, does not appear to be significantly at play because PR expression, which depends on ERα DBD function (Supplementary Figure S2, available at Carcinogenesis Online), was retained in the cervix of most K14E7/ERα N/f mice (Figure 5B). In addition, the E207A/G208A mutant does not inhibit expression of an ERE-dependent reporter gene even when overexpressed 10 times more than wt ERα (18). Nonetheless, the fact that PR expression was absent in cervical epithelial cells of three K14E7/ERα N/f mice (Figure 5B) suggests that a dominant-negative effect may arise in some contexts. Another possibility is that the mutant ERα results in epigenetic changes in, at least, a subset of classical pathway target genes necessary for cancer promotion. We would argue that such an epigenetic effect becomes more pronounced over time because significant impact on cell proliferation was observed after 6-month E2 treatment but not after 4-week treatment in K14E7/ERα N/f mice (Figure 4). The third possibility is that a balance between classical (i.e. ERE binding) and non-classical pathway (i.e. interaction with other transcription factors such as AP1 and Sp1) is important for cervical carcinogenesis. We favor this possibility because the mutant ERα (E207A/G208A) used in this study activates an AP1 reporter gene and non-ERE target genes (18), and because microarray analyses have suggested that it augments non-classical activity compared with wt ERα (41). Although a precise mechanism for inhibition of cervical carcinogenesis by the E207A/G208A mutant remains to be determined, the result further supports our conclusion that the classical pathway is necessary for cervical carcinogenesis.
A role of the classical pathway in ERα-dependent cancers
Our results are the first to show that ERα DBD is necessary for the earliest stage of cervical carcinogenesis, which we had shown previously to be ERα dependent in mice (16). A role of ERα in cancer is best characterized in breast cancer and selective ER modulator tamoxifen is most widely used for treatment of ERα-positive breast cancer (42). However, those cancers often develop tamoxifen resistance and tamoxifen promotes growth of those resistant tumors. Disulfide benzamide, an ER zinc finger inhibitor, restores tamoxifen sensitivity in tamoxifen-resistant breast cancer models (31). Interestingly, it correlates to inhibition of tamoxifen-induced ERα binding to EREs but not to non-EREs, suggesting that ERE binding (i.e. classical pathway) is crucial for tamoxifen-mediated proliferation of drug-resistant breast cancer cells. C4-12 cell line cloned from MCF7 breast cancer cells does not express ERα, yet overexpression of the receptor promotes proliferation upon estrogen treatment (30). Human ERα DBD mutant (E203A/G204A; this mutant is equivalent to mouse ERα E207A/G208A expressed in NERKI mice) promotes proliferation of C4-12 cells; however, their proliferation is more strongly promoted by wt ERα (30). Combined with our results, these observations support the idea that the classical pathway is crucial for growth of ERα-dependent cancers.
In summary, our results provide strong genetic evidence for the requirement of ERα DBD for initiation of cervical carcinogenesis. Our results also suggest that selective inhibition of the ERα classical pathway or preferential activation of non-classical pathway may be effective in treating cervical cancer.
Supplementary material
Supplementary Figures S1 and S2 and Table SI can be found at http://carcin.oxfordjournals.org/
Funding
University of Houston (Small Grant Program Award to S.-H.C.); Cancer Prevention and Research Institute of Texas [Imaging Core seed grant (RP120617) to S.-H.C.]; National Institutes of Health (CA141583, CA022443 to P.F.L.); State of Texas [Texas Emerging Technology Fund (Agreement no. 300-9-1958) to Center for Nuclear Receptors and Cell Signaling].
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- ASM
atypical squamous metaplasia
- BrdU
bromo-deoxyuridine
- CIN
cervical intraepithelial neoplasia
- DBD
DNA-binding domain
- E2
17β-estradiol
- ERα
estrogen receptor α
- ERE
estrogen-responsive element
- FGF
fibroblast growth factor
- HPV
human papillomavirus
- IGF1
insulin-like growth factor 1
- NTG
non-transgenic
- PR
progesterone receptor
- wt
wild-type.
