1.0 INTRODUCTION
Working memory refers to a collection of sub-processes that enables the short-term maintenance and manipulation of information required for complex cognitive tasks such as reasoning, comprehension, and decision-making (Baddeley, 1992). These sub-processes encompass the maintenance, overwriting, and selective updating of information stored in working memory. While the neural mechanisms underlying the pure maintenance and manipulation of information have been extensively studied, the neural mechanisms underlying the updating of information, specifically selective updating, have not been studied as extensively.
Updating in general refers to the process of rapidly encoding and maintaining new information as an individual’s environment changes, whereas selective updating refers to the rapid encoding of information into working memory content while selectively maintaining goal-relevant information (Hazy et al., 2006). Previous neuroimaging studies have demonstrated that updating processes engage the prefrontal cortices (PFC), dorsal striatum, posterior parietal cortices, and inferior parietal cortices (Bledowski et al., 2009; Lenartowicz et al., 2010; Leung et al., 2007; Montojo and Courtney, 2008; Roth and Courtney, 2007; Roth et al., 2006; Sorqvist and Saetrevik, 2010; Takahama et al., 2010). Within the PFC, the dorsolateral portions are reliably recruited during working memory updating (Bledowski et al., 2010) and specifically track updating demands (Leung et al., 2007; Takahama et al., 2010). However, the above studies predominantly studied general updating mechanisms as opposed to more selective updating processes.
Computational models of working-memory have proposed that the selective updating of information into working memory is accomplished via the gating of information into the PFC. Mechanistically, these gating properties are thought to be executed via neuromodulatory projections from the dopaminergic system. This mechanism of gating has been proposed in a variety of domains including the execution of cognitive control over active representations in the prefrontal cortex (Braver et al., 1999; Braver and Cohen, 1999), dopaminergic midbrain neuromodulation of PFC neuronal firing properties during working memory maintenance (Durstewitz et al., 2000), and the most relevant to our work during the selective updating of information into working memory content (Hazy et al., 2006). In this latter model, the authors propose that dopaminergic signals arising from midbrain nuclei, specifically the substantia nigra and ventral tegmental area (SN/VTA), tune gating mechanisms in the dorsal striatum to mediate prefrontal cortical activity. This selective gating allows the prefrontal cortex to rapidly switch between encoding new information from the environment while maintaining internal representations of information (Frank et al., 2001; Hazy et al., 2006; O'Reilly and Frank, 2006). This mechanism of gating accounts for the specific overwriting of targeted information while continuing to maintain task-relevant information, the cognitive process under study. All of the above models propose that engagement of the SN/VTA midbrain nuclei increases as the demands for prefrontal gating increase. However, to date this feature of working memory updating has not been explicitly studied. Anatomical and computational models have demonstrated dopamine-mediated connectivity between the striatum and DLPFC (Alexander et al., 1986; Goldman-Rakic, 1990, 1995; Gruber et al., 2006). Functional imaging studies have shown engagement of both the dorsal striatum and DLPFC during tasks engaging selective working memory updating (Dahlin et al., 2008; Lewis et al., 2004; McNab and Klingberg, 2008; Tan et al., 2007; Tanaka et al., 2004), however, these studies did not isolate this process. Furthermore, converging evidence from studies using dopaminergic genes (de Frias et al., 2009; Tan et al., 2007; Zhang et al., 2007), dopaminergic drug challenges (Clatworthy et al., 2009; Landau et al., 2009), and patients with disorders of the dopaminergic system (Lewis et al., 2003; Marklund et al., 2009; Wolf et al., 2008), provide supporting evidence for the modulatory role of the midbrain dopaminergic system on the cortico-striatal circuitry. Together these findings suggest that the selective updating of working memory content should engage a cortico-striatal network that includes activation and functional coupling of the caudate, DLPFC, and the dopaminergic midbrain nuclei.
Utilizing a novel behavioral paradigm and functional magnetic resonance imaging (fMRI), the study reported herein isolated the neural circuitry underlying the selective updating of working memory content while controlling for other working memory sub-processes including maintenance and nonspecific overwriting of information into working memory stores. We found that the selective updating of working memory content elicits activity in the meso-cortico-striatal network including a midbrain region encompassing the SN/VTA, as well as parietal, occipito-temporal, cerebellar, and cingulate regions. Furthermore, we provide initial evidence that this midbrain SN/VTA region acts in concert with the caudate and DLPFC, consistent with computational models of working memory. Finally, we illustrate that differences in network connectivity during the updating of working memory content separate high and low performing individuals.
