Summary
Findings from our study of prediagnostic circulating estrogen metabolism profiles suggest that increased hydroxylation along the 2- or 4-pathways may be associated with reduced breast cancer risk among postmenopausal women.
Abstract
Although elevated circulating estrogens are associated with increased postmenopausal breast cancer risk, less is known regarding the role of estrogen metabolism in breast carcinogenesis. We conducted a case–cohort study within the Breast and Bone Follow-up to the Fracture Intervention Trial to assess serum estrogens and estrogen metabolites (EMs) in 407 incident breast cancer cases diagnosed during follow-up and a subcohort of 496 women. In 1992–93, women completed a baseline questionnaire and provided blood samples. Hazard ratios (HRs) and 95% confidence intervals (CIs), adjusted for geography and trial participation status, were estimated using Cox proportional hazard regression. Serum concentrations of EMs were measured by liquid chromatography–tandem mass spectrometry. EMs (quintiles, Q) were analyzed individually, as metabolic pathways (C-2, -4 or -16) and as ratios. Elevated circulating estradiol was associated with increased breast cancer risk (HRQ5vsQ1 = 1.86; 95% CI: 1.19–2.90; P trend = 0.04). An elevated ratio of the 2-hydroxylation pathway (HRQ5vsQ1 = 0.69; 95% CI: 0.46–1.05; P trend = 0.01) and 4-hydroxylation pathway (HRQ5vsQ1 = 0.61; 95% CI: 0.40–0.93; P trend = 0.004) to parent estrogens (estradiol and estrone) was inversely associated with risk. A higher ratio of the 2/16-hydroxylation pathways was associated with reduced risk (HRQ5vsQ1 = 0.60; 95% CI: 0.40–0.90; P trend = 0.002). Increased 2- or 4-hydroxylation of parent estrogens may lower risk of postmenopausal breast cancer. Analyses of metabolic pathways may help elucidate the role of estrogen metabolism in breast carcinogenesis.
Introduction
Evidence suggests that circulating estrogens are associated with increased postmenopausal breast cancer risk (1). However, less is known regarding the role of estrogen metabolism in breast carcinogenesis. Estrogen metabolism occurs via irreversible hydroxylation at the C-2, -4 or -16 positions of the steroid ring (2), resulting in metabolites (Supplementary Figure 1, available at Carcinogenesis Online) with suggested varying genotoxic and mitogenic effects (3,4). Earlier laboratory studies of two metabolites, 2-hydroxyestrone (2-OHE1) and 16α-hydroxyestrone (16α- OHE1), suggested differing mitogenic properties based on their affinity for estrogen receptor binding, with 16α-OHE1 binding covalently to the estrogen receptor (5) and resulting in adverse cell proliferation (6,7). Estrogen metabolites (EMs) may also contribute to potential genotoxic damage (3). Hydroxylation at the C-2 and C-4 positions of the steroid ring leads to the formation of the catechol estrogens 2-OHE1, 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestrone (4-OHE1), which can be further oxidized to form mutagenic quinone products (3,8). Alternatively, methylation of these catechol estrogens prevents the formation of these potentially mutagenic products (9).
Earlier prospective studies of estrogen metabolism and breast cancer risk primarily focused on the evaluation of 2-OHE1 and 16α-OHE1 and their ratio, as measured by enzyme-linked immunosorbent assays. Findings from these studies of urinary (10–14) or circulating (15,16) 2-OHE1 and 16α-OHE1 in relation to breast cancer risk among postmenopausal women (not currently on hormone therapy) have mainly suggested no association.
Despite the multiple hypothesized biological mechanisms, epidemiological studies of estrogen metabolism have been restricted by the limitations of available immunoassays (17,18). The recent development of a liquid chromatography–tandem mass spectrometry assay, which simultaneously measures 15 estrogens and EMs (19) with high specificity, affords an opportunity to assess estrogen metabolism profiles among postmenopausal women and to extend epidemiological studies of estrogen metabolism and breast cancer beyond the assessment of the 2-OHE1 and 16α-OHE1 metabolites. To date, only two prospective studies (20,21) have examined this comprehensive circulating estrogen metabolism profile in relation to postmenopausal breast cancer risk. Findings from both studies suggest that increased hydroxylation of parent estrogens along the 2-pathway may be inversely related to breast cancer risk. Although results from Fuhrman et al. suggest that a higher ratio of catechols to methylated catechols in the 4-pathway is positively associated with breast cancer risk, Falk et al. did not observe similar relationships. Despite these two prior studies, it remains unclear whether estrogen metabolism profiles provide additional information beyond individual metabolites or parent estrogens alone. To further investigate the role of estrogen metabolism, we conducted a large case–cohort study within the Breast and Bone Follow-up to the Fracture Intervention Trial (B~FIT) to evaluate 15 prediagnostic serum estrogens and EMs in relation to postmenopausal breast cancer risk.
Materials and methods
We conducted a prospective case–cohort study within B~FIT, a longitudinal cohort of participants screened for the Fracture Intervention Trial (FIT). FIT, which has previously been described (22), was a randomized, placebo-controlled trial designed to test whether alendronate, a bisphosphonate, would reduce the rate of fractures in women with low bone mineral density (BMD). In 1992–93, 22695 postmenopausal women (aged 55–80) were screened for participation at 11 clinical centers in the USA. Potential participants underwent a BMD scan, donated a baseline serum sample, provided clinical examination data (including measured anthropometry and blood pressure) and completed an extensive health history questionnaire that ascertained information on demographic, lifestyle, hormonal and reproductive factors. Serum samples were originally stored at −20°C for 3 years and then transferred to −70°C for long-term storage. Primary results from FIT were reported in 1996 (23) and 1998 (24), and a subset of participants who had used alendronate for at least 3 years were invited to participate in an extension of the trial (FLEX, the FIT Long-Term Extension Trial) (25). B~FIT is comprised of female volunteers originally screened for FIT (N = 15 595) at 10 of the original 11 FIT clinical centers; one clinic declined to participate in the follow-up study. Women who refused, withdrew or had their questionnaire removed by the Institutional Review Board (IRB) were excluded (n = 7100). Vital status and cause of death of screenees was determined using the National Death Index (NDI). From 2001 to 2004, surviving screenees were contacted by mail and/or telephone and invited to complete a follow-up questionnaire (64% of eligible women completed the B~FIT questionnaire), which asked about cancer diagnoses, other health outcomes and reproductive surgeries that occurred since they completed the FIT baseline questionnaire, family history of cancer, detailed hormone use and preventive screening procedures. Women who reported an incident cancer or fracture were asked to give permission for medical record review of those events. In addition, women from clinical sites located in states with cancer registries (Florida, Maryland, North Carolina, Oregon and Tennessee) or in Surveillance Epidemiology and End Results (SEER) registry areas (Northern California, Washington and Iowa) were linked to the registry (through 2002–04) to identify and confirm cancer diagnoses (73% of subjects resided in areas with registry linkage, of which 29% were SEER registry areas). Approximately 90% of the breast cancer cases diagnosed among B~FIT participants were confirmed by medical record or linkage. Information on tumor behavior was available for 79.1% of breast cancer cases (n = 268 invasive, 54 in situ and 85 cases with missing tumor behavior). All women provided written informed consent. B~FIT was approved by the IRB of each participating site and the University of California, San Francisco Coordinating Center, as well as the National Cancer Institute.
Eligibility criteria and subcohort selection
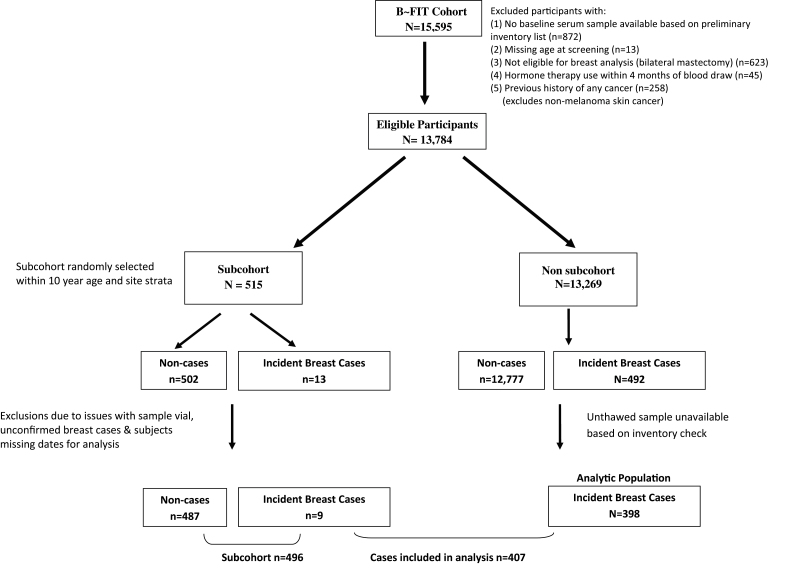
Women were eligible for inclusion in the present study (Figure 1) if they had an available unthawed baseline serum sample, no history of bilateral mastectomy, no reported use of postmenopausal estrogens (oral, injection or patch) in the 4 months before their baseline FIT interview/blood draw and no prior history of any cancer (other than non-melanoma skin cancer) before FIT baseline. Participants randomized for FIT and women who were ineligible for or declined FIT randomization were included.
Fig. 1.
Case–cohort study design.
The subcohort (N = 515) was randomly selected from the overall B~FIT cohort, within 10-year age and geographical clinic strata, irrespective of case status (Figure 1). Within the subcohort, women were excluded based on the following: missing dates for analysis (n = 1 case, 3 non-cases), issues with sample vials (n = 14 non-cases) and unconfirmed breast cancer diagnoses (n = 1 case). The final study population includes 894 distinct postmenopausal women, including 407 incident breast cancer cases, of which 9 occurred in women sampled as part of the subcohort, and 487 subcohort members who did not develop breast cancer during follow-up.
Laboratory assays.
Stable isotope dilution liquid chromatography–tandem mass spectrometry was used to simultaneously measure 15 serum estrogens and EMs including parent estrogens (estrone and estradiol) and EMs in the 2-hydroxylation pathway (2-hydroxyestrone, 2-hydroxyestradiol, 2-hydroxyestrone-3-methyl ether, 2-methoxyestrone and 2-methoxyestradiol), the 4-hydroxylation pathway (4-hydroxyestrone, 4-methoxyestrone and 4-methoxyestradiol) and the 16-hydroxylation pathway (16α-hydroxyestrone, 17-epiestriol, estriol, 16-epiestriol and 16-ketoestradiol). Details on this method have previously been published (19). In brief, the estrogens and EMs measured included both conjugated (attached to sulfate or glucuronide moieties) and unconjugated forms. Six stable isotopically labeled standards were used including: deuterated 2-hydroxyestradiol, 2-methoxyestradiol and estriol (C/D/N Isotopes, Pointe-Claire, Quebec, Canada); deuterated 16-epiestriol (Medical Isotopes, Pelham, NH) and 13C-labeled estrone and estradiol (Cambridge Isotope Laboratories, Andover, MA). These standards were added to 0.5ml of serum, followed by enzymatic hydrolysis, using a preparation from Helix pomatia with β-glucuronidase and sulfatase activity (Sigma Chemical Co., St Louis, MO), to enable the measurement of the sum of the unconjugated and conjugated forms of each estrogen or EM.
Samples were randomized across the batches irrespective of case status; laboratory personnel were blinded to the case status. Three blinded quality control samples were included within each batch. Coefficients of variation (within and between-batch) from masked quality control samples were <3% for all analytes. The published lower limit of quantitation for these serum estrogens is 26.5–29.6 pmol/l (19); however, our results suggest that even lower limits can reliably be achieved with this assay. All estrogens and EMs were detected; there were no samples with undetectable levels.
Statistical analysis
Differences in baseline characteristics and EM levels between cases and subcohort members were assessed using t-tests, chi-square tests or Wilcoxon rank sum tests. Spearman correlation coefficients were estimated for the relationships between parent estrogens and all metabolites among the subcohort. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationship between each estrogen exposure and breast cancer risk were estimated using Cox proportional hazard regression with robust variance adjustment to account for the case–cohort design (26). The time scale was defined as age at baseline (entry) and age at event or censoring (exit). For cases in the non-subcohort (n = 398), follow-up started 6 months prior to their age of breast cancer diagnosis, contributing information only to their risk set (26). For the subcohort, follow-up started at age at baseline and ended at age at breast cancer diagnosis or censoring, defined as age at death or end of follow-up. The appropriateness of the proportional hazards assumption was visually examined by Kaplan–Meier survival curve and tested using time-dependent interactions between estrogen exposures and age.
Quintile categories for each EM were determined based on the distribution among the subcohort, with the lowest quintile (Q1) as the referent group. Estrogens and EMs (pmol/l) were analyzed individually, as metabolic pathways (C-2, -4 or -16), as ratios of metabolic pathways and as ratios of metabolic pathways relative to the parent estrogens (estradiol and estrone). Metabolic pathways were created by summing the individual EM within each pathway (Supplementary Table 1, available at Carcinogenesis Online). Tests for trend were performed by modeling each estrogen exposure as an ordinal variable. Models were adjusted for (i) study design variables only: clinic (10 geographical sites) and trial participation status (screenee-only, FIT or FLEX) and (ii) additional adjustment for baseline covariates known to affect breast cancer risk: race, education, age at menarche, parity/age at first live birth combination variable, breast feeding, body mass index (BMI), frequency of alcohol consumption in the past month, prior estrogen therapy use, years since menopause and family history of breast cancer in first-degree relatives. Adjustment for the above breast cancer risk factors did not alter the HR estimates by >10% and were not included in the final models. Additionally, adjustment for estradiol, year of blood draw or time since blood draw did not affect the estimates and were not included in final models. Therefore, results from parsimonious models, adjusted for clinic and trial participation, status are presented.
We conducted sensitivity analyses that (i) restricted the study population to screenees (n = 696 women who were screened for FIT but did not participate due to ineligibility), (ii) excluded 14 cases and 4 subcohort participants whose total estrogens and metabolite values were in the top 2%, which suggested potential exogenous hormone use and (iii) excluded non-confirmed breast cancer cases (n = 40).
The P values presented were not adjusted for multiple comparisons. All statistical analyses were performed using the SAS software package, version 9.2 (SAS Institute, Cary, NC). Forest plots of HRs for quintiles of serum concentrations of EMs were created using SigmaPlot 11.0.
Results
The distributions of baseline characteristics by case and subcohort status are presented in Table I. The study population was comprised mostly of Caucasian women (95%) who were, on average, 67 years of age at blood draw (SD, cases: 5.7; controls: 6.2), with a mean follow-up of 8.1 years (SD: 3.4). For cases, the average time between blood draw and diagnosis was 6 years (SD: 3.0). Cases and subcohort members were similar with regard to reproductive factors, prior estrogen therapy use, and lifestyle factors such as alcohol consumption and smoking status. However, compared with controls, cases were slightly more overweight, more educated, had higher total hip BMD and were more likely to report a family history of breast cancer in a first-degree relative.
Table I.
Distribution of baseline descriptive characteristics by case and subcohort status (n = 903)
| Characteristic | Cases (n = 407) | Subcohorta (n = 496) | P valueb | ||
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Age (years) at: | |||||
| Blood draw | 67.2±5.7 | 67.3±6.2 | 0.93 | ||
| Menarche | 12.7±1.5 | 12.8±1.4 | 0.35 | ||
| Breast cancer diagnosis | 73.3±6.3 | — | — | ||
| Education (years) | 16.2±4.4 | 15.3±4.2 | 0.003 | ||
| Years between blood draw and breast cancer diagnosis | 6.1±3.0 | — | — | ||
| Years since menopause | 19.8±8.1 | 20.7±8.9 | 0.10 | ||
| BMI (kg/m2) | 26.7 (22.1, 35.4)c | 25.8 (21.0, 34.7)c | 0.03c | ||
| Waist (cm) | 97.1±13.6 | 96.1±15.2 | 0.35 | ||
| Neck BMD | 0.67±0.10 | 0.66±0.11 | 0.06 | ||
| Total hip BMD | 0.79±0.12 | 0.77±0.13 | 0.02 | ||
| Characteristic | N | % | N | % | P valueb |
| Ethnicity | |||||
| Asian/Pacific Islander | 3 | 0.7 | 5 | 1.0 | |
| Black or African American | 9 | 2.2 | 8 | 1.6 | |
| Hispanic or Latino | 3 | 0.7 | 8 | 1.6 | |
| White Caucasian | 386 | 94.8 | 470 | 94.8 | |
| Other | 6 | 1.5 | 5 | 1.0 | 0.66 |
| Trial participation status | |||||
| Screenee-only | 324 | 79.6 | 372 | 75.0 | |
| FIT | 69 | 17.0 | 98 | 19.8 | |
| FLEX | 14 | 3.4 | 26 | 5.2 | 0.20 |
| Family history of breast cancer in first-degree relatives | |||||
| No | 318 | 78.1 | 420 | 84.7 | |
| Yes | 81 | 19.9 | 61 | 12.3 | 0.002 |
| BMI category (kg/m2) | |||||
| <25 | 148 | 36.4 | 210 | 42.3 | |
| 25–29.9 | 139 | 34.2 | 156 | 31.5 | |
| 30–34.9 | 73 | 17.9 | 81 | 16.3 | |
| ≥35 | 43 | 10.6 | 45 | 9.1 | 0.34 |
| Parity/age at first live birth (years) | |||||
| Nulliparous | 50 | 12.3 | 45 | 9.1 | |
| <20 | 35 | 8.6 | 50 | 10.1 | |
| 20–24 | 160 | 39.3 | 213 | 42.7 | |
| 25–29 | 122 | 30.0 | 127 | 25.6 | |
| ≥30 | 38 | 9.3 | 60 | 12.1 | 0.18 |
| Ever breastfed | |||||
| No | 165 | 40.5 | 211 | 42.5 | |
| Yes | 227 | 55.8 | 271 | 54.6 | 0.62 |
| Prior estrogen therapy used (years) | |||||
| Never | 256 | 62.9 | 330 | 66.5 | |
| <1 year | 39 | 9.6 | 48 | 9.7 | |
| 1–4 | 63 | 15.5 | 64 | 12.9 | |
| 5–9 | 17 | 4.2 | 27 | 5.4 | |
| ≥10 | 26 | 6.4 | 23 | 4.6 | 0.48 |
| Alcohol consumption in past month | |||||
| Not at all | 164 | 40.3 | 208 | 41.9 | |
| Once | 50 | 12.3 | 57 | 11.5 | |
| 2–3 times | 69 | 17.0 | 78 | 15.7 | |
| 1–2 days per week | 50 | 12.3 | 45 | 9.1 | |
| 3–4 days per week | 26 | 6.4 | 40 | 8.1 | |
| 5–6 days per week | 21 | 5.2 | 31 | 6.3 | |
| Every day | 27 | 6.6 | 37 | 7.5 | 0.65 |
| Smoking status | |||||
| Never | 215 | 52.8 | 257 | 51.8 | |
| Former | 153 | 37.6 | 183 | 36.9 | |
| Current | 32 | 7.9 | 53 | 10.7 | 0.38 |
Missing values included in the denominator for calculation of above percentages. Frequency of missing values (cases, subcohort): family history of breast cancer (n = 8, 15); BMI (n = 4, 4); parity/age first live birth (n = 2, 1); ever breastfed (n = 15, 14); prior estrogen use (n = 6, 4); smoking status (n = 7, 3).
aIncludes nine incident breast cancer cases (n = 487+9 incident cases).
b P values calculated using t-tests for continuous variables and χ2 for categorical variables.
cMedian (10th, 90th); P value calculated using Wilcoxon rank sum.
dEstrogen pill use; years of use among former users.
Table II summarizes the median and interdecile ranges (10th, 90th percentiles) of each analyte by case status. Overall, cases had higher circulating parent estrogens as compared with the subcohort (Table II), with a median (10th, 90th) of 380.3 (159.5, 1026.0) versus 331.7 (134.2, 852.8) pmol/l, respectively. Median levels of individual metabolites were significantly higher among the cases (P < 0.05) with the exception of 2-methoxyestradiol, 2-hydroxyestrone-3-methyl-ether and 4-methoxyestrone. Among the subcohort, estrogens and EMs were significantly correlated with each other (all P values < 0.0001), with correlation coefficients ranging from 0.33 to 0.96, depending on the analyte (Supplementary Table 1, available at Carcinogenesis Online).
Table II.
Serum concentrations of estrogens and EMs (pmol/l) among postmenopausal breast cancer cases and subcohort members (N = 903)
| Estrogen measures (pmol/l) | Cases (n = 407) | Subcohorta (n = 496) | P valueb | Average percent contributionc | ||||
|---|---|---|---|---|---|---|---|---|
| Median | 10th | 90th | Median | 10th | 90th | |||
| Parent estrogens | 380.3 | 159.5 | 1026.0 | 331.7 | 134.2 | 852.8 | 0.001 | |
| Estrone | 334.0 | 133.8 | 935.9 | 285.7 | 113.3 | 761.3 | 0.002 | 86.9 |
| Estradiol | 44.5 | 21.0 | 96.0 | 38.5 | 17.8 | 91.5 | 0.003 | 13.1 |
| 2-Hydroxylation pathway | 127.0 | 80.9 | 279.0 | 119.3 | 75.4 | 244.4 | 0.02 | |
| 2-Pathway catechols | 80.1 | 48.5 | 177.6 | 75.7 | 46.0 | 159.0 | 0.03 | |
| 2-Hydroxyestrone | 67.7 | 40.4 | 149.3 | 62.4 | 37.8 | 140.5 | 0.04 | 52.1 |
| 2-Hydroxyestradiol | 12.9 | 7.6 | 27.3 | 12.2 | 7.4 | 24.6 | 0.04 | 10.3 |
| 2-Pathway methylated catechols | 49.5 | 26.2 | 104.5 | 47.0 | 24.5 | 92.6 | 0.008 | |
| 2-Methoxyestrone | 31.9 | 14.3 | 65.1 | 28.1 | 13.9 | 57.3 | 0.005 | 22.9 |
| 2-Methoxyestradiol | 13.8 | 7.1 | 28.0 | 13.3 | 6.8 | 24.5 | 0.05 | 10.8 |
| 2-Hydroxyestrone-3-methyl ether | 4.2 | 2.4 | 13.9 | 4.0 | 2.3 | 10.4 | 0.07 | 3.9 |
| 4-Hydroxylation pathway | 17.7 | 12.0 | 39.0 | 16.9 | 11.3 | 34.3 | 0.02 | |
| 4-Hydroxyestrone (catechol) | 10.9 | 6.8 | 22.3 | 10.6 | 6.4 | 21.1 | 0.04 | 59.7 |
| 4-Pathway methylated catechols | 7.1 | 4.2 | 16.6 | 6.8 | 3.8 | 14.1 | 0.05 | |
| 4-Methoxyestrone | 4.0 | 2.2 | 10.5 | 3.8 | 2.2 | 8.3 | 0.14 | 23.7 |
| 4-Methoxyestradiol | 3.1 | 2.2 | 6.7 | 2.8 | 1.2 | 5.9 | 0.02 | 16.6 |
| 16-Hydroxylation pathway | 346.1 | 183.9 | 896.1 | 309.1 | 173.0 | 730.6 | 0.002 | |
| Estriol | 277.8 | 140.0 | 663.9 | 244.5 | 130.1 | 559.7 | 0.001 | 77.1 |
| 16α-Hydroxyestrone | 29.0 | 18.2 | 72.2 | 28.3 | 17.6 | 69.2 | 0.03 | 9.2 |
| 16-Epiestriol | 4.5 | 2.3 | 19.5 | 4.0 | 1.8 | 14.4 | 0.003 | 1.6 |
| 17-Epiestriol | 3.6 | 1.4 | 13.8 | 3.5 | 1.4 | 9.3 | 0.008 | 1.2 |
| 16-Ketoestradiol | 34.3 | 18.5 | 109.2 | 30.2 | 18.0 | 90.7 | 0.003 | 10.9 |
aIncludes nine incident breast cancer cases.
bWilcoxon rank sum test.
cAverage percent contribution of each estrogen or EM to its respective pathway.
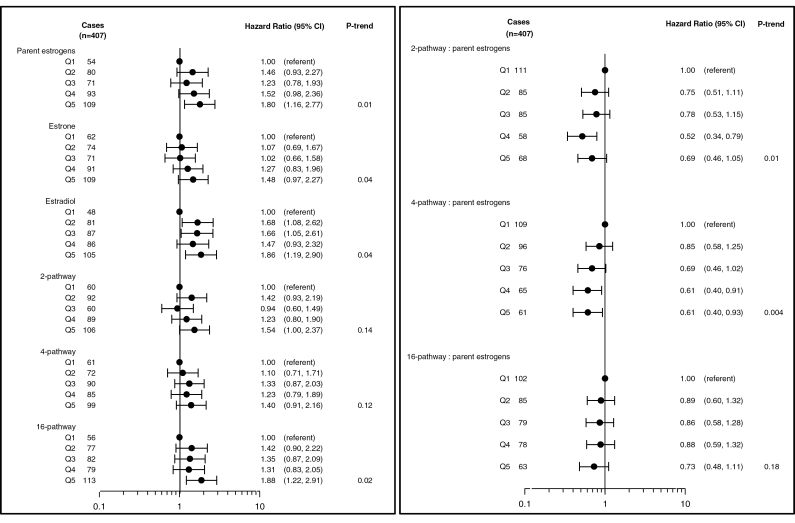
Breast cancer risk increased with increasing levels of parent estrogens (estrone and estradiol) and was significantly elevated for women in the highest quintile of parent estrogens as compared with the subcohort (HRQ5vsQ1 = 1.80; 95% CI: 1.16–2.77; P trend = 0.01) (Figure 2). This significant increase in risk was also observed when both estrone (HRQ5vsQ1 = 1.48; 95% CI: 0.97–2.27; P trend = 0.04) and estradiol (HRQ5vsQ1 = 1.86; 95% CI: 1.19–2.90; P trend = 0.04) were analyzed individually. No significant patterns were observed when examining either the 2 or 4-hydroxylation pathway as a whole but elevated levels of 16-pathway metabolites were associated with increased risk (HRQ5vsQ1 = 1.88; 95% CI: 1.22–2.91; P trend = 0.02) (Figure 2). When examining each hydroxylation pathway as a ratio to the parent estrogens, an elevated ratio of the 2-hydroxylation pathway (HRQ5vsQ1 = 0.69; 95% CI: 0.46–1.05; P trend = 0.01) and 4-hydroxylation pathway (HRQ5vsQ1 = 0.61; 95% CI: 0.40–0.93; P trend = 0.004) to parent estrogens was significantly associated with a reduction in risk. The ratio of 16-hydroxylation pathway to parents was not associated with risk (P trend = 0.18).
Fig. 2.
HRs and 95% CIs for the association between parent estrogens, hydroxylation pathways and the ratio of each hydroxylation pathway to parent estrogens in relation to breast cancer risk. HR estimates are denoted by the black circles, whereas the solid horizontal lines reflect the CI. Quintile cutpoints (Q) were based on the distribution among the subcohort. Estimates are adjusted for geographical clinic site and trial participation status.
Results for individual metabolites within each hydroxylation pathway are presented in Table III. No significant associations were observed with the catechol estrogens in the 2-pathway; however, elevated 2-methoxyestrone was associated with increased risk (HRQ5vsQ1 = 1.60; 95% CI: 1.05–2.42; P trend = 0.03). Within the 4-pathway (Table III), no associations were observed with 4-hydroxyestrone or the 4-pathway methylated catechols (P trend = 0.27 and 0.08, respectively). With regard to individual metabolites within the 16-pathway, elevated estriol and 16-ketoestradiol levels were associated with significant increases in breast cancer risk (HRQ5vsQ1 = 1.98; 95% CI: 1.28–3.0; P trend = 0.01 and HRQ5vsQ1 = 1.51; 95% CI: 1.01–2.27; P trend = 0.02, respectively).
Table III.
HRs and 95% CIs for the association between individual serum EMs in the 2-, 4- and 16-hydroxylation pathways and breast cancer risk among postmenopausal women
| Estrogen metabolites (pmol/l) |
Quintilea | Cases (n = 407) | HR (95% CI)b | P trend |
|---|---|---|---|---|
| 2-Hydroxylation pathway | ||||
| 2-Pathway catechols | Q1 | 64 | 1.00 (referent) | |
| Q2 | 82 | 1.32 (0.86, 2.03) | ||
| Q3 | 79 | 1.15 (0.74, 1.78) | ||
| Q4 | 80 | 1.06 (0.68, 1.64) | ||
| Q5 | 102 | 1.42 (0.92, 2.17) | 0.32 | |
| 2-Hydroxyestrone | Q1 | 64 | 1.00 (referent) | |
| Q2 | 81 | 1.30 (0.85, 1.98) | ||
| Q3 | 76 | 1.07 (0.69, 1.66) | ||
| Q4 | 86 | 1.14 (0.74, 1.75) | ||
| Q5 | 100 | 1.38 (0.90, 2.12) | 0.28 | |
| 2-Hydroxyestradiol | Q1 | 73 | 1.00 (referent) | |
| Q2 | 74 | 1.04 (0.68, 1.59) | ||
| Q3 | 69 | 0.84 (0.54, 1.29) | ||
| Q4 | 81 | 0.97 (0.64, 1.48) | ||
| Q5 | 110 | 1.31 (0.87, 1.98) | 0.25 | |
| 2-Pathway methylated catechols | Q1 | 64 | 1.00 (referent) | |
| Q2 | 73 | 1.16 (0.75, 1.80) | ||
| Q3 | 81 | 1.31 (0.85, 2.02) | ||
| Q4 | 66 | 0.99 (0.64, 1.53) | ||
| Q5 | 123 | 1.80 (1.19, 2.72) | 0.02 | |
| 2-Methoxyestrone | Q1 | 68 | 1.00 (referent) | |
| Q2 | 74 | 1.07 (0.69, 1.65) | ||
| Q3 | 64 | 0.99 (0.63, 1.54) | ||
| Q4 | 82 | 1.10 (0.73, 1.68) | ||
| Q5 | 119 | 1.60 (1.05, 2.42) | 0.03 | |
| 2-Methoxyestradiol | Q1 | 66 | 1.00 (referent) | |
| Q2 | 88 | 1.29 (0.85, 1.96) | ||
| Q3 | 65 | 0.94 (0.60, 1.47) | ||
| Q4 | 92 | 1.37 (0.90, 2.11) | ||
| Q5 | 96 | 1.36 (0.89, 2.09) | 0.17 | |
| 2-Hydroxyestrone-3- methyl ether |
Q1 | 69 | 1.00 (referent) | |
| Q2 | 86 | 1.20 (0.79, 1.82) | ||
| Q3 | 75 | 1.12 (0.73, 1.73) | ||
| Q4 | 70 | 0.95 (0.62, 1.46) | ||
| Q5 | 107 | 1.50 (0.99, 2.26) | 0.19 | |
| 4-Hydroxylation pathway | ||||
| 4-Hydroxyestrone (catechol) | Q1 | 67 | 1.00 (referent) | |
| Q2 | 78 | 1.15 (0.75, 1.78) | ||
| Q3 | 80 | 0.99 (0.64, 1.53) | ||
| Q4 | 77 | 0.97 (0.63, 1.50) | ||
| Q5 | 105 | 1.38 (0.91, 2.10) | 0.27 | |
| 4-Pathway methylated catechols | Q1 | 53 | 1.00 (referent) | |
| Q2 | 90 | 1.68 (1.08, 2.60) | ||
| Q3 | 85 | 1.57 (1.01, 2.45) | ||
| Q4 | 85 | 1.58 (1.02, 2.47) | ||
| Q5 | 94 | 1.69 (1.08, 2.65) | 0.08 | |
| 4-Methoxyestrone | Q1 | 75 | 1.00 (referent) | |
| Q2 | 75 | 1.03 (0.68, 1.58) | ||
| Q3 | 76 | 1.01 (0.66, 1.54) | ||
| Q4 | 83 | 1.08 (0.71, 1.64) | ||
| Q5 | 98 | 1.28 (0.85, 1.93) | 0.24 | |
| 4-Methoxyestradiol | Q1 | 64 | 1.00 (referent) | |
| Q2 | 65 | 0.98 (0.63, 1.51) | ||
| Q3 | 95 | 1.40 (0.92, 2.13) | ||
| Q4 | 89 | 1.32 (0.86, 2.02) | ||
| Q5 | 94 | 1.37 (0.89, 2.10) | 0.08 | |
| 16-Hydroxylation pathway | ||||
| 16α-Hydroxyestrone | Q1 | 59 | 1.00 (referent) | |
| Q2 | 82 | 1.31 (0.85, 2.02) | ||
| Q3 | 91 | 1.45 (0.94, 2.24) | ||
| Q4 | 80 | 1.14 (0.73, 1.78) | ||
| Q5 | 95 | 1.39 (0.90, 2.16) | 0.33 | |
| Estriol | Q1 | 53 | 1.00 (referent) | |
| Q2 | 79 | 1.44 (0.92, 2.27) | ||
| Q3 | 82 | 1.42 (0.92, 2.21) | ||
| Q4 | 74 | 1.29 (0.82, 2.02) | ||
| Q5 | 119 | 1.98 (1.28, 3.08) | 0.01 | |
| 17-Epiestriol | Q1 | 76 | 1.00 (referent) | 0.03 |
| Q2 | 61 | 0.84 (0.54, 1.32) | ||
| Q3 | 70 | 0.93 (0.61, 1.43) | ||
| Q4 | 80 | 1.01 (0.66, 1.53) | ||
| Q5 | 120 | 1.46 (0.97, 2.20) | ||
| 16-Ketoestradiol | Q1 | 72 | 1.00 (referent) | 0.02 |
| Q2 | 55 | 0.87 (0.56, 1.36) | ||
| Q3 | 83 | 1.20 (0.79, 1.82) | ||
| Q4 | 89 | 1.21 (0.80, 1.82) | ||
| Q5 | 108 | 1.51 (1.01, 2.27) | ||
| 16-Epiestriol | Q1 | 65 | 1.00 (referent) | 0.02 |
| Q2 | 71 | 1.10 (0.72, 1.71) | ||
| Q3 | 77 | 1.09 (0.71, 1.68) | ||
| Q4 | 85 | 1.37 (0.90, 2.09) | ||
| Q5 | 109 | 1.54 (1.01, 2.35) | ||
aQuintile cutpoints (Q) were based on the EM distribution among the subcohort.
bEstimates are adjusted for geographical clinic site and trial participation status.
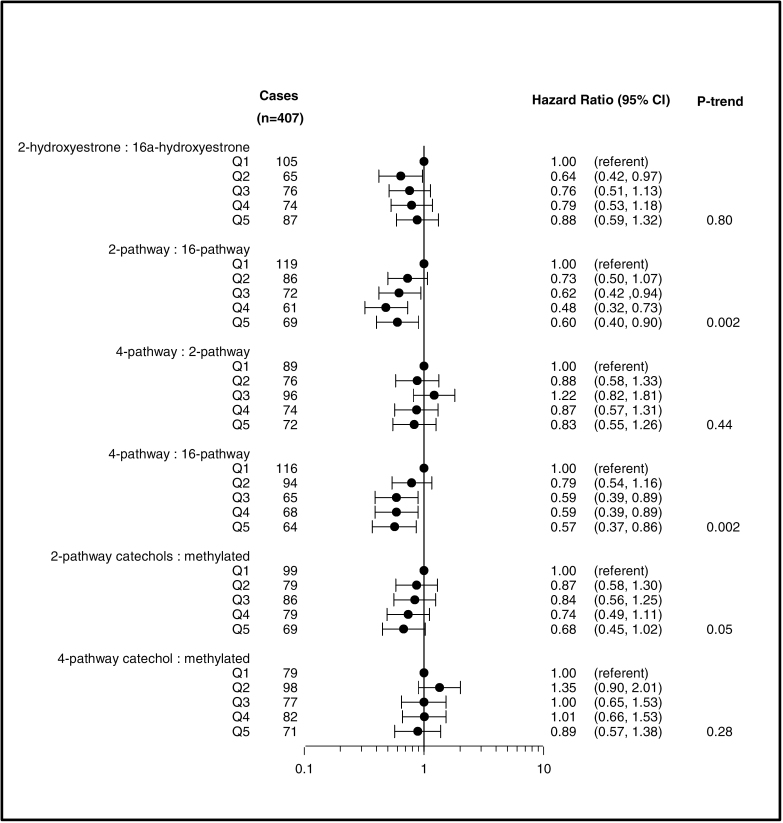
EMs were also examined as ratios based on metabolic pathways (Figure 3). Although the ratio of the individual metabolites, 2-OHE1 to 16α-OHE1, was not associated with breast cancer risk (P trend = 0.80), an elevated ratio of the 2-pathway to the 16-pathway was inversely related to risk (HRQ5vsQ1 = 0.60; 95% CI: 0.40–0.90; P trend = 0.002). A higher ratio of 4-pathway to 16-pathway hydroxylation was also associated with a significant reduction in risk (HRQ5vsQ1 = 0.57; 95% CI: 0.37–0.86; P trend = 0.002). No other significant associations were observed when examining ratios of metabolic pathways.
Fig. 3.
HRs and 95% CIs for the association between metabolic ratios and breast cancer risk. HR estimates are denoted by the black circles, whereas the solid horizontal lines reflect the CI. Quintile cutpoints (Q) were based on the EM distribution among the subcohort. Estimates are adjusted for geographical clinic site and trial participation status.
Sensitivity analyses restricting to screenees or to confirmed breast cancer cases yielded similar results to those presented herein. Additionally, results from sensitivity analyses excluding women with estrogen and EM values in the top 2% of the distribution (due to potential hormone use) also yielded similar patterns.
Discussion
In this case–cohort study, elevated serum levels of estrone and estradiol were significantly associated with increased breast cancer risk among postmenopausal women, with an almost 2-fold increase observed among women in the highest quintile of circulating estradiol to those in the lowest. With regard to EMs, increased hydroxylation at the C-2 and C-4 sites, relative to concentrations of their parent estrogens (estradiol and estrone), was suggestive of reduced breast cancer risk. We also observed a significant inverse association among women with an increasing ratio of the 2-pathway to 16-pathway and 4-pathway to 16-pathway.
Overall, the most consistent finding across all three prospective studies conducted to date is the inverse association with increasing 2-hydroxylation relative to the parent estrogens as well as reductions in risk with an increasing ratio of 2-pathway to 16-pathway metabolism. In the prior nested case–control study conducted among postmenopausal women (n = 277 invasive breast cancer cases) within the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (20), a similar reduction in risk was observed with an elevated ratio of the 2-hydroxylation pathway to parent estrogens but no significant associations were observed with the ratio of the 4- or 16-hydroxylation pathways to parent estrogens. Results from another nested case–control study conducted among postmenopausal women using serum samples from the Columbia, Missouri Serum blood bank (n = 215 breast cancer cases) also support a non-significant reduction in risk among women with increased 2- or 4-hydroxylation relative to parent estrogens (21). Less is known regarding the biological properties of the 4-pathway metabolites, aside from the potential genotoxic effects that result from further oxidation of 4-hydroxyestrone (3). Although one can argue that increased hydroxylation in general, irrespective of the C-2, C-4 or C-16 site, may reflect increased metabolism and ultimately secretion of potentially harmful estrogens, we did not observe reduced risks with the ratio of the 16-pathway to parent estrogens. Furthermore, the strong inverse association observed with the ratio of the 2-pathway to 16-pathway and 4-pathway to 16-pathway supports the notion of differential risk associations by hydroxylation pathways. Further research is needed to better understand the role of site-specific hydroxylation in breast carcinogenesis.
The evaluation and interpretation of this comprehensive profile of EMs is complex, given that there are multiple biological hypotheses underlying the potential role of estrogen metabolism in breast carcinogenesis. The hypothesis proposed by Bradlow et al. and Fishman et al. (27) suggests that EMs, specifically 2-hydroxyestrone and 16α-hydroxyestrone, may operate through estrogen-mediated cell signaling whereby 16α-hydroxyestrone is thought to bind more covalently to the receptor than 2-hydroxyestrone (5). As estrogen receptor binding is activated, cell proliferation increases and the chance a mutation will replicate prior to repair may also increase. Early laboratory-based studies also suggested a protective role for 2-hydroxyestrone and 2-hydroxyestradiol based on their potential antiestrogenic properties (28,29) and increased clearance rate from circulation (30). These catechols represent a large portion of the metabolites that are derived from the 2-hydroxylation pathway and their suggested biological properties may, in part, explain the reduction in risk observed with a higher ratio of the 2-hydroxylation pathway to parent estrogens and to the 16-pathway.
The second hypothesis surrounding estrogen metabolism suggests a potential genotoxic role for catechol estrogens derived from the 2- and 4-hydroxylation pathways (31). These catechol estrogens can be deactivated by the addition of a methyl group to the steroid ring, a process catalyzed by catechol-O-methyltransferase (32). Studies suggested a potential protective role for 2-methoxyestradiol based on observed antiangiogenic activity in animal models and induction of apoptosis in breast cancer cell lines (4,33,34). However, epidemiological investigations of catechol estrogens, particularly 4-hydroxyestrone, and methylated estrogens in the 2- and 4-pathways have been limited due to the lack of available assays.
Findings from the PLCO and Columbia, MO studies have been inconsistent with regards to associations with catechol and methylated estrogens. Falk et al. observed no significant patterns when analyzing the ratio of catechol estrogens to methylated catechols in relation to postmenopausal breast cancer risk. Fuhrman et al. reported that an elevated ratio of 4-hydroxyestrone (catechol estrogen) to methylated 4-pathway metabolites was significantly associated with increased breast cancer risk, whereas no association was observed when examining 2-pathway catechol estrogens relative to methylated catechols (20). In contrast, we observed a significant inverse trend with an increasing ratio of 2-pathway catechol estrogens to methylated catechols. Possible explanations for these disparate results are unclear but may include differences in the activation of catechol-O-methyltransferase and genetic polymorphisms (35). Furthermore, given the number of statistical tests, we cannot dismiss the potential for some of our findings, and those of prior studies, to be due to chance. Whether the proposed biological mechanisms work independently or in tandem is unclear; more research is needed to disentangle the mechanisms by which estrogen metabolism may influence breast cancer risk.
It is important to note key differences regarding the estrogen exposure assessment in the present study as compared with the PLCO and Columbia, MO studies, which utilized the same liquid chromatography–tandem mass spectrometry assay. In our study, the EM exposures represent both unconjugated and conjugated forms and thus, we were unable to adjust our results for unconjugated estradiol, the potentially active form in circulation. Both the PLCO and Columbia, MO studies presented some of their findings adjusted for unconjugated estradiol. Differences in median estrone and estradiol levels across the studies (B~FIT: 285.7, 38.5 pmol/l; Columbia: 226.8, 22.9 pmol/l; PLCO: 390, 36.0 pmol/l, respectively) and the range of exposures, which was somewhat narrow in PLCO, may also explain potential differences in risk associations. Additionally, in the present study, we did not observe striking differences in the case/subcohort distribution of many traditional breast cancer risk factors. Although case status was significantly associated with known risk factors such as family history of breast cancer, higher education and slightly higher levels of BMI and BMD, we did not observe significant differences with regard to classical reproductive factors. This may reflect the older age of our study population, in which the average age at blood draw was ~67 years. Prior studies have observed weaker or less consistent associations between reproductive factors and breast cancer risk among older women (36,37).
Notable strengths to this analysis include the use of prediagnostic serum, the prospective case–cohort design, the large sample size and the measurement of a comprehensive profile of endogenous EMs shown to have high specificity and sensitivity among postmenopausal women (19), who generally have lower concentrations of circulating estrogens. Despite these strengths, the generalizability of our results may be limited, as the study population is comprised of women who volunteered to be screened for participation in a randomized clinical trial aimed at reducing fracture risk. However, our findings with the parent estrogens are consistent with previous reports among postmenopausal women (1) and furthermore, the mean femur neck BMD in this study population is comparable to that of women who participated in the Third National Health and Nutrition Examination Survey (1988–94) (38), which presumably reflects the inclusion of women not eligible for FIT and limits this concern. There is also the possibility of case and non-case misclassification. Women living in non-registry states who did not complete the follow-up questionnaire may have been misclassified as a non-case, given that case information could not be ascertained. However, this scenario applies to only 7.4% of the study population. Although some of the self-reported breast cancer diagnoses that were not confirmed by linkage or medical record adjudication (n = 40) may have been misclassified, analyses excluding these cases yielded similar results. The measurement of estrogens and EMs occurred at one time point; although this is a common limitation in studies of circulating markers, the representativeness and stability of these metabolites over time remains unknown. Given limited numbers, we were unable to assess associations by tumor characteristics including behavior (invasive or in situ) or hormone receptor status; future studies should consider whether estrogen metabolism profiles vary by tumor characteristics.
In summary, our findings suggest that increased metabolism favoring the 2-hydroxylation pathway may be associated with reduced breast cancer risk among postmenopausal women and that the analysis of pathways may provide additional information beyond that of parent estrogens or individual metabolites alone. Given the limited data available on the relationship between estrogen metabolism profiles and postmenopausal breast cancer risk, additional studies are needed to help disentangle the role of individual metabolites and their pathways in relation to breast cancer overall, by hormone receptor status and among other subgroups. Pooling of existing and future breast cancer studies will help facilitate the examination of this comprehensive estrogen metabolism profile.
Supplementary material
Supplementary Table 1 and Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
Merck Research Laboratories (to the original FIT study); National Cancer Institute, National Institutes of Health (contract no. N02-CP-01019 to B~FIT.
Supplementary Material
Acknowledgements
We thank Stephanie Litwack-Harrison, MPH (UCSF, San Francisco, CA), Eric Boyd (IMS, Silver Spring, MD) and Vicky Chia for their invaluable assistance with study and data management and the B~FIT investigators and participants for their contributions to this study. We also thank Ms Munira Gunja for her assistance with the graphical presentation of results. B~FIT Research Group members: (i) Coordinating Center – University of California, San Francisco, CA: Douglas C.Bauer MD, Trisha F.Hue PhD MPH, Stephanie Litwack-Harrison MPH, Susan Rubin MPH, Jeffrey A.Tice MD; (ii) Clinical Centers – Group Health Cooperative, Seattle, WA: Diana Buist PhD and Andrea Z.LaCroix PhD; Kaiser Permanente Center for Health Research, Portland, OR: Emily Harris PhD; Stanford Medical Center, Palo Alto, CA: William L.Haskell PhD; University of California, San Diego, CA: Elizabeth Barrett-Connor MD; University of Iowa, Iowa City, IA: James C.Torner PhD; University of Maryland, Baltimore, MD: Marc C.Hochberg MD; University of Miami Medical School: Silvina Levis MD; University of Pittsburgh: Jane Cauley DrPH; University of Tennessee, Memphis, TN: Suzanne Satterfield MD MPH; Wake Forest University, Winston-Salem, NC: Sara. A.Quandt PhD and (iii) National Cancer Institute: Louise Brinton PhD and Jim Lacey Jr PhD MPH.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- B~FIT
Breast and Bone Follow-up to the Fracture Intervention Trial
- BMD
bone mineral density
- BMI
body mass index
- CI
confidence interval
- EM
estrogen metabolite
- HR
hazard ratio
- PLCO
Prostate, Lung, Colorectal and Ovarian.
References
- 1. Key T., et al. Endogenous Hormones and Breast Cancer Collaborative Group (2002). Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl. Cancer Inst., 94, 606–616 [DOI] [PubMed] [Google Scholar]
- 2. Lippert T.H., et al. (2000). The impact of endogenous estradiol metabolites on carcinogenesis. Steroids, 65, 357–369 [DOI] [PubMed] [Google Scholar]
- 3. Cavalieri E., et al. (2006). Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta, 1766, 63–78 [DOI] [PubMed] [Google Scholar]
- 4. Mueck A.O., et al. (2002). Estradiol metabolism and malignant disease. Maturitas, 43, 1–10 [DOI] [PubMed] [Google Scholar]
- 5. Swaneck G.E., et al. (1988). Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc. Natl Acad. Sci. USA, 85, 7831–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seeger H., et al. (2006). Comparison of possible carcinogenic estradiol metabolites: effects on proliferation, apoptosis and metastasis of human breast cancer cells. Maturitas, 54, 72–77 [DOI] [PubMed] [Google Scholar]
- 7. Lippert C., et al. (2003). The effect of endogenous estradiol metabolites on the proliferation of human breast cancer cells. Life Sci., 72, 877–883 [DOI] [PubMed] [Google Scholar]
- 8. Liehr J.G. (1990). Genotoxic effects of estrogens. Mutat. Res., 238, 269–276 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y., et al. (2007). Cytochrome P450 isoforms catalyze formation of catechol estrogen quinones that react with DNA. Metabolism., 56, 887–894 [DOI] [PubMed] [Google Scholar]
- 10. Meilahn E.N., et al. (1998). Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br. J. Cancer, 78, 1250–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muti P., et al. (2000). Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology, 11, 635–640 [DOI] [PubMed] [Google Scholar]
- 12. Kabat G.C., et al. (1997). Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol. Biomarkers Prev., 6, 505–509 [PubMed] [Google Scholar]
- 13. Wellejus A., et al. (2005). Urinary hydroxyestrogens and breast cancer risk among postmenopausal women: a prospective study. Cancer Epidemiol. Biomarkers Prev., 14, 2137–2142 [DOI] [PubMed] [Google Scholar]
- 14. Dallal C.M., et al. (2012). Urinary estrogen metabolites and breast cancer: a combined analysis of individual level data. Int J Biol Markers, 28, 3–16 [DOI] [PubMed] [Google Scholar]
- 15. Cauley J.A., et al. (2003). Estrogen metabolites and the risk of breast cancer in older women. Epidemiology, 14, 740–744 [DOI] [PubMed] [Google Scholar]
- 16. Eliassen A.H., et al. (2008). Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol. Biomarkers Prev., 17, 2029–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klug T.L., et al. (1994). Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids, 59, 648–655 [DOI] [PubMed] [Google Scholar]
- 18. Bradlow H.L., et al. (1998). Application of an improved ELISA assay to the analysis of urinary estrogen metabolites. Steroids, 63, 406–413 [DOI] [PubMed] [Google Scholar]
- 19. Xu X., et al. (2007). Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal. Chem., 79, 7813–7821 [DOI] [PubMed] [Google Scholar]
- 20. Fuhrman B.J., et al. (2012). Estrogen metabolism and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst., 104, 326–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falk R.T., et al. (2013). Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res., 15, R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Black D.M., et al. (1993). Design of the Fracture Intervention Trial. Osteoporos. Int., 3 (suppl. 3), S29–S39 [DOI] [PubMed] [Google Scholar]
- 23. Black D.M., et al. (1996). Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet, 348, 1535–1541 [DOI] [PubMed] [Google Scholar]
- 24. Cummings S.R., et al. (1998). Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA, 280, 2077–2082 [DOI] [PubMed] [Google Scholar]
- 25. Black D.M., et al. FLEX Research Group (2006). Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA, 296, 2927–2938 [DOI] [PubMed] [Google Scholar]
- 26. Barlow W.E., et al. (1999). Analysis of case-cohort designs. J. Clin. Epidemiol., 52, 1165–1172 [DOI] [PubMed] [Google Scholar]
- 27. Fishman J., et al. (1984). Increased estrogen-16 alpha-hydroxylase activity in women with breast and endometrial cancer. J. Steroid Biochem., 20(4B), 1077–1081 [DOI] [PubMed] [Google Scholar]
- 28. Zhu B.T., et al. (2006). Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: insights into the structural determinants favoring a differential subtype binding. Endocrinology, 147, 4132–4150 [DOI] [PubMed] [Google Scholar]
- 29. Schneider J., et al. (1984). Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J. Biol. Chem., 259, 4840–4845 [PubMed] [Google Scholar]
- 30. Kono S., et al. (1983). Radioimmunoassay and metabolic clearance rate of catecholestrogens, 2-hydroxyestrone and 2-hydroxyestradiol in man. J. Steroid Biochem., 19(1B), 627–633 [DOI] [PubMed] [Google Scholar]
- 31. Cavalieri E.L., et al. (2004). A unifying mechanism in the initiation of cancer and other diseases by catechol quinones. Ann. N. Y. Acad. Sci., 1028, 247–257 [DOI] [PubMed] [Google Scholar]
- 32. Zahid M., et al. (2006). The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem. Res. Toxicol., 19, 164–172 [DOI] [PubMed] [Google Scholar]
- 33. Zhu B.T., et al. (1998). Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis, 19, 1–27 [DOI] [PubMed] [Google Scholar]
- 34. Mueck A.O., et al. (2010). 2-Methoxyestradiol–biology and mechanism of action. Steroids, 75, 625–631 [DOI] [PubMed] [Google Scholar]
- 35. Thompson P.A., et al. (2000). Molecular epidemiology of genetic polymorphisms in estrogen metabolizing enzymes in human breast cancer. J. Natl Cancer Inst Monogr., 27, 125–134 [DOI] [PubMed] [Google Scholar]
- 36. Clavel-Chapelon F., et al. (1995). Reproductive factors and breast cancer risk. Effect of age at diagnosis. Ann. Epidemiol., 5, 315–320 [DOI] [PubMed] [Google Scholar]
- 37. Cauley J.A., et al. (1999). Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann. Intern. Med., 130(4 Pt 1), 270–277 [DOI] [PubMed] [Google Scholar]
- 38. Looker A.C., et al. (2012). Changes in femur neck bone density in US adults between 1988-1994 and 2005-2008: demographic patterns and possible determinants. Osteoporos. Int., 23, 771–780 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.