References
- 1. Ferlay J., et al. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer, 127, 2893–2917 [DOI] [PubMed] [Google Scholar]
- 2. zur Hausen H. (2002). Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer, 2, 342–350 [DOI] [PubMed] [Google Scholar]
- 3. Schiller J.T., et al. (2012). Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol., 10, 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quinn M.A., et al. (2006). Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet., 95 (suppl. 1), S43–103 [DOI] [PubMed] [Google Scholar]
- 5. Arbyn M., et al. (2008). Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ, 337, a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strati K., et al. (2008). Human papillomavirus association with head and neck cancers: understanding virus biology and using it in the development of cancer diagnostics. Expert Opin. Med. Diagn., 2, 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johung K., et al. (2007). Human papillomavirus E7 repression in cervical carcinoma cells initiates a transcriptional cascade driven by the retinoblastoma family, resulting in senescence. J. Virol., 81, 2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jabbar S.F., et al. (2012). Cervical cancers require the continuous expression of the human papillomavirus type 16 E7 oncoprotein even in the presence of the viral E6 oncoprotein. Cancer Res., 72, 4008–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jabbar S.F., et al. (2009). Persistence of high-grade cervical dysplasia and cervical cancer requires the continuous expression of the human papillomavirus type 16 E7 oncogene. Cancer Res., 69, 4407–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moreno V., et al. ; International Agency for Research on Cancer. Multicentric Cervical Cancer Study Group (2002). Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet, 359, 1085–1092 [DOI] [PubMed] [Google Scholar]
- 11. Rinaldi S., et al. (2011). Endogenous sex steroids and risk of cervical carcinoma: results from the EPIC study. Cancer Epidemiol. Biomarkers Prev., 20, 2532–2540 [DOI] [PubMed] [Google Scholar]
- 12. Elson D.A., et al. (2000). Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res., 60, 1267–1275 [PubMed] [Google Scholar]
- 13. Riley R.R., et al. (2003). Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res., 63, 4862–4871 [PubMed] [Google Scholar]
- 14. Brake T., et al. (2003). Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res., 63, 8173–8180 [PubMed] [Google Scholar]
- 15. Chung S.H., et al. (2009). Prevention and treatment of cervical cancer in mice using estrogen receptor antagonists. Proc. Natl Acad. Sci. USA, 106, 19467–19472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung S.H., et al. (2008). Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res., 68, 9928–9934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung S.H., et al. (2010). Estrogen and ERalpha: culprits in cervical cancer? Trends Endocrinol. Metab., 21, 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jakacka M., et al. (2002). An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo . Mol. Endocrinol., 16, 2188–2201 [DOI] [PubMed] [Google Scholar]
- 19. Syed F.A., et al. (2005). Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J. Bone Miner. Res., 20, 1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chokalingam K., et al. (2012). Examination of ERα signaling pathways in bone of mutant mouse models reveals the importance of ERE-dependent signaling. Endocrinology, 153, 5325–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herber R., et al. (1996). Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J. Virol., 70, 1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winuthayanon W., et al. (2010). Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc. Natl Acad. Sci. USA, 107, 19272–19277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayashi S., et al. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol., 244, 305–318 [DOI] [PubMed] [Google Scholar]
- 24. Lubahn D.B., et al. (1993). Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl Acad. Sci. USA, 90, 11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin M.K., et al. (2012). Inactivating all three rb family pocket proteins is insufficient to initiate cervical cancer. Cancer Res., 72, 5418–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung S.H., et al. (2013). Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm. Cancer, 4, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin M.K., et al. (2009). Human papillomavirus E7 oncoprotein overrides the tumor suppressor activity of p21Cip1 in cervical carcinogenesis. Cancer Res., 69, 5656–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strati K., et al. (2006). Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc. Natl Acad. Sci. USA, 103, 14152–14157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jozwik K.M., et al. (2012). Pioneer factors in hormone-dependent cancers. Nat. Rev. Cancer, 12, 381–385 [DOI] [PubMed] [Google Scholar]
- 30. DeNardo D.G., et al. (2007). Estrogen receptor DNA binding is not required for estrogen-induced breast cell growth. Mol. Cell. Endocrinol., 277, 13–25 [DOI] [PubMed] [Google Scholar]
- 31. Wang L.H., et al. (2006). Disruption of estrogen receptor DNA-binding domain and related intramolecular communication restores tamoxifen sensitivity in resistant breast cancer. Cancer Cell, 10, 487–499 [DOI] [PubMed] [Google Scholar]
- 32. Thomas C., et al. (2011). The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer, 11, 597–608 [DOI] [PubMed] [Google Scholar]
- 33. Zhai Y., et al. (2010). Loss of estrogen receptor 1 enhances cervical cancer invasion. Am. J. Pathol., 177, 884–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwasniewska A., et al. (2011). Estrogen and progesterone receptor expression in HPV-positive and HPV-negative cervical carcinomas. Oncol. Rep., 26, 153–160 [DOI] [PubMed] [Google Scholar]
- 35. Hewitt S.C., et al. (2010). Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J. Biol. Chem., 285, 2676–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu X., et al. (2003). Serum levels of insulin-like growth factor I and risk of squamous intraepithelial lesions of the cervix. Clin. Cancer Res., 9, 3356–3361 [PubMed] [Google Scholar]
- 37. Bourdeau V., et al. (2004). Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol. Endocrinol., 18, 1411–1427 [DOI] [PubMed] [Google Scholar]
- 38. Kawase R., et al. (2010). Expression of fibroblast growth factor receptor 2 IIIc in human uterine cervical intraepithelial neoplasia and cervical cancer. Int. J. Oncol., 36, 331–340 [PubMed] [Google Scholar]
- 39. Zheng J., et al. (1995). Keratinocyte growth factor is a bifunctional regulator of HPV16 DNA-immortalized cervical epithelial cells. J. Cell Biol., 129, 843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bulut G., et al. (2011). Beta-catenin accelerates human papilloma virus type-16 mediated cervical carcinogenesis in transgenic mice. PLoS One, 6, e27243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hewitt S.C., et al. (2009). Selective disruption of ER{alpha} DNA-binding activity alters uterine responsiveness to estradiol. Mol. Endocrinol., 23, 2111–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jordan V.C. (2007). Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat. Rev. Cancer, 7, 46–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.