Abstract
The bioactive lipid sphingosine-1-phosphate (S1P) is involved in multiple cellular signalling systems and has a pivotal role in the control of immune cell trafficking. As such, S1P has been implicated in disorders such as cancer and inflammatory diseases. This Review discusses the ways in which S1P might be therapeutically targeted — for example, via the development of chemical inhibitors that target the generation, transport and degradation of S1P and via the development of specific S1P receptor agonists. We also highlight recent conflicting results observed in preclinical studies targeting S1P and discuss ongoing clinical trials in this field.
Since the discovery of the sphingolipid metabolite sphingosine-1-phosphate (S1P) as a bioactive signalling molecule more than 20 years ago1, a plethora of its functions that are important for health and disease have been identified. The numerous biological functions of S1P include regulation of cellular proliferation, survival, migration, invasion, differentiation and cellular architecture, as well as the control of immune cell trafficking, angiogenesis and vascular integrity2–6. Therefore, it is not surprising that S1P affects the immune system, central nervous system and cardiovascular system and has been implicated in a broad range of diseases, including atherosclerosis, respiratory distress, diabetes and, most importantly, cancer7 and inflammatory disorders8. The control of immune cell trafficking is one of the hallmarks of the involvement of S1P in these diseases9.
S1P is formed intracellularly by the phosphorylation of sphingosine (which is derived from the deacylation of ceramide), a process that is catalysed by two sphingosine kinases: SPHK1 and SPHK2. S1P is then exported out of cells where it can act on five specific G protein-coupled receptors (S1P receptor 1 (S1PR1) to S1PR5) and can also act on some direct intracellular targets before being broken down by S1P lyase. Each of these steps that makes up the so-called S1P axis could be therapeutically targeted (BOX 1). Given the large number of roles of S1P, it is crucial that S1PRs or tissue-specific S1P production or degradation are specifically targeted to ensure specificity and reduce side effects.
Box 1. The sphingosine-1-phosphate axis.
The sphingosine-1-phosphate (S1P) axis refers to the signalling molecule S1P, its receptors and intracellular targets, as well as the proteins that synthesize, transport and degrade S1P. Many stimuli have been shown to activate S1P synthesis inside cells, which can then either act on intracellular targets or be secreted to act on cell surface receptors. The latter process is termed ‘inside-out’ signalling and occurs when S1P acts in an autocrine and/or paracrine fashion. An S1P gradient also exists, with high S1P levels in the circulation and low S1P levels in tissues. This gradient is maintained by a balance between the synthesis of S1P — which probably occurs in red blood cells, platelets and endothelial cells — and the degradation of S1P in tissues. The S1P gradient promotes the trafficking of haematopoietic cells from lymphoid tissues into the blood and is dependent on the expression of S1P receptors.
Although it has been suggested since the mid-1990s that compounds targeting the S1P axis would be of therapeutic benefit10, several S1P modulators have only recently reached the clinic and demonstrated the utility of targeting the S1P axis. Indeed, fingolimod (also known as FTY720), which acts as a functional antagonist of S1PR1, is an oral therapeutic that is approved for the treatment of multiple sclerosis11. In this Review we discuss the development of chemical inhibitors targeting S1P generation, transport and degradation, and highlight the development of specific agonists and antagonists that target cell surface S1PRs. We focus on inflammatory disorders and cancer in which there is the strongest evidence for the importance of the S1P axis, and also describe ongoing clinical trials.
Cell surface receptors and intracellular targets
Most of the functions of S1P have been attributed to its activation of the cell surface receptors S1PR1 to S1PR5. These receptors are coupled to several — often overlapping — heterotrimeric G proteins, which accounts for both the diversity and, at times, the opposing effects of S1P on cells6. Although much of the research to date has focused on S1P signalling through S1PRs, for many years there have been observations suggesting the existence of direct intracellular targets.
More recently, several proteins have been shown to directly bind to S1P, demonstrating important roles for S1P as a localized second messenger within cells; these proteins include TNF receptor-associated factor 2 (TRAF2; an E3 ubiquitin ligase that is a key component of the nuclear factor-κB (NF-κB) pathway)12 as well as the histone deacetylases HDAC1 and HDAC2 (REF. 13), which regulate gene expression. The activity of other proteins, including the β-amyloid precursor protein cleaving enzyme 1 (BACE1), which has been implicated in Alzheimer’s disease14, has been shown to be modulated in vitro by S1P. Therefore, the potential clinical implications of targeting the S1P axis and its receptors have attracted much attention.
S1P synthesis, degradation and export
Cellular levels of S1P are controlled by its synthesis and degradation. S1P is irreversibly degraded by S1P lyase, an enzyme that is localized in the endoplasmic reticulum and cleaves the sphingoid base into ethanolamine phosphate and hexadecenal. S1P can also be dephosphorylated by two phosphatases localized in the endoplasmic reticulum: S1P phosphatase 1 (SGPP1) and SGPP2, which are members of the lipid phosphate phosphohydrolase (LPP) family. It is possible that other phosphatases are also able to dephosphorylate S1P. The resultant sphingosine can be reused for the synthesis of ceramide and complex sphingolipids (FIG. 1).
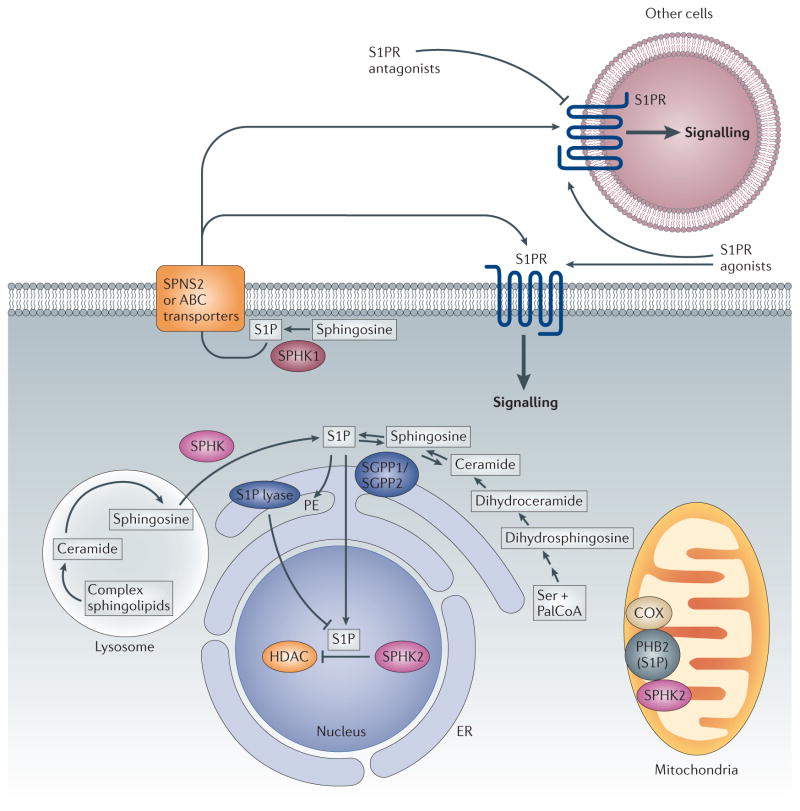
Figure 1. S1P biosynthesis, degradation, export and signalling.
Sphingosine, the substrate of sphingosine kinases (SPHKs), is not generated de novo but through the degradation of complex sphingolipids and ceramide, which can occur in the lysosome as well as on the endoplasmic reticulum (ER) and other membranes. SPHK1 is mainly located in the cytosol and is translocated to the plasma membrane upon activation. This leads to the formation of sphingosine-1-phosphate (S1P), which can be exported out of the cell by specific transporters. Binding to S1P receptors (S1PRs) initiates downstream signalling pathways. SPHK2 is localized to the ER, mitochondria and nucleus. At the ER, S1P is irreversibly degraded by S1P lyase or dephosphorylated by S1P phosphatases to sphingosine, which is reused for the synthesis of ceramide. S1P produced in the mitochondria and nucleus by SPHK2 also has direct intracellular targets. These include prohibitin 2 (PHB2), which stabilizes cytochrome c oxidase (COX), and histone deacetylases (HDACs), which remove acetyl groups from histones. ABC, ATP-binding cassette; PalCoA, palmitoyl-CoA; PE, phosphoethanolamine; SGPP1, S1P phosphatase 1; SGPP2, S1P phosphatase 2.
Signalling of S1P through its cell surface S1PRs is further controlled through localization: S1P formed inside the cell must be secreted or flopped out of the cytoplasm to bind to and activate these receptors in paracrine or autocrine manners. Although it has been shown that several ABC transporters, including ABCA1, ABCC1 and ABCG2 (REF. 15), transport S1P, the identity of the transporter in red blood cells or platelets remains unclear. Interestingly, recent studies have demonstrated that the SPNS2 protein, which belongs to the large major facilitator superfamily of transporters, regulates S1P release from endothelial and lymphendothelial cells, and controls S1P levels in plasma and lymph16–20. Because lymphocyte egress is suppressed in Spns2-deficient mice16–20, targeting SPNS2 could be a new therapeutic avenue for autoimmune diseases.
Experimental evidence suggests that the S1P axis is controlled by synthesis, secretion and degradation. The activation of SPHKs and subsequent S1P-dependent activation of S1PRs is required for the full effects of many signalling molecules such as growth factors and cytokines7,8; this is referred to as ‘inside-out’ signalling (BOX 1). In addition, the S1P gradient between the blood and lymphoid organs is required for S1PR1-mediated egress of lymphocytes, and either disruption of this gradient or S1PR1 inhibition (using fingolimod) induces lymphopenia and immunosuppression in mice9. Moreover, recent studies have demonstrated that the secretion of S1P by SPNS2 expressed on endothelial cells regulates T and B lymphocyte egress from their respective primary lymphoid organs17–20. It has also been suggested that LPP3 promotes efficient export of mature T cells from the thymus into the circulation by destroying thymic S1P21.
Together, these studies — which were carried out in mice — demonstrate that the generation, destruction and secretion of S1P is tightly regulated and that the S1P gradient is crucial for lymphocyte trafficking. Therefore, as we discuss below, the S1P axis could be targeted at the level of synthesis, secretion and degradation.
Targeting S1P synthesis and degradation
Studies from knockout mice have provided valuable information about the effects of disrupting the activity of enzymes involved in the synthesis of S1P. Mice in which either SPHK1 or SPHK2 is knocked out (Sphk1- or Sphk2-knockout mice) are viable and fertile with no obvious phenotypic changes, but circulating levels of S1P are decreased in Sphk1-knockout mice and elevated in Sphk2-knockout mice, probably owing to compensatory upregulation of SPHK1 (REF. 22). Double knockout mice (in which both SPHK1 and SPHK2 are knocked out) are embryonic lethal, which indicates that the ability to synthesize S1P is essential for development23. However, studies using isoform-specific silencing RNAs and knockout mice have indicated that SPHK1 and SPHK2 do have some distinct and non-redundant functions involved in pathophysiology. This has spurred the search for more effective isoform-specific inhibitors of SPHK1 and SPHK2.
SPHK1
Several studies, involving overexpression, small interfering RNA (siRNA) knockdown and inhibitors (TABLE 1) in cell culture and animal tumour models, have indicated that increased SPHK1 activity promotes cell growth and inhibits apoptosis. The studies have also shown that increased SPHK1 activity regulates several pathological processes such as inflammation and cancer, and that upregulation of SPHK1 correlates with poor cancer prognosis3,4,8. These results have substantiated the sphingolipid rheostat concept, in which SPHK1 produces S1P that promotes growth and inhibits apoptosis while decreasing levels of the precursors sphingosine and ceramide that inhibit growth and promote apoptosis10. Consequently, several studies have investigated the possibility of tipping the balance from S1P production towards sphingosine and ceramide production in order to promote apoptosis and inhibit growth (reviewed in REF. 24).
Table 1.
Compounds that target the S1P axis
| Compounds (alternative names) | Targets | Mechanism of action | Preclinical effects in animal models of disease | Refs |
|---|---|---|---|---|
| SKI-I | SPHK1 | SPHK1-specific inhibitor (Ki = 10 μM) | Decreases cancer progression, angiogenesis, lymphangiogenesis and airway hyperresponsiveness | 28,33,106,107 |
| Safingol | SPHK1, SPHK2 | SPHK1 (Ki = 5 μM) and PKC inhibitor | Decreases cancer progression | 108 |
| SKi (2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole or SKI-II) | SPHK1, SPHK2 | SPHK inhibitor (IC50 = 16 μM for SPHK1; IC50 = 8 μM for SPHK2) | Decreases cancer progression | 109 |
| PF-543 | SPHK1 | SPHK1-specific inhibitor (Ki = 3.6 nM) | No effect observed on cell growth | 35 |
| ABC294640 | SPHK2 | SPHK2-specific inhibitor (Ki = 9.8 μM), partial oestrogen receptor antagonist | Decreases cancer progression, liver transplant graft injury and rheumatoid arthritis | 42,110,111 |
| LX3305 and LX2931 | S1P lyase | Both compounds inhibit S1P lyase activity | Reduces rheumatoid arthritis and cerebral malaria | 57,112 |
| THI (2-acetyl-4-tetrahydroxybutylimidazole) | S1P lyase | Inhibits S1P lyase activity | Reduces muscular dystrophy | 60 |
| Fingolimod and phosphorylated fingolimod | S1PR1, S1PR3, S1PR4, S1PR5 | S1PR1 agonist and functional antagonist (IC50 = 0.2–6 nM for S1PR1, S1PR3, S1PR4 and S1PR5) | Suppresses EAE, inhibits lymphocyte trafficking, prevents transplant rejection and decreases colitis and cancer progression | 11,22,25,63, 113,114 |
| KRP-203 and phosphorylated KRP-203 | S1PR1, S1PR4 | S1PR1 agonist and functional antagonist (ED50 = 0.84 nM) | Decreases rejection of heart allografts, colitis, atherosclerosis and renal injury | 115–119 |
| AUY954 | S1PR1 | Agonist (EC50 = 1.2 nM) | Decreases experimental autoimmune neuritis, heart transplant rejection and EAE | 120,121 |
| SEW2871 | S1PR1 | Agonist (EC50 = 14–140 nM) | Decreases ischaemic renal failure and blocks diabetic nephropathy | 122–124 |
| CS-0777 and phosphorylated CS-0777 | S1PR1 | S1PR1 agonist and functional antagonist (EC50 = 1.1 nM) | Decreases EAE | 125 |
| AAL(R) and phosphorylated AAL(R) | S1PR1, S1PR3, S1PR4, S1PR5 | Agonist (EC50 = 1 nM) | Inhibits cytokine storm | 64,126 |
| TASP0277308 | S1PR1 | Antagonist (IC50 2 nM) | Ameliorates collagen-induced arthritis | 127 |
| CYM-5442 | S1PR1 | Agonist (EC50 = 1.35 nM) | Inhibits cytokine storm resulting from viral infection and decreases EAE | 66,76,128 |
| VPC23019 | S1PR1, S1PR3 | Antagonist (pKi = 7.9 for S1PR1; pKi = 5.9 for S1PR3) | Used for receptor function testing in cells and ex vivo tissue preparations | 129 |
| W146 | S1PR1 | Antagonist (Ki = 10–20 nM) | Induces lymphopenia and inhibits hyperalgesia | 75,130,131 |
| VPC44116 | S1PR1 | Antagonist (Ki = 30 nM) | Decreases Hodgkin’s lymphoma | 132,133 |
| JTE-013 | S1PR2 | Antagonist (Ki = 17 nM) | Decreases osteoporosis and atherosclerosis | 90,134,135 |
| Ponesimod (ACT-128800) | S1PR1 | S1PR1-specific agonist (EC50 = 5–9.1 nM) | Decreases delayed-type hypersensitivity and arthritis | 70,136 |
| ASONEP and iSONEP | S1P | S1P-blocking antibody (Kd = 100 pM) | Decreases cancer progression, angiogenesis and choroidal neovascularization | 137,138 |
| Siponimod (BAF312) | S1PR1, S1PR5 | Agonist (EC50 = 0.4 nM for S1PR1) | Decreases EAE | 68 |
| ONO-4641 | S1PR1, S1PR5 | Agonist (EC50 = 0.03 nM S1PR1) | Decreases EAE and colitis | 69,139 |
| VPC23153 | S1PR4 | Agonist (Kd = 38 nM) | Induces vasoconstriction | 140,141 |
| W-061 | S1PR1, S1PR4, S1PR5 | Agonist (Ki = 4 μM for S1PR1; 65 μM for S1PR4; 10 μM for S1PR5) | Decreases colitis and graft-versus-host disease | 139,142 |
| NIBR-0213 | S1PR1 | Antagonist (IC50 = 2 nM) | Decreases EAE | 73 |
EAE, experimental autoimmune encephalomyelitis; EC50, half-maximal effective concentration; ED50, half-maximal effective dose; IC50, half-maximal inhibitory concentration; Kd, dissociation constant; Ki, inhibition constant; PKC, protein kinase C; pKi, negative log of the Ki value; S1P, sphingosine-1-phosphate; S1PR, S1P receptor; SPHK, sphingosine kinase.
SPHK1 and S1P in cancer and inflammation
More recent studies have indicated that the actions of SPHK1 and S1P are complex, especially with regard to the involvement of SPHK1 and S1P in inflammation and cancer. Upregulation of SPHK1 increases the production of S1P, and this molecule links chronic intestinal inflammation to colitis-associated cancer through the stimulation of S1PR1, which leads to the activation of the master transcription factors NF-κB and STAT3 (signal transducer and activator of transcription 3) in a malicious feed-forward amplification loop22. This S1PR1–STAT3 signalling axis has been previously found in breast cancer25 and lymphomas26 and is also crucial for myeloid cell colonization at future metastatic sites in prostate cancer and melanoma27. Of particular relevance, RNA-based downregulation of SPHK1 or S1PR1 has been shown to block the persistent activation of STAT3 and reduce cancer progression and levels of inflammatory mediators in animal models22,27.
These studies raise several questions. For example, what cell types produce S1P — is it the tumour cells, cells of the tumour microenvironment or tumour-associated immune cells? What is the role of systemic S1P in cancer and inflammation? Earlier studies using mouse models of cancer28,29 and patient samples30–32 suggested that the tumours themselves, in which SPHK1 is upregulated, may be a key source of S1P. However, a recent study has suggested that local tumour growth is regulated by both S1P from the tumour and systemic S1P, whereas lung colonization and metastasis is selectively controlled via systemic S1P and downregulation of breast cancer metastasis suppressor 1 (BRMS1; a master suppressor of metastasis) through S1PR2 signalling29.
These findings have implications for targeting the S1P axis. Indeed, neutralization of systemic S1P with a specific monoclonal antibody (known as sphingomab) suppressed lung metastasis, which suggests a new therapeutic strategy to prevent cancer metastasis137. Sonepcizumab, the humanized version of sphingomab, has recently completed Phase I clinical trials in cancer (TABLE 2) and advanced into Phase II safety and efficacy trials. Thus, targeting S1P production in the tumour and the host would help reduce both growth and metastasis, respectively.
Table 2.
Drugs in clinical trials targeting the S1P axis
| Drug | Mechanism of action | Indications | ClinicalTrials.gov identifier | Phase |
|---|---|---|---|---|
| Fingolimod (Gilenya; Novartis) | S1PR modulator, S1PR1 functional antagonist | Relapsing–remitting multiple sclerosis | - | Approved |
| Acute, non-infectious intermediate, posterior and pan-uveitis | NCT01791192 | II | ||
| Amyotrophic lateral sclerosis | NCT01786174 | II | ||
| Schizophrenia | NCT01779700 | I | ||
| Acute demyelinating optic neuritis | NCT01757691 | II | ||
| Relapsing–remitting multiple sclerosis with depression, in combination with antidepressants | NCT01436643 | IV | ||
| Chronic inflammatory demyelinating polyradiculoneuropathy | NCT01625182 | III | ||
| Kidney transplant | NCT00099801 | III | ||
| Safingol | Sphingosine derivative, PKC inhibitor | Solid tumours, combined with fenretinide | NCT01553071 | I |
| Solid tumours, combined with cisplatin | NCT00084812 | I (completed) | ||
| Sonepcizumab | S1P-specific monoclonal antibody | Exudative age-related macular degeneration | NCT01414153 | II |
| Pigment epithelial detachment | NCT01334255 | I (terminated) | ||
| Neovascular age-related macular degeneration | NCT00767949 | I | ||
| Solid tumours | NCT00661414 | I (completed) | ||
| Unresectable and refractory renal cell carcinoma | NCT01762033 | II | ||
| ABC294640 | SPHK2 inhibitor | Pancreatic cancer | NCT01488513 | I |
| KRP203 | S1PR1 agonist | Sub-acute cutaneous lupus erythematosus | NCT01294774 | II (terminated) |
| Ulcerative colitis | NCT01375179 | II (terminated) | ||
| Haematological malignancies | NCT01830010 | I | ||
| Siponimod (BAF312) | S1PR1 and S1PR5 modulator | Hepatic impairments | NCT01565902 | I |
| Relapsing–remitting multiple sclerosis | NCT00879658 | II | ||
| Relapsing–remitting multiple sclerosis | NCT01185821 | II | ||
| Secondary progressive multiple sclerosis | NCT01665144 | III | ||
| Polymyositis, dermatomyositis | NCT01148810 | II (terminated) | ||
| RPC1063 | S1PR1 modulator | Relapsing–remitting multiple sclerosis | NCT01628393 | II |
| Ulcerative colitis | NCT01647516 | II | ||
| ONO-4641 | S1PR1 and S1PR5 agonist | Multiple sclerosis | NCT01226745 | II |
| LX3305 | S1P lyase inhibitor | Rheumatoid arthritis | NCT00847886 | I (completed) |
| Rheumatoid arthritis | NCT00903383 | II (completed) | ||
| GSK2018682 | S1PR1 agonist | Relapsing–remitting multiple sclerosis | NCT01466322 | I (completed) |
| Relapsing–remitting multiple sclerosis | NCT01431937 | I (completed) | ||
| Ponesimod ACT-128800 | S1PR1 agonist | Plaque psoriasis | NCT00852670 | II (completed) |
| Relapsing–remitting multiple sclerosis | NCT01093326 | II | ||
| Psoriasis | NCT01208090 | II (completed) | ||
| Relapsing–remitting multiple sclerosis | NCT01006265 | II (completed) |
PKC, protein kinase C; S1P, sphingosine-1-phosphate; S1PR, S1P receptor; SPHK, sphingosine kinase.
Encouraging results such as these have driven the pursuit of effective SPHK1 inhibitors for cancer chemotherapy. Although several SPHK1 inhibitors have shown promise in preclinical studies7,33, two new selective SPHK1 inhibitors had no effect on cancer cell growth, which seems to contradict the current dogma. An amidine-based inhibitor that had 15-fold higher selectivity for SPHK1 over SPHK2 and a Ki (inhibition constant) value of 100 nM34 rapidly reduced S1P levels in cells but did not potently inhibit cell growth. Its administration to mice resulted in a rapid decrease in S1P levels in the blood, which indicates that there is a rapid turnover of circulating S1P levels34; however, the effects of this amidine-based inhibitor on tumour growth in vivo have not yet been reported. An even more potent SPHK1 inhibitor, PF-543, which has a nanomolar Ki value and 100-fold selectivity for SPHK1 over SPHK2, has been identified35. PF-543 also rapidly reduced S1P levels in cells, but it too had no effect on cell growth at the same or higher concentrations.
The lack of effect of PF-543 on cell growth might be due to its inability to increase levels of pro-apoptotic ceramide, as was typically observed when SPHK1 was inhibited or downregulated7,33. It is also possible that the potency of SPHK1 inhibitors may depend in part on their ability to induce the proteasomal degradation of SPHK1, as has been demonstrated for some of these inhibitors such as Ski36 (2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole; also known as SKI-II). Alternatively, S1P that is produced and secreted as a result of SPHK1 upregulation may promote cancer progression by tumour-induced angiogenesis and lymphangiogenesis28, and it could be crucial for myeloid cell colonization at future metastatic sites27 in vivo without affecting tumour growth. Although PF-543 appears to be a useful tool for inhibiting SPHK1 in vitro, its effects were not investigated in vivo.
The recently solved X-ray crystal structure of SPHK1 revealed that the active site is located in a cleft between two domains with a hydrophobic lipid-binding pocket buried in the carboxy-terminal domain37. It remains to be determined how the substrate sphingosine — which has a hydrocarbon tail that may be associated with membranes — can tunnel into this site in a tail-to-head manner. Elucidation of the structural basis of SPHK1 substrate recognition and catalysis will lead to a better understanding of how this important enzyme can be regulated. In addition, it might clarify the seemingly contradictory findings observed with different SPHK1 inhibitors and could accelerate the development of high-potency inhibitors for therapeutic uses. Owing to the many important roles of S1P in physiological processes, further studies are also needed to determine whether there are any adverse effects associated with the long-term inhibition of SPHK1 and decrease in S1P levels.
SPHK2
Knowledge of the pathophysiological roles of SPHK2 is not as advanced as that of SPHK1, perhaps owing to the fewer numbers of studies carried out on SPHK2, its localization in several subcellular compartments and its ambiguous nature in promoting pathology in some disorders and preventing it in others5. Although SPHK1 is generally localized in the cytosol and is translocated to the plasma membrane upon activation, SPHK2 is expressed in several organelles, including the nucleus in many cell types. S1P produced by the actions of SPHK2 in the nucleus binds to and inhibits HDAC1 and HDAC2, which suggests that S1P is an endogenous HDAC inhibitor that contributes to epigenetic regulation of gene expression13.
Although it has been reported that knockdown of SPHK2 induces apoptosis7, somewhat surprisingly it was recently suggested that mitochondrial SPHK2 is pro-apoptotic; it produces S1P that is degraded by S1P lyase to hexadecenal, which then binds to the apoptosis regulator BAX, promoting its oligomerization and the release of cytochrome c38. However, contrary to the view that SPHK2 is pro-apoptotic, studies of Sphk2-null mice have revealed that it is required for ischaemic pre- and post-conditioning as well as cardioprotection39,40. Moreover, S1P produced by mitochondrial SPHK2 binds to the scaffold protein prohibitin 2 (REF. 41) — a protein that is important for respiration and the assembly of complex IV. Studies involving conditional deletions of SPHK2 might clarify the functions of this protein and help determine whether specific SPHK2 inhibitors might be clinically useful.
Targeting SPHK2
An SPHK2 inhibitor, ABC294640, has been described that inhibits the growth of cancer cells in culture and reduces S1P levels and the growth of mammary tumours in nude mice42. However, care should be taken in interpreting results obtained from this compound, as more recent research has shown that ABC294640 also binds to the oestrogen receptor and has anti-oestrogenic effects43. A newer SPHK2 inhibitor, SLR080811, decreased S1P levels in fibroblasts44, but administration of the compound to mice caused an unexpected rapid increase in blood S1P levels. Although this elevation in S1P levels could be due to the off-target effects of SLR080811 on S1P transporters, this observation resembles the increase in circulating basal levels of S1P seen in Sphk2-null mice22, which indicates that deletion as well as inhibition of SPHK2 leads to compensatory increases in the activity of SPHK1.
This increase in SPHK1 levels caused by the downregulation or deletion of SPHK2 might explain why the data on the roles of SPHK2 in inflammation are also conflicting. There was greater disease severity in Sphk2-knockout mice with colitis22 (induced by dextran sulphate sodium); disease severity was also higher in severe combined immunodeficient (SCID) mice adoptively transferred with Sphk2-null T cells45 than with wild-type T cells. Conversely, inhibition of SPHK2 with ABC294640 reduced the severity of colitis46 and colitis-associated cancer47. Several studies have also examined the role of SPHK2 in models of inflammatory arthritis. Although genetic ablation of SPHK2 had no effect on arthritis progression, administration of ABC294640 increased the severity of arthritis in one study48 and protected against the development of arthritis in another study49. Such observations remain puzzling. Together, these results suggest that ABC29460 may not provide a valid approach for investigating the role of SPHK2 in vivo and so the development of new specific inhibitors is eagerly awaited.
HDAC inhibitors are used in psychiatry and various brain disorders, and are being investigated as potential treatments for several other diseases, particularly cancer50–52. Because SPHK2 produces nuclear S1P that inhibits HDACs13, SPHK2 inhibitors could have opposite effects to HDAC inhibitors, which might not be beneficial. The development of sphingosine analogues that are phosphorylated in vivo by SPHK2 to produce S1P mimetics is an approach that might inhibit HDACs with greater specificity than current clinically used pan-HDAC inhibitors. Overall, however, targeting SPHK2 should be approached with caution.
S1P lyase
S1P lyase catalyses the irreversible cleavage of S1P into phosphoethanolamine and hexadecenal, which are precursors for phospholipid synthesis (FIG. 1). A recent report demonstrated that aldehyde dehydrogenase family 3 member A2 (ALDH3A2), the causative mammalian gene for Sjögren–Larsson syndrome, is responsible for the conversion of hexadecenal to hexadecenoic acid, which suggests that the accumulation of hexadecenal may contribute to neurological and cognitive defects, as well as ichthyosis, in the pathogenesis of Sjögren–Larsson syndrome53. It remains to be determined whether S1P lyase inhibition could reduce symptoms in affected patients.
S1P lyase regulates the cellular pool of S1P that is available for signalling in S1P-dependent physiological and pathological processes54. As the terminal step in the degradation of all sphingolipids, it not only controls levels of bioactive sphingolipid metabolites but is also the link between sphingolipid and phospholipid metabolism. S1P lyase deficiency (by gene ablation) or RNA-based inhibition is associated with elevated nuclear S1P levels and reduced HDAC activity55. In addition to enhanced histone acetylation, it is possible that downregulation of HDAC isoenzymes may contribute to the dysregulation of calcium homoeostasis that is observed in S1P lyase-null cells55.
S1P lyase in lymphocyte function and immunosuppression
Seminal studies by Schwab and Cyster56 showed that lymphocyte egress from lymphoid organs is mediated by S1P gradients — that are maintained at least in part by S1P lyase — between the circulation and tissues. They found that S1P levels in lymphoid tissues are low and are dramatically increased following the administration of THI (2-acetyl-4-tetrahydroxybutylimidazole; a food colourant), which is a compound that inhibits the degradation of S1P by S1P lyase. Moreover, increased cellular levels of S1P and disruption of the S1P gradient induced lymphopenia probably through the downregulation of S1PR1 expression on lymphocytes56. This study indicates that S1P lyase may represent a novel immunosuppressant drug target.
Indeed, derivatives of THI have been developed that prevent the development and reduce the severity of rheumatoid arthritis in mice57. A Phase II clinical trial was recently completed for one of these compounds, LX3305 (TABLE 2), examining its efficacy in the treatment of rheumatoid arthritis. However, the mechanism by which THI or any of its derivatives inhibit S1P lyase in vivo merits additional studies as none of these compounds has been shown to directly inhibit lyase activity in vitro.
The crystal structure of the yeast S1P lyase highlighted residues that are involved in activity and substrate binding58, and so this knowledge might aid the development of inhibitors that specifically target the S1P lyase active site rather than its pyridoxal cofactor (which could induce side effects by inhibiting other pyridoxal-dependent enzymes).
S1P lyase and muscle function
Suppression of S1P lyase may also be an effective way to promote muscle regeneration. S1P is a trophic factor for muscle regeneration and can activate quiescent muscle stem cells known as satellite cells59, which maintain muscle homeostasis and are needed for muscle repair. Intriguingly, S1P lyase is upregulated in injured skeletal muscle and in muscles of mdx mice60. THI treatment elevated muscle S1P levels, resulting in enhanced recruitment and proliferation of satellite cells as a result of S1PR2 and STAT3 activation, which led to suppression of cell cycle inhibitors and skeletal muscle regeneration60.
Studies in Drosophila melanogaster have shown that genetic elevation of S1P (caused by the deletion of S1P lyase) suppresses dystrophic muscle phenotypes61. Because there are no known S1PR homologues in D. melanogaster, it was suggested that localized intracellular S1P elevation directly promotes the suppression of muscle wasting in fruitflies61. Thus, it is possible that inhibitors of S1P lyase may provide a new therapeutic strategy for myopathies. However, further work needs to be carried out to understand the role of S1P in mammalian muscle development and regeneration.
Targeting S1PRs
First- and second-generation agonists and antagonists that are specific for one or a subset of S1PRs have been developed (TABLE 1) and are discussed below. Fingolimod has been clinically approved for the treatment of relapsing and remitting multiple sclerosis in the United States and Europe11, and several other compounds are in clinical trials (TABLE 2).
Efforts to develop highly specific and efficacious drugs will be greatly enhanced by the recent report of the crystal structure of S1PR1 complexed with an antagonist62. Intriguingly, this structure indicates that, at least for this member of the S1PR family, the ligand binding pocket is covered by an amino-terminal helix. This suggests that to access the binding pocket S1P must slide laterally within the plane of the bilayer between a pair of transmembrane helixes. Ultimately, this structure will both assist with the development of S1PR1 targeted compounds that have greater specificity and provide a basis for determining the structure of other S1PRs.
S1PR1 agonism and antagonism
Fingolimod is a sphingosine analogue that is phosphorylated primarily by SPHK2 to form phosphorylated fingolimod, which is an agonist at all of the S1PRs except for S1PR2 (REFS 63,64). However, persistent activation of S1PR1 by phosphorylated fingolimod causes S1PR1 internalization and degradation, and so fingolimod acts as a functional antagonist at this receptor11,65. Drug-induced downregulation of the expression of cell surface S1PRs on lymphocytes prevents their egress from lymphoid organs and induces lymphopenia and immunosuppression11,66; these effects are advantageous for the treatment of autoimmune diseases such as multiple sclerosis.
Although phosphorylated fingolimod targets multiple S1PRs, several next-generation agonists and antagonists have been developed and are in clinical trials that more specifically target S1PR1 (TABLE 2). The use of fingolimod as a lead compound and its optimization for potency at S1PR1 (REF. 67) led to the development of siponimod (also known as BAF312)68, which is now in Phase III trials for multiple sclerosis. Other examples of S1PR1-directed drugs include ONO-4641 (REF. 69), which is a novel selective agonist for both S1PR1 and S1PR5, and ponesimod (ACT-128800)70, which is a potent selective S1PR1 modulator; both of these drugs have been effective in rodent models and are now in Phase II clinical trials for multiple sclerosis and moderate-to-severe chronic plaque psoriasis, respectively (TABLE 2). Other modulators of S1PRs are being investigated in several preclinical disease models (TABLE 1), including viral responses, cancer treatments and modulation of angiogenesis.
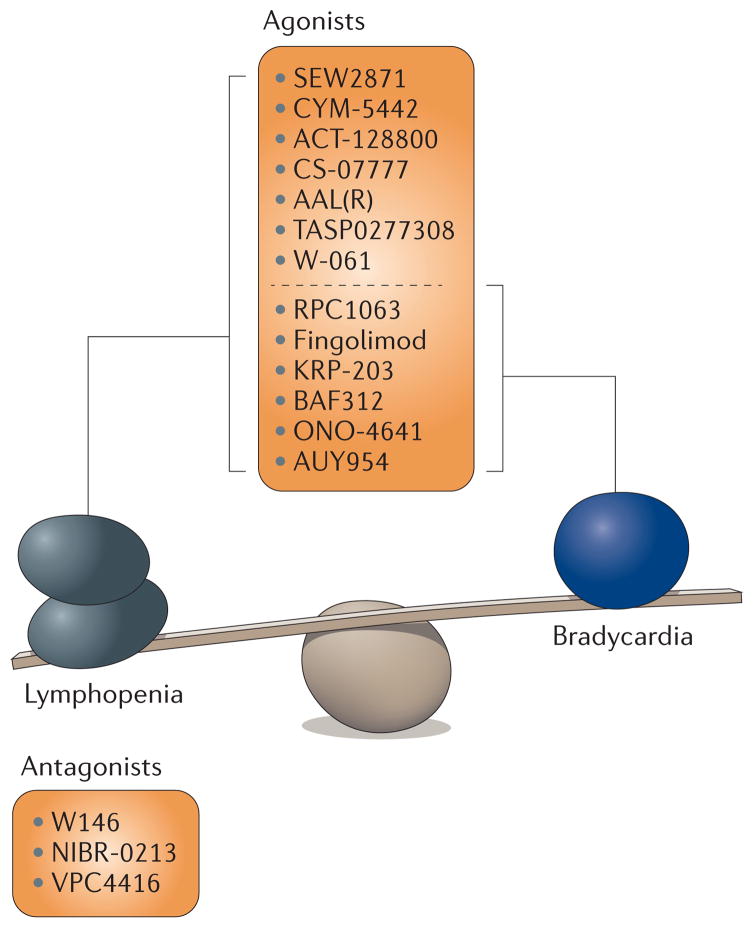
The issue of receptor specificity must be borne in mind, as transient bradycardia is the major adverse effect of fingolimod in humans and is also observed with siponimod and other S1PR1 modulators. This suggests that S1PR1 modulators contribute to this effect (FIG. 2). Therefore, S1PR1 modulators could potentially have the same adverse effects in patients as have been reported for fingolimod, including first-dose bradycardia, macular oedema and infection71,72. However, S1PR1 modulators might still be an effective treatment option for patients with serious disease, provided they are selected and monitored appropriately71. A major remaining challenge is to gain a deeper knowledge of any beneficial as well as adverse side effects of targeting S1PRs and to understand how potential therapeutics modulate the functions and mechanisms of action of S1PRs.
Figure 2. The balance between beneficial and detrimental effects of S1PR1 agonists and antagonists.
Agonists and antagonists of sphingosine-1-phosphate receptor 1 (S1PR1) can induce both lymphopenia and the bradycardic side effects. Fingolimod acts as both an agonist and functional antagonist of S1PR1.
The understanding of the mechanism of action of S1PR1 modulators focuses on preventing S1PR1 function on lymphocytes, either by functional antagonism (for example, phosphorylated fingolimod)11,66 or with a competitive antagonist (for example, NIBR-0213), in order to prevent lymphocytes from recognizing S1P egress signals73. However, it has been suggested that some of the advantageous effects of the compounds that modulate S1PR1-mediated functions are independent of their effects on lymphocytes74. Using the experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis, it was shown that loss of S1PR1 in astrocytes alone reduced disease severity, demyelination, axonal loss and astrogliosis, and made the mice non-responsive to fingolimod treatment74. So, in addition to inhibiting the migration of specific lymphocyte subsets into the central nervous system, the therapeutic activity of fingolimod could be due to its direct effects on neural cells, particularly astrocytes11.
It is also assumed that some of the reported adverse effects of S1PR1 modulators, such as macular oedema, might be due to vascular leakage as S1PR1 is important for the maintenance of vascular integrity. This effect may limit the therapeutic window of some antagonists more than that of agonists75. In this regard, the selective S1PR1 agonist CYM-5442 modulates S1PR1 on the pulmonary endothelium, inhibiting the cytokine storm and enhancing survival — following the infection of mice with human pathogenic influenza virus — independently of lymphocyte S1PR1 activation76.
There is no doubt that an increased understanding of S1PR1 signalling and of the cell types that mediate the effects of S1PR1 signalling will advance the development of additional S1P-based therapeutics for the treatment of multiple sclerosis and potentially other diseases. However, the potential for S1P analogues to have multiple targets remains a challenge for pharmacological intervention.
S1PR-independent functions of fingolimod
It has been known for several years that the prodrug fingolimod itself can induce apoptosis of several types of cancer cells independently of its phosphorylation or its S1PR-dependent effects67,77–79. More recently, fingolimod was shown to activate protein phosphatase 2A (PP2A), a tumour suppressor that dephosphorylates many oncogenic signalling proteins including AKT, leading to mitochondria-dependent apoptosis. This led to the suggestion that fingolimod might be an alternative treatment for blast crisis in chronic myelogenous leukaemia and Philadelphia chromosome-positive acute lymphocytic leukaemia — two BCR–ABL-driven leukaemias against which ABL kinase inhibitors fail to induce a long-term response67,78,79.
Recently, it was found that fingolimod, but not phosphorylated fingolimod, directly binds to the PP2A inhibitor (I2PP2A; also known as SET) — a protein that inhibits PP2A function and thus results in PP2A reactivation — and, surprisingly, causes caspase-independent death of lung cancer cells through RIPK1 (receptor-interacting serine/threonine protein kinase 1)-dependent necroptosis. It was known that RIPK1 activates RIPK3 and, subsequently, leads to increased phosphorylation and activation of mixed lineage kinase domain-like protein (MLKL). MLKL, in turn, activates mitochondrial phosphoglycerate mutase family member 5 (PGAM5), which leads to dynamin 1-like protein (DNM1L; also known as DRP1)-dependent mitochondrial fission and subsequent cell death80,81. However, PP2A-dependent necroptosis occurs independently of PGAM5, which indicates that fingolimod might induce a novel type of necrotic cell death programme. Further studies to elucidate this pathway may be of great importance as fingolimod administration has been shown to suppress tumour progression in vivo36.
S1PR1 and STAT3
Several studies have suggested that S1PR1 has a crucial role in the persistent activation of STAT3 (REFS 22,25–27), a transcription factor that is constitutively active in — and associated with — multiple types of cancers. Historically, it has been difficult to therapeutically target STAT3 (REFS 82–84). STAT3 induces the transcription of S1PR1, which then reciprocally activates STAT3, resulting in its persistent activation and interleukin-6 (IL-6) production25 (FIG. 3). This unique S1PR1-dependent axis may be an attractive target for intervention, as several reports have indicated that disruption of S1PR1 signalling abrogates this cycle of STAT3 amplification22,82–84.
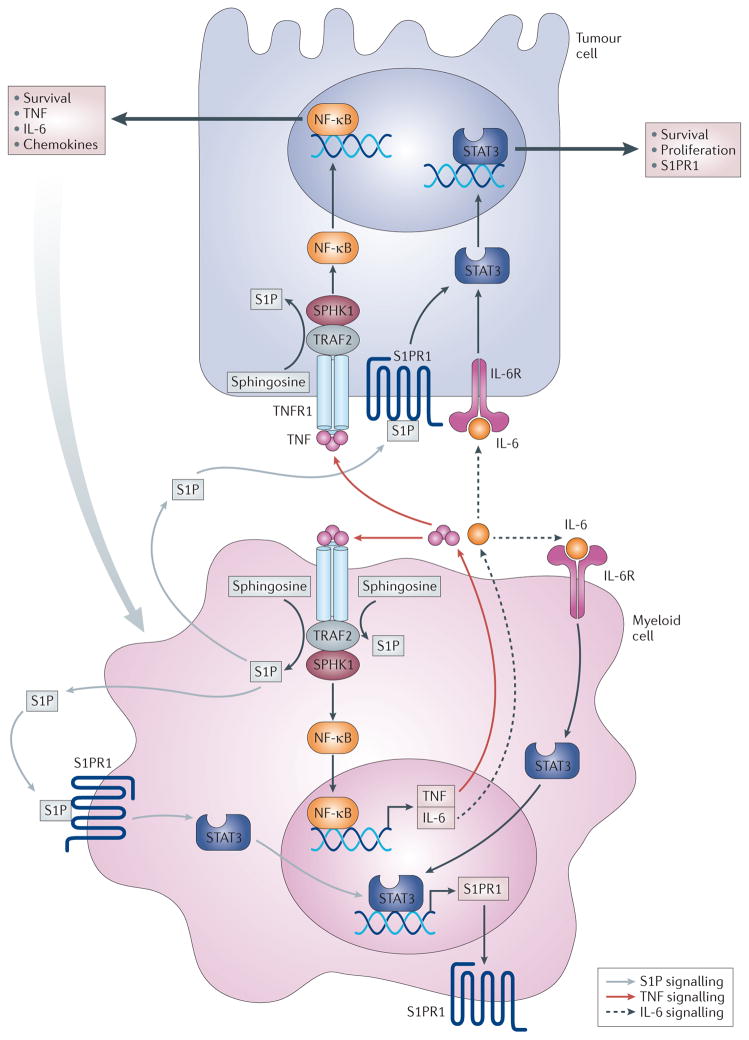
Figure 3. The S1PR1–STAT3 axis linking inflammation and cancer.
Sphingosine kinase 1 (SPHK1) is upregulated in tumour cells to produce sphingosine-1-phosphate (S1P); this activates S1P receptor 1 (S1PR1), which leads to the activation of signal transducer and activator of transcription 3 (STAT3). Reciprocally, STAT3 enhances the transcription of its target genes, including S1PR1. S1P is also involved in the activation of nuclear factor-κB (NF-κB), which regulates the transcription of the pro-inflammatory cytokines tumour necrosis factor (TNF) and interleukin-6 (IL-6). TNF stimulates SPHK1 to further maintain NF-κB activation, and IL-6 induces STAT3 activation. In addition to upregulating SPHK1 in tumour cells, inflammation upregulates SPHK1 in inflammatory and/or myeloid cells in a manner similar to that in tumour cells. Communication among tumour cells, the host microenvironment and inflammatory cells via systemic S1P regulates metastasis. Targeting SPHK1 and S1PR1 — for example, with fingolimod — interferes with these amplification cascades and cancer progression. IL-6R, IL-6 receptor; TNFR, TNF receptor; TRAF2, TNF receptor-associated factor 2.
Targeting of S1PR1 using short hairpin RNA (shRNA) or via the administration of fingolimod reduces S1PR1 expression and downregulates STAT3 activity in the activated B cell-like subtype of diffuse large B cell lymphoma, which reduces the growth of lymphoma tumour cells in vitro and in vivo26. Moreover, SPHK1 and S1PR1 were upregulated in chronic intestinal inflammation and associated cancers26. Indeed, S1P was essential for the production of the multifunctional NF-κB-regulated cytokine IL-6, the persistent activation of STAT3 and the consequent upregulation of S1PR1. In this case, treatment with fingolimod decreased not only S1PR1 expression but also SPHK1 levels and, by doing so, it eliminated the NF-κB–IL-6–STAT3 amplification cascade and the development of colitis-associated cancer in mice (FIG. 3). Therefore, it was suggested that the SPHK1–S1P–S1PR1 axis forms the nexus between NF-κB and STAT3, which connects chronic inflammation with colitis-associated cancer, and that fingolimod may be useful in treating colon cancer in patients with colitis22.
A more recent study introduced the notion that the S1PR1–STAT3 signalling axis is involved in tumour metastasis; namely, it suggested that levels of signalling molecules involved in this axis are elevated in distant organs before the arrival of tumour cells, which empowers myeloid cells to invade, proliferate and resist apoptosis at pre-metastatic sites27. These myeloid cells then influence other cells, such as fibroblasts, to produce factors that facilitate the formation of pre-metastatic niches for tumour cell metastasis. An important aspect of this notion is the therapeutic potential of targeting the S1PR1–STAT3 signalling axis to eliminate and/or reduce preformed pre-metastatic niches, thereby preventing tumour metastasis27.
Constitutive activation of STAT3 through S1PR1 therefore allows for both a survival advantage and an increased metastatic potential. Targeting S1PR1 with an antagonist can thus potentially decrease two clinically relevant functions of cancer development at the same time; that is, it can inhibit tumour cell proliferation and survival while simultaneously decreasing the ability of the cancer to spread. Further studies using other specific S1PR1 modulators are needed to confirm these possibilities.
S1PR1 and angiogenesis
Angiogenesis has multiple physiological roles in development and disease. The importance of S1PR1 in angiogenesis and vascular maturation was first realized with the observation that S1PR1-knockout animals die in utero owing to severe haemorrhaging and incomplete vasculogenesis85. It has recently been shown that S1PR1 is involved in the termination of sprouting angiogenesis86 (FIG. 4) and that endothelial S1PR1 stabilizes the primary vascular network during development and homeostasis87,88. Severe aberrations in vessel size and excessive sprouting were observed in the limbs of mice in which S1PR1 was deleted on endothelial cells, which indicates that the effect of S1PR1 on sprouting is endothelial cell-autonomous. Similar effects were observed with S1PR1 knockdown in zebrafish, which suggests that this is an evolutionarily conserved mechanism86.
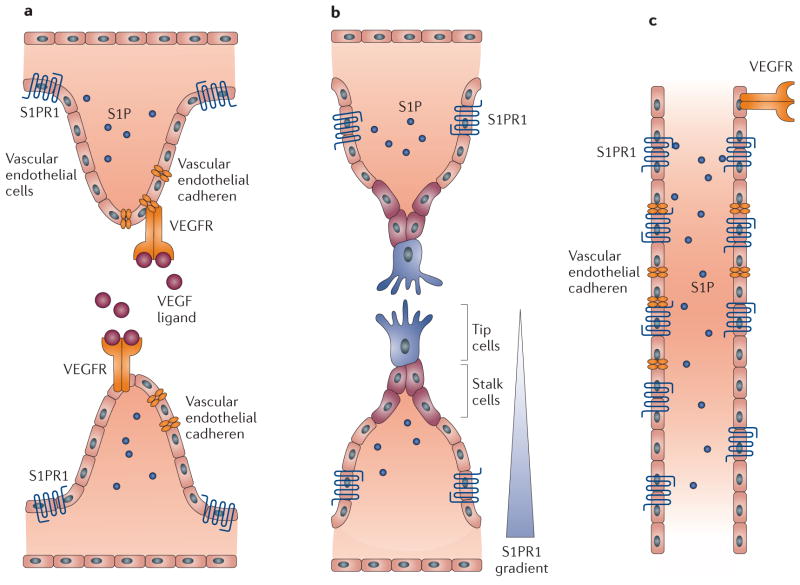
Figure 4. S1PR1-mediated suppression of sprouting angiogenesis and stabilization of blood vessels.
a | Sphingosine-1-phosphate receptor 1 (S1PR1) is expressed in vascular endothelial cells and colocalizes with vascular endothelial cadherin in regions of normal blood flow, but is internalized in regions with turbulent blood flow (not shown). b | Engagement of vascular endothelial growth factor (VEGF) signalling in the growing vascular front induces the formation of sprouts consisting of tip cells that extend out from the blood vessel and stalk cells. The sprouting vascular front, the tip and stalk cell region express very low levels of S1PR1 (REF. 88); alternatively, S1PR1 is expressed on tip cells but they are not exposed to S1P present in the bloodstream86. c | After its fusion into a primary vascular loop, which is a key step in the initiation of blood flow, S1P activates S1PR1. Activation of S1PR1 enhances the formation of adherens junctions, inhibits VEGF signalling, suppresses sprouting and stabilizes new vascular connections. VEGFR, VEGF receptor.
S1PR1 activation counteracts vascular endothelial growth factor (VEGF) function and positively regulates endothelial cell–cell adhesion. These results establish a functional antagonism between S1PR1 and VEGF receptor 2 (VEGFR2) at the levels of sprouting angiogenesis and junctional stability87. Moreover, it was shown that vascular endothelial cadherin is a crucial downstream mediator of S1PR1 function. Together, these studies suggest that S1P carried by blood flow closes a negative feedback loop that inhibits sprouting angiogenesis once the vascular bed is established and functional.
In addition to responding to S1P, S1PR1 responds to laminar shear stress to transduce blood flow-mediated signalling involving the extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase (MAPK) pathway and the AKT–endothelial nitric oxide (eNOS) pathway in endothelial cells in vitro and in vivo. Moreover, activation of these pathways was suppressed by fingolimod88. Interestingly, the same study showed that S1PR1 can be activated in a ligand-independent manner by laminar shear stress, which suggests that S1PR1 can respond not only to blood-borne S1P but also to biomechanical signals independently of its ligand. This raises several intriguing questions. If S1PR1 is always mechanosensitive, what is the function of S1P binding? And what is the relationship between mechanotransduction and sprouting?
The regulation of vascular hypersprouting by S1PR1 may have implications for the clinical use of S1PR1 modulators. For example, it is possible that exaggerated VEGF signalling in pathological conditions — such as tumour angiogenesis, age-related macular degeneration and rheumatoid arthritis — could be reduced by agonists that activate S1PR1 signalling. However, it is still not clear whether S1PR1 functions similarly in pathology-driven angiogenesis as it does during development. This is an important area that merits further study.
Targeting other S1PRs
In contrast to the large amount of information that is available on the functions of S1PR1, less is known about the biological and pathological roles of the other S1PRs, and so substantially less progress has been made in developing specific agonists and antagonists of these receptors. A few clues have surfaced that suggest that targeting other S1PRs might be beneficial in certain diseases. Below, we address the emerging pathophysiological roles of specific, individual S1PRs that still need receptor-specific pharmacological validation.
S1PR2 in osteoporosis
Bone is continuously remodelled throughout life by the balanced actions of osteoblasts that form bone and osteoclasts that resorb it. In conditions such as osteoporosis, osteolysis and rheumatoid arthritis, this balance is tipped in favour of osteoclasts. Osteoclasts are derived from precursor monocytes that dynamically migrate from the blood into bone and back into the blood. This migration is dependent on S1P-mediated ligation of specific S1PRs. S1PR1 promotes the migration of osteoclasts from the bone to the blood along an upward S1P gradient89, whereas S1PR2 inhibits their migration, leading to increased numbers of osteoclasts on bone and subsequent bone resorption90. Accordingly, it was recently shown that the steroid hormone calcitriol — the active form of vitamin D, which is an established treatment for osteoporosis — acts in part by reducing the expression of S1PR2 on osteoclast precursor cells91.
Effective antagonism of S1PR2 might be useful for the treatment of osteoporosis as inhibition of S1PR2 with the S1PR2 antagonist JTE-013 relieved osteoporosis in a mouse model by limiting the location of osteoclast precursor cells and reducing the number of mature osteoclasts that were attached to the bone surface90. However, although many studies have shown JTE-013 to be useful for targeting S1PR2, this compound also antagonizes S1PR4 (REF. 92), and other studies have suggested that it might also have off-target effects93. Therefore, the results obtained with JTE-013 should be validated by genetic deletion or downregulation of S1PR2.
S1PR3 in cancer and sepsis
Several reports have implicated S1PR3 in cancer. Early studies showed that estradiol stimulates SPHK1 in human breast cancer cells and that ligation of S1PR3 by released S1P transactivated the epidermal growth factor receptor in a matrix metalloproteinase-dependent manner94. Thus, these findings reveal that SPHK1 has a key role in the coupling of signals among three membrane-spanning events induced by estradiol, S1P and epidermal growth factor94. Moreover, increased expression of S1PR3 correlated with shorter disease-free survival times in patients with oestrogen receptor-positive breast cancer95. The notion that S1PR3 might be involved in cancer progression is supported by the observation that an S1PR3-specific inhibitory monoclonal antibody (7H9) decreased tumour growth in a xenograft model of breast cancer96.
In addition to cancer, S1PR3 has been implicated in promoting sepsis. S1PR3 activation promotes lipopolysaccharide (LPS)-induced vascular leakage and lung oedema97, as well as regulating the amplification of inflammation in sepsis syndrome98. Moreover, whereas S1PR1 was shown to be crucial for endothelial barrier enhancement, S1PR3 expression was involved in barrier disruption97. Recent studies have shown that increased S1PR3 expression is associated with the mortality of severely ill patients with sepsis or acute lung injury; these studies suggest that S1PR3 is a candidate biomarker and a target for future therapeutic strategies against acute lung injury99. The potential of S1PR3 inhibition in sepsis treatment was substantially advanced when it was shown that the S1PR3-specific inhibitory monoclonal antibody 7H9 increased the survival of LPS-treated mice96. Further studies with a humanized version of this antibody are required to demonstrate possible efficacy in patients with sepsis or cancer.
S1PR4 and dendritic cell function
Although it is predominantly expressed on lymphocytic and haematopoietic cells, the role of S1PR4 in immune homeostasis is still poorly understood. S1PR4 is involved in the regulation of dendritic cell function and TH17 cell differentiation100, and has been shown to modify the course of several immune diseases in murine models100. Moreover, this receptor could be involved in neutropenia and inflammation101. Therefore, S1PR4 may be an interesting target for influencing the course of several autoimmune pathologies using newly developed selective S1PR4 antagonists102.
S1PR5 and natural killer cells
S1PR5 is required for the trafficking of natural killer cells — which are involved in the clearance of infectious agents and antitumour surveillance — from the bone marrow and lymph nodes into tissue103. This indicates that S1PR5 agonists may be useful for promoting natural killer cell-dependent clearance of tumours, whereas S1PR5 antagonists may reduce transplant rejection. However, there is currently no evidence to support this notion. Although some selective and orally active S1PR5 agonists have been synthesized104, their in vivo effects have not yet been reported.
Concluding remarks and future perspectives
Given the success of targeting S1PR1, we expect that a new generation of selective S1PR-targeted drugs will be developed as the roles of these receptors in health and disease become more apparent. However, some of the pathogenic roles of the S1P axis have been linked to multiple S1PRs, possibly owing to the coupling of different S1PRs to the same Gα proteins in particular cell types105. Thus, for further progress in this area it is necessary to bear in mind the need for molecular and pharmacological validation of the specific roles of individual S1PRs.
Based on the recently solved X-ray crystal structure of S1PR1, we expect that the structures of the other S1PRs will soon be solved. A better understanding of structure–function relationships within this subfamily will enable the rational design of more selective and potent modulators with useful pharmacokinetics. The development of these tools will provide a fundamental understanding of S1P signalling as well as new therapeutics for treating many human disorders.
Acknowledgments
Work in the laboratory of S.S. is supported by grants R37GM043880 and RO1CA61774 from the US National Institutes of Health (NIH). The work of G.T.K. is supported by the NIH grant T32 HL094290.
Glossary
- Angiogenesis
The development of new blood vessels. Angiogenesis is required in development and tissue repair, as well as pathologically for tumour progression
- Fingolimod
A sphingosine-1-phosphate receptor (S1PR) agonist. However, sustained activation of S1PR1 by fingolimod leads to degradation of S1PR1. Thus, fingolimod is often referred to as a ‘functional antagonist’ of S1PR1, particularly in its role in lymphocyte trafficking
- Histone deacetylases
(HDACs). Proteins that remove acetyl groups from specific histone lysine residues, thus altering gene transcription
- ABC transporters
(ATP-binding cassette transporters). A family of proteins that transport small molecules across the membrane, including drugs and lipids. Several of these proteins have been shown to transport sphingosine-1-phosphate
- ‘Inside-out’ signalling
A model whereby agonists such as growth factors promote the production of sphingosine-1-phosphate (S1P) within the cell. This S1P is then exported outside the cell to signal through cell surface S1P receptors in an autocrine and/or paracrine manner
- Lymphopenia
An abnormally low level of lymphocytes in the blood
- Sphingolipid rheostat concept
A concept that describes how the metabolic balance between sphingosine-1-phosphate (S1P) and ceramide regulates cell fate. S1P-mediated signals mostly regulate cell survival and proliferation, whereas ceramide-mediated signals regulate growth inhibition and apoptosis
- Myeloid cell
A blood cell type that includes macrophages, monocytes, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes and dendritic cells but not lymphocytes
- Lymphangiogenesis
The development of new lymph vessels. Lymphangiogenesis is required in development and for tissue repair, as well as for the metastasis of some tumours, such as breast cancer tumours
- Sjögren–Larsson syndrome
An autosomal recessive form of ichthyosis (scaly, dry, thickened skin) that is characterized by spastic paraplegia and mild to moderate intellectual disability
- Ichthyosis
A family of mostly genetic skin disorders characterized by dry, thickened, scaly skin, often with cracks
- mdx mice
A mouse model of Duchenne muscular dystrophy, which is a muscle-wasting disease that is caused by a mutation in the X-linked dystrophin gene, leading to loss of expression of the dystrophin protein
- Plaque psoriasis
An autoimmune disorder of the skin in which the patient typically presents with scaly patches of skin with a red and/or white hue
- Bradycardia
A decreased resting heart rate that results in in dizziness, weakness and fatigue
- Macular oedema
The accumulation of fluid and protein in the macula (visual field), leading to swelling and loss of vision
- Experimental autoimmune encephalomyelitis
(EAE). An animal model of inflammation-induced demyelinating disease, often used as a proxy for human multiple sclerosis
- Cytokine storm
A potentially fatal immune reaction consisting of a positive feedback loop between highly elevated levels of many cytokines with immune cells
- Sprouting angiogenesis
The process of developing new blood vessels in which angiogenic factors bind to receptors on endothelial cells of a blood vessel. These cells grow out and form sprouts connecting to other blood vessels
- Laminar shear stress
The stress on tissues derived from the flow of a fluid through the vessel
- Osteoporosis
A bone-thinning disease that is characterized by overactive bone resorption by osteoclasts, reduced bone formation by osteoblasts, or both
- Sepsis syndrome
A life-threatening systemic response to severe infection that is characterized by vascular leakage and oedema, hypo- or hyperthermia, low blood pressure and reduced lung function
- Dendritic cell
A cell type that has a central role in the adaptive immune response by presenting antigens to lymphocytes and causing their activation
- TH17 cell
T helper 17 cell; a subset of T helper cells that secrete pro-inflammatory cytokines.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
ClinicalTrials.gov website: http://www.clinicaltrials.gov
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Zhang H, et al. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. This paper provided the first evidence to demonstrate that S1P is a bioactive lipid produced from sphingosine that regulates cellular proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nature Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyne S, Pyne NJ. Translational aspects of sphingosine 1-phosphate biology. Trends Mol Med. 2011;17:463–472. doi: 10.1016/j.molmed.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Orr Gandy KA, Obeid LM. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim Biophys Acta. 2013;1831:157–166. doi: 10.1016/j.bbalip.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem. 2013;82:637–662. doi: 10.1146/annurev-biochem-062411-130916. [DOI] [PubMed] [Google Scholar]
- 7.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nature Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 10.Cuvillier O, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. This paper established the concept of the sphingolipid rheostat complex, in which the balance between ceramide (the pro-apoptotic precursor of S1P) and the anti-apoptotic S1P determines cell fate. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann V, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. This was the first demonstration that HDACs are direct intracellular targets of S1P and are formed in the nucleus by SPHK2, thus linking nuclear sphingolipid metabolism to gene regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takasugi N, et al. BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. J Neurosci. 2011;31:6850–6857. doi: 10.1523/JNEUROSCI.6467-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahara A, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. This study showed that SPNS2 is an S1P transporter that has an important role in cardiac development in zebrafish. This study led to the later demonstration that mammalian SPNS2 analogues are also S1P transporters. [DOI] [PubMed] [Google Scholar]
- 17.Nagahashi M, et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27:1001–1011. doi: 10.1096/fj.12-219618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuhara S, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza A, et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2:1104–1110. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breart B, et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med. 2011;208:1267–1278. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizugishi K, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nature Med. 2010;16:1421–1428. doi: 10.1038/nm.2250. This study showed that persistent activation of STAT3 in tumours involves S1P and S1PR1 in a malicious feedforward cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, et al. S1PR1 is an effective target to block STAT3 signaling in activated B cell-like diffuse large B-cell lymphoma. Blood. 2012;120:1458–1465. doi: 10.1182/blood-2011-12-399030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng J, et al. S1PR1–STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642–654. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagahashi M, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by tumor-induced angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponnusamy S, et al. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med. 2012;4:761–775. doi: 10.1002/emmm.201200244. This paper highlighted that S1P is involved in the communication between tumours and hosts to regulate metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruckhaberle E, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 31.Kawamori T, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2008;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyne S, Edwards J, Ohotski J, Pyne NJ. Sphingosine 1-phosphate receptors and sphingosine kinase 1: novel biomarkers for clinical prognosis in breast, prostate, and hematological cancers. Front Oncol. 2012;2:168. doi: 10.3389/fonc.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paugh SW, et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharel Y, et al. Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Biochem J. 2011;440:345–353. doi: 10.1042/BJ20110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnute ME, et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem J. 2012;444:79–88. doi: 10.1042/BJ20111929. [DOI] [PubMed] [Google Scholar]
- 36.Pyne S, Bittman R, Pyne NJ. Sphingosine kinase inhibitors and cancer: seeking the golden sword of Hercules. Cancer Res. 2011;71:6576–6582. doi: 10.1158/0008-5472.CAN-11-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure. 2013;21:798–809. doi: 10.1016/j.str.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Chipuk JE, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez L, et al. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Bas Res Cardiol. 2011;106:1341–1353. doi: 10.1007/s00395-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vessey DA, et al. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:961059. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strub GM, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.French KJ, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antoon JW, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151:5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharel Y, et al. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem J. 2012;447:149–157. doi: 10.1042/BJ20120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samy ET, et al. Cutting edge: modulation of intestinal autoimmunity and IL-2 signaling by sphingosine kinase 2 independent of sphingosine 1-phosphate. J Immunol. 2007;179:5644–5648. doi: 10.4049/jimmunol.179.9.5644. [DOI] [PubMed] [Google Scholar]
- 46.Maines LW, Fitzpatrick LR, Green CL, Zhuang Y, Smith CD. Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn’s disease. Inflammopharmacology. 2010;18:73–85. doi: 10.1007/s10787-010-0032-x. [DOI] [PubMed] [Google Scholar]
- 47.Chumanevich AA, et al. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J Immunol. 2010;185:2570–2579. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzpatrick LR, et al. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology. 2011;19:75–87. doi: 10.1007/s10787-010-0060-6. [DOI] [PubMed] [Google Scholar]
- 50.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nature Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 51.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Nakahara K, et al. The Sjogren–Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol Cell. 2012;46:461–471. doi: 10.1016/j.molcel.2012.04.033. This study showed that the S1P metabolite hexadecenal is implicated in the pathogenesis of Sjögren–Larsson syndrome as a result of mutations in ALDH3A2, which is needed to metabolize the hexedecanal produced from S1P by S1P lyase. [DOI] [PubMed] [Google Scholar]
- 54.Aguilar A, Saba JD. Truth and consequences of sphingosine-1-phosphate lyase. Adv Biol Regul. 2012;52:17–30. doi: 10.1016/j.advenzreg.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ihlefeld K, Claas RF, Koch A, Pfeilschifter JM, Meyer Zu Heringdorf D. Evidence for a link between histone deacetylation and Ca2+ homoeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts. Biochem J. 2012;447:457–464. doi: 10.1042/BJ20120811. [DOI] [PubMed] [Google Scholar]
- 56.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. This important study established that lymphocyte egress is mediated by S1P gradients that are established by S1P lyase activity. [DOI] [PubMed] [Google Scholar]
- 57.Bagdanoff JT, et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53:8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 58.Bourquin F, Riezman H, Capitani G, Grutter MG. Structure and function of sphingosine-1-phosphate lyase, a key enzyme of sphingolipid metabolism. Structure. 2010;18:1054–1065. doi: 10.1016/j.str.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Nagata Y, Partridge TA, Matsuda R, Zammit PS. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol. 2006;174:245–253. doi: 10.1083/jcb.200605028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loh KC, et al. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE. 2012;7:e37218. doi: 10.1371/journal.pone.0037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pantoja M, Fischer KA, Ieronimakis N, Reyes M, Ruohola-Baker H. Genetic elevation of sphingosine 1-phosphate suppresses dystrophic muscle phenotypes in Drosophila. Development. 2013;140:136–146. doi: 10.1242/dev.087791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanson MA, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. The first X-ray crystal structure of an S1PR, namely S1PR1, was reported in this paper. This structure provided a detailed view of the binding site of S1P and paves the way for the development of more specific agonists and antagonists that could be clinically useful. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. This study elucidated the mechanism of immunosuppression induced by fingolimod and showed that its phosphorylated form signals through S1PR1 to interfere with lymphocyte trafficking. [DOI] [PubMed] [Google Scholar]
- 64.Brinkmann V, et al. The immune modulator, FTY720, targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 65.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Cabrera PJ, et al. S1P1 receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol Pharmacol. 2012;81:166–174. doi: 10.1124/mol.111.076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neviani P, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gergely P, et al. The selective S1P receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate: translation from preclinical to clinical studies. Br J Pharmacol. 2012;167:1035–1047. doi: 10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Komiya T, et al. Efficacy and immunomodulatory actions of ONO-4641, a novel selective agonist for sphingosine 1-phosphate receptors 1 and 5, in preclinical models of multiple sclerosis. Clin Exp Immunol. 2013;171:54–62. doi: 10.1111/j.1365-2249.2012.04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piali L, et al. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther. 2011;337:547–556. doi: 10.1124/jpet.110.176487. [DOI] [PubMed] [Google Scholar]
- 71.Willis MA, Cohen JA. Fingolimod therapy for multiple sclerosis. Semin Neurol. 2013;33:37–44. doi: 10.1055/s-0033-1343794. [DOI] [PubMed] [Google Scholar]
- 72.Nolan R, Gelfand JM, Green AJ. Fingolimod treatment in multiple sclerosis leads to increased macular volume. Neurology. 2013;80:139–144. doi: 10.1212/WNL.0b013e31827b9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quancard J, et al. A potent and selective S1P1 antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem Biol. 2012;19:1142–1151. doi: 10.1016/j.chembiol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 74.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanna MG, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 76.Teijaro JR, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. This study showed that S1PR1 agonists can suppress cytokines and innate immune cell recruitment, and it identified endothelial cells as central regulators of the cytokine storm induced by viral infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol. 2003;138:1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Q, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts KG, et al. Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res. 2010;70:5438–5447. doi: 10.1158/0008-5472.CAN-09-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 81.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 82.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 83.Ding BB, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. This is the first demonstration of the essential role of S1PR1 in vascular integrity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shoham AB, et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development. 2012;139:3859–3869. doi: 10.1242/dev.078550. [DOI] [PubMed] [Google Scholar]
- 87.Gaengel K, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–599. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Jung B, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell. 2012;23:600–610. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kikuta J, et al. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc Natl Acad Sci USA. 2013;110:7009–7013. doi: 10.1073/pnas.1218799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Long JS, et al. Sphingosine 1-phosphate receptor 4 uses HER2 (ERBB2) to regulate extracellular signal regulated kinase-1/2 in MDA-MB-453 breast cancer cells. J Biol Chem. 2010;285:35957–35966. doi: 10.1074/jbc.M110.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salomone S, Waeber C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptormediated effects. Front Pharmacol. 2011;2:9. doi: 10.3389/fphar.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sukocheva O, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watson C, et al. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol. 2010;177:2205–2215. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harris GL, Creason MB, Brulte GB, Herr DR. In vitro and in vivo antagonism of a G protein-coupled receptor (S1P3) with a novel blocking monoclonal antibody. PLoS ONE. 2012;7:e35129. doi: 10.1371/journal.pone.0035129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sammani S, et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43:394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niessen F, et al. Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 99.Sun X, et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol. 2012;47:628–636. doi: 10.1165/rcmb.2012-0048OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulze T, et al. Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25:4024–4036. doi: 10.1096/fj.10-179028. [DOI] [PubMed] [Google Scholar]
- 101.Allende ML, et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2011;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urbano M, et al. SAR analysis of innovative selective small molecule antagonists of sphingosine-1-phosphate 4 (S1P4) receptor. Bioorg Med Chem Lett. 2011;21:5470–5474. doi: 10.1016/j.bmcl.2011.06.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jenne CN, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mattes H, et al. Design and synthesis of selective and potent orally active S1P5 agonists. ChemMedChem. 2010;5:1693–1696. doi: 10.1002/cmdc.201000253. [DOI] [PubMed] [Google Scholar]
- 105.Yang L, et al. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol. 2013;59:114–123. doi: 10.1016/j.jhep.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 106.Kapitonov D, et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Price MM, et al. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent mouse model of allergic asthma. J Allergy Clin Immunol. 2012;131:501–511. doi: 10.1016/j.jaci.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dickson MA, et al. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin Cancer Res. 2011;17:2484–2492. doi: 10.1158/1078-0432.CCR-10-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao P, Peterson YK, Smith RA, Smith CD. Characterization of isoenzyme-selective inhibitors of human sphingosine kinases. PLoS ONE. 2012;7:e44543. doi: 10.1371/journal.pone.0044543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Antoon JW, et al. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. J Mol Endocrinol. 2011;46:205–216. doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Q, et al. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS ONE. 2012;7:e41834. doi: 10.1371/journal.pone.0041834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Finney CA, et al. S1P is associated with protection in human and experimental cerebral malaria. Mol Med. 2011;17:717–725. doi: 10.2119/molmed.2010.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scuto A, et al. STAT3 inhibition is a therapeutic strategy for ABC-like diffuse large B-cell lymphoma. Cancer Res. 2011;71:3182–3188. doi: 10.1158/0008-5472.CAN-10-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saddoughi SA, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimizu H, et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation. 2005;111:222–229. doi: 10.1161/01.CIR.0000152101.41037.AB. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki C, et al. Efficacy of mycophenolic acid combined with KRP-203, a novel immunomodulator, in a rat heart transplantation model. J Heart Lung Transplant. 2006;25:302–309. doi: 10.1016/j.healun.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 117.Song J, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 118.Wenderfer SE, Stepkowski SM, Braun MC. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 2008;74:1319–1326. doi: 10.1038/ki.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poti F, et al. Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R−/−) mice. Vascul Pharmacol. 2012;57:56–64. doi: 10.1016/j.vph.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 120.Zhang ZY, et al. AUY954, a selective S1P1 modulator, prevents experimental autoimmune neuritis. J Neuroimmunol. 2009;216:59–65. doi: 10.1016/j.jneuroim.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 121.Galicia-Rosas G, et al. A sphingosine-1-phosphate receptor 1-directed agonist reduces central nervous system inflammation in a plasmacytoid dendritic cell-dependent manner. J Immunol. 2012;189:3700–3706. doi: 10.4049/jimmunol.1102261. [DOI] [PubMed] [Google Scholar]
- 122.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 123.Awad AS, et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int. 2011;79:1090–1098. doi: 10.1038/ki.2010.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jo E, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 125.Nishi T, et al. Discovery of CS-0777: a potent, selective, and orally active S1P1 agonist. ACS Med Chem Lett. 2011;2:368–372. doi: 10.1021/ml100301k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marsolais D, et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci USA. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fujii Y, et al. Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J Immunol. 2012;188:206–215. doi: 10.4049/jimmunol.1101537. [DOI] [PubMed] [Google Scholar]
- 128.Gonzalez-Cabrera PJ, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-Like headgroup interactions. Mol Pharmacol. 2008;74:1308–1318. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9633–9641. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 130.Tarrason G, et al. The sphingosine-1-phosphate receptor-1 antagonist, W146, causes early and short-lasting peripheral blood lymphopenia in mice. Int Immunopharmacol. 2011;11:1773–1779. doi: 10.1016/j.intimp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 131.Finley A, et al. Sphingosine 1-phosphate mediates hyperalgesia via a neutrophil-dependent mechanism. PLoS ONE. 2013;8:e55255. doi: 10.1371/journal.pone.0055255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kluk MJ, et al. Sphingosine-1-phosphate receptor 1 in classical Hodgkin lymphoma: assessment of expression and role in cell migration. Lab Invest. 2013;93:462–471. doi: 10.1038/labinvest.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Foss FW, Jr, et al. Synthesis and biological evaluation of γ-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Skoura A, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ikeda H, et al. Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology. 2003;124:459–469. doi: 10.1053/gast.2003.50049. [DOI] [PubMed] [Google Scholar]
- 136.Bolli MH, et al. 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem. 2010;53:4198–4211. doi: 10.1021/jm100181s. [DOI] [PubMed] [Google Scholar]
- 137.Visentin B, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 138.Caballero S, et al. Anti-sphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sanada Y, et al. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS ONE. 2011;6:e23933. doi: 10.1371/journal.pone.0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clemens JJ, Davis MD, Lynch KR, Macdonald TL. Synthesis of benzimidazole based analogues of sphingosine-1-phosphate: discovery of potent, subtype-selective S1P4 receptor agonists. Bioorg Med Chem Lett. 2004;14:4903–4906. doi: 10.1016/j.bmcl.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 141.Ota H, et al. S1P4 receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm Circ. 2011;1:399–404. doi: 10.4103/2045-8932.87309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Song J, Hagiya H, Kurata H, Mizuno H, Ito T. Prevention of GVHD and graft rejection by a new S1P receptor agonist, W-061, in rat small bowel transplantation. Transpl Immunol. 2012;26:163–170. doi: 10.1016/j.trim.2011.12.005. [DOI] [PubMed] [Google Scholar]