2.0 Methods
2.1 Subjects
49 healthy participants recruited through the National Institutes of Health normal volunteer office completed this study. Recruitment evaluation included a complete medical history and physical examination, a detailed neurological exam, the Structured Clinical Interview for DSM-IV, and the Wechsler Adult Intelligence Scale (WAIS-R). Inclusion criteria were: absence of any neurological and psychiatric disorders or any serious medical conditions, absence of any pharmacological treatments potentially having influence on cerebral metabolism or blood flow, age < 45 years, and right-handedness [Edinburgh Handedness Inventory scores > 0.5 (Oldfield, 1971)]. All participants gave written informed consent to the study after the procedure was fully explained to them. The National Institute of Mental Health Institutional Review Board approved the protocol (National Institutes of Health, protocol 95-M-0150). 5 participants were excluded because of excessive head-motion and 2 other participants were removed for poor behavioral performance (i.e. at chance during any of the task conditions), resulting in 42 participants (25 males, age ± SEM 27.2 ± 1.0, IQ ± SEM 109 ± 1.7) for the final analysis.
2.2 Behavioral Paradigm
While undergoing BOLD fMRI, participants performed a novel, working memory task designed to isolate the selective updating component of working memory processes (Fig.1). Accuracy and reaction time measures were collected for each of the task components. During each trial, subjects initially encoded four digits each presented in a blue box (encoding phase - 3.8 seconds). Following this, participants were shown three to five sequential presentations (presented on the screen for 2.0 seconds each) of four black boxes that either contained digits and/or asterisks (experimental phase - 6.6–11.2 seconds). Participants were instructed that if the box contained an asterisk they should continue to store the number previously presented in the box, however, if the box contained a new digit they should disregard the previous number and store the new digit. After these serial presentations of four black boxes, participants were presented with four red boxes containing four digits (response phase - 3.0 seconds). During the response phase, participants were instructed to respond if the presented digit-set matched the digit-set they were currently maintaining. For fifty percent of trials, the digits presented during the response phase matched what the participants should have been maintaining in working memory, warranting a correct response, while in the other half of the trials a different set of digits were presented, warranting an incorrect response. Importantly, on non-matching trials only one digit was changed, to ensure that participants were attending to all four digits during task performance. Participants had 3 seconds to respond with left and right button presses for incorrect and correct matches, respectively.
Figure 1.
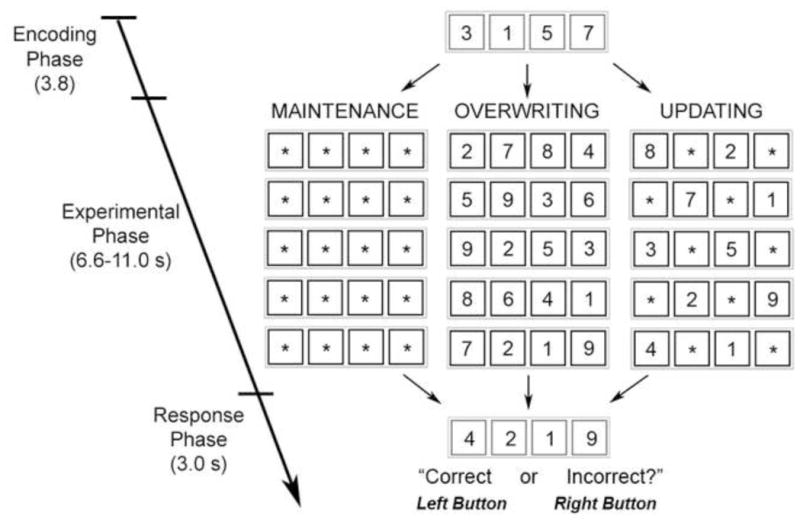
Schematic diagram of the working memory task. Participants began each trial with an encoding phase in which they had to encode four digits. Then during the experimental phase, participants were instructed to maintain the previous number they saw if they were presented with an asterisk, and to encode the new digit if presented with one. In maintenance conditions, participants only received asterisks, in overwriting conditions participants only received digits, and during updating conditions participants saw 1–3 asterisks. Then during a response phase participants had to decide whether the four digits presented matched participant’s internal representation stored in working memory.
Experimental conditions were manipulated by changing the contents of the boxes, i.e. changing the number of asterisks relative to digits. In the maintenance condition (MAI), participants were shown 5 serial presentations of black boxes containing only asterisks (11.2 seconds). Hence during these trial types, participants maintained the 4 digits they originally encoded until the response phase. During the overwriting condition (OVR), participants were shown 3–5 serial presentations of black boxes containing only digits (6.6–11.2 seconds). Hence, during these trial types, participants continually encoded four new digits, without maintaining any of the previous digits, until they decided if the most current set of encoded digits matched those presented in the red boxes. During this condition, the number of presentations of black boxes varied to assure that participants were attending to all sequential presentations. During the updating condition (UPD), participants were shown 5 serial presentations of black boxes containing 1–3 digits with the rest of the boxes containing asterisks (11.2 seconds). Hence, during these trials, participants selectively maintained some of the digits, while clearing the others to encode the new digits, i.e. selectively updating information in the working memory store. Critically, the updating condition differed from the overwriting condition in that the replacement of information into participant’s working memory store in the updating condition was selective and specific, whereas the overwriting condition involved the general re-encoding of a novel digit set without necessitating any selective storage of previous information. Prior to fMRI scanning participants completed a practice session in which they performed 3 trials of each condition.
Participants were presented with 9 maintenance, 9 overwriting, and 27 updating (9 trials each of 1, 2, and 3 digit presentations) trials over 3 experimental runs, with each run lasting 5 minutes, 20 seconds. Trial order was pseudo-randomized across each run. Between trials, a fixation crosshair was presented at interstimulus intervals (ISI) of 3.1 ± 1.2 seconds.
2.3 BOLD fMRI
BOLD fMRI was performed on a GE Signa 3T scanner (gradient echo-planar imaging sequence, TR/TE = 2000/28; 26 interleaved slices, thickness = 4mm, gap = 1mm, voxel size = 3.75 x 3.75 x 5 mm; scan repetitions, 160 scans/run; flip angle = 90°, FOV = 24 cm; matrix = 64x64 mm) while participants performed the task described above. Stimuli were presented via a back-projection system, and the responses (button presses indicating yes or no) were recorded through a fiber optic response box, which allowed measurement of accuracy (% correct responses) and reaction time (msec) for each trial. 2.4 Behavioral Analysis. Behavioral data [accuracy and reaction time (RT)] were compared using repeated-measures ANOVA with task condition (OVR, UPD, MAI) as a within-subjects factor. Tukey Honest Significance Difference (HSD) was used for post-hoc analysis. All statistical analyses were set to a threshold of p< 0.05, and post-hoc analysis were Bonferonni corrected for multiple comparisons.
2.4 Functional Imaging Analysis
Image analysis was completed using SPM5 (www.fil.ion.ucl.ac.uk/spm). The first four scans were discarded to allow for signal saturation. All images underwent slice timing, realignment across all three runs, spatial normalization to the MNI template using a 4th Degree B-spline interpolation, and smoothing using an isotropic 8-mm3 Full-Width-Half maximum kernel. Each individual data set was then carefully screened for data quality via inspection for image artifacts and excessive head motion (> 3 mm head motion or 2 degrees head rotation).
fMRI responses were modeled using the General Linear Model (GLM) across all three runs modeled as separate blocks with a canonical hemodynamic response function (HRF) convolved for the length of each phase (encoding, experimental, and response), normalized to the global signal across the whole brain across the entire session, and high-pass filtered (less than 124 s). Regressors of interest were modeled for the experimental phase during correct OVR, MAI, and UPD trials separately. Data from incorrect trials were modeled separately and hence were not included within these regressors of interest. Other regressors of no interest that were modeled included the encoding phase and retrieval phase of each task component and 6 head motion regressors. Using the GLM, individual maps of parameter estimates were generated for contrasts of interest: the UPD experimental phase > baseline, the MAI experimental phase > baseline, and the OVR experimental phase > baseline. These individual first level maps of parameter estimate contrasts were entered into the second level analyses.
Second level random-effects analyses were performed using one-sample t-tests to explore the main effect of each task condition during the experimental phase. Statistical thresholds for one-sample t-tests were set at p < 0.001, a relatively liberal threshold to explore all regions activated by the task. Whole-brain repeated-measures, random-effects ANCOVAs in SPM5 corrected for non-sphericity were performed to assess relative differences in brain activity between the three conditions while controlling for individual differences in Accuracy and RT. To explore differences in activation in task-relevant regions only, ANCOVAs were inclusively masked by a mask that was created by taking the union of activation maps for the experimental phase of the UPD, MAI, and OVR conditions, each individually thresholded at p< 0.05, uncorrected. Statistical thresholds for ANCOVAs were set at p < 0.05, family-wise errors (FWE)-corrected with small volume correction (SVC) within the caudate and the VTA/SN ROIs (described below). SVC within the caudate was performed within anatomical regions of interest encompassing the left and right caudate (using WFU PICKATLAS), whereas SVC within the VTA/SN midbrain nuclei was performed within a sphere with a radius of 8 mm from coordinates derived from (Adcock et al., 2006). Conjunction analyses were performed with the conjunction option in SPM5.
ROI analyses were performed by extracting eigenvariates of beta-parameters from each individual regressor of interest within anatomical regions of interest (ROI) in the left and right caudate using MARSBAR toolbox (http://marsbar.sourceforge.net), separately (using WFU PICKATLAS), and the VTA/SN midbrain nuclei using a combination of two spheres with a radius of 4 mm derived from coordinates derived from (Adcock et al., 2006). A 4 mm radius was selected given the relatively small size of the VTA/SN. These extracted values were analyzed with repeated-measures ANOVAs with task-conditions as a within-subjects factor using Tukey’s HSD test using SPSS 16 for Macintosh (www.spss.com). ROI statistical analyses were set to a threshold of p< 0.05.
To assess SN/VTA-caudate-DLPFC interactions, network regressions across participants were performed (as both computational models and previous neuroimaging studies implicate engagement of this network during working memory updating). First eigenvariates of beta-parameters from the experimental phase of UPD condition within ROIs of the caudate, midbrain, and the DLPFC (defined as lateral portions of BA 9,46) were entered into simple regressions across participants. Construction of midbrain and caudate ROIs are described above and the DLPFC ROI was constructed by generating a sphere with an 8 mm radius derived from the peak cluster within the DLPFC (as identified in WFU Pickatlas; peak coordinates: -52,26,26) from a conjunction analysis of UPD > OVR and UPD > MAI contrasts (p< 0.05, FWE-corrected). Statistical thresholds for simple regressions were set at p < 0.05.
To evaluate the relationship of these networks with performance, these same correlations were performed separately in high-performing and low-performing groups of participants. The high performing (HPs) group consisted of 15 participants that performed above median performance determined by accuracy > 85% during the updating condition, while the low performing group consisted of 17 participants that had below median performance with accuracy < 85% during the updating condition. Thus, this analysis excluded 10 participants that performed at 85% accuracy during the updating condition. There were no significant differences in the age, gender, head motion, functional MRI signal-to-noise ratios, performance on any other condition, or reaction time during the updating condition (p>0.10), however, there was a significant difference in IQ with high performers having a greater IQ than low performers (p=0.04).
To assess mesolimbic functional connectivity, within-subjects functional connectivity analysis was performed using single-trial/beta-series analysis, described elsewhere in detail (Rissman et al., 2004). In short, each individual UPD trial was modeled in a GLM independently, and single-trial beta parameter values from a seed-region were correlated across the whole brain during the UPD condition. For this analysis, seeds were placed in anatomical ROIs of the left and right caudate, independently. A second level random-effects analysis as performed using a one-sample t-test to explore striatal connectivity with other brain regions within the inclusive mask of updating-related brain activity (UPD>baseline, p<0.05, uncorrected). Statistical thresholds for this analysis were set a p < 0.05, FWE-corrected, SVC in the VTA/SN (as described above).
3.0 RESULTS
3.1 Behavioral Results
Accuracy and reaction time was analyzed in an ANOVA with condition [Updating (UPD), Maintenance (MAI), or Overwriting (OVR)] as a within-subjects factor. The main effect of accuracy across condition was significant [UPD: Accuracy ± SD = 83.60 ± 1.47; OVR: Accuracy ± SD = 96.34 ± 1.36; MAIN: Accuracy ± SD = 96.83 ± 0.87; F (2,67.5) = 38.18, p<0.01]. Post-hoc analyses revealed significantly decreased accuracy during the UPD condition compared to both the MAI and OVR condition (p<0.01), with no significant differences between MAI and OVR. The main effect of reaction time (RT) across conditions was significant [UPD: RT ± SD = 1.531 s ± 0.049; OVR: RT ± SD = 1.420 s ± 0.046; MAI: RT ± SD = 1.366 ± 0.048; F (2,82.0) = 23.68, p<0.01]. Post-hoc analyses revealed significantly slower reaction times during the UPD condition compared to both the MAI and OVR condition (p<0.01), and a trend towards slower reaction times during the OVR condition compared to the MAI condition (P = 0.09). Analysis of updