Abstract
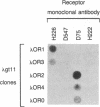
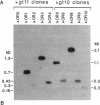
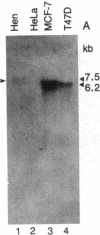
Poly(A)+ RNA isolated from the human breast cancer cell line MCF-7 was fractionated by sucrose gradient centrifugation and fractions enriched in estrogen receptor (ER) mRNA were used to prepare randomly primed cDNA libraries in the lambda gt10 and lambda gt11 vectors. Clones corresponding to ER sequence were isolated from both libraries after screening with either ER monoclonal antibodies (lambda gt11) or synthetic oligonucleotide probes designed from two peptide sequences of purified ER (lambda gt10). Five cDNA clones were isolated by antibody screening and five were isolated after screening with synthetic oligonucleotides. The two largest ER cDNA clones, lambda OR3 (1.3 kilobase pairs) and lambda OR8 (2.1 kilobase pairs), isolated by using antibodies and oligonucleotides, respectively, were able to enrich selectively for ER mRNA by hybrid-selection. Furthermore, lambda OR8 contains the DNA sequence expected from the two ER peptides and crosshybridizes with each of the other ER cDNA clones. These results demonstrate that the clones isolated correspond to the ER mRNA sequence. Use of lambda OR8 as a hybridization probe revealed a single poly(A)+ RNA band of approximately equal to 6.2 kilobase pairs in the ER-containing human breast cancer cell lines MCF-7 and T47D. In contrast, no hybridization was seen in the human ER-negative cell line HeLa. The same probe hybridizes to a chicken gene that is expressed in oviduct tissue as a 7.5-kilobase-pair poly(A)+ RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Brooks S. C., Locke E. R., Soule H. D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973 Sep 10;248(17):6251–6253. [PubMed] [Google Scholar]
- Chambon P., Dierich A., Gaub M. P., Jakowlev S., Jongstra J., Krust A., LePennec J. P., Oudet P., Reudelhuber T. Promoter elements of genes coding for proteins and modulation of transcription by estrogens and progesterone. Recent Prog Horm Res. 1984;40:1–42. doi: 10.1016/b978-0-12-571140-1.50005-0. [DOI] [PubMed] [Google Scholar]
- Dean D. C., Gope R., Knoll B. J., Riser M. E., O'Malley B. W. A similar 5'-flanking region is required for estrogen and progesterone induction of ovalbumin gene expression. J Biol Chem. 1984 Aug 25;259(16):9967–9970. [PubMed] [Google Scholar]
- Govindan M. V., Spiess E., Majors J. Purified glucocorticoid receptor-hormone complex from rat liver cytosol binds specifically to cloned mouse mammary tumor virus long terminal repeats in vitro. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5157–5161. doi: 10.1073/pnas.79.17.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Nolan C., Engler J. P., Jensen E. V. Monoclonal antibodies to human estrogen receptor. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5115–5119. doi: 10.1073/pnas.77.9.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Sobel N. B., King W. J., Jensen E. V. Immunochemical studies of estrogen receptors. J Steroid Biochem. 1984 Jan;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Canellos G. P. Cancer of the breast: the past decade (second of two parts). N Engl J Med. 1980 Jan 10;302(2):78–90. doi: 10.1056/NEJM198001103020203. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. Is a functional estrogen receptor always required for progesterone receptor induction in breast cancer? J Steroid Biochem. 1981 Dec;15:209–217. doi: 10.1016/0022-4731(81)90277-6. [DOI] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Long E. O., Gross N., Wake C. T., Mach J. P., Carrel S., Accolla R., Mach B. Translation and assembly of HLA-DR antigens in Xenopus oocytes injected with mRNA from a human B-cell line. EMBO J. 1982;1(5):649–654. doi: 10.1002/j.1460-2075.1982.tb01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes H. W., Zenke W. M., Grundström T., Staub A., Wintzerith M., Chambon P. Simultaneous rapid chemical synthesis of over one hundred oligonucleotides on a microscale. EMBO J. 1984 Apr;3(4):801–805. doi: 10.1002/j.1460-2075.1984.tb01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland A., Kühn L. C., Ruddle F. H. The human transferrin receptor gene: genomic organization, and the complete primary structure of the receptor deduced from a cDNA sequence. Cell. 1984 Dec;39(2 Pt 1):267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Okret S., Wikström A. C., Wrange O., Gustafsson J. A., Yamamoto K. R. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984 Dec 20;312(5996):779–781. doi: 10.1038/312779a0. [DOI] [PubMed] [Google Scholar]
- Mulvihill E. R., LePennec J. P., Chambon P. Chicken oviduct progesterone receptor: location of specific regions of high-affinity binding in cloned DNA fragments of hormone-responsive genes. Cell. 1982 Mar;28(3):621–632. doi: 10.1016/0092-8674(82)90217-3. [DOI] [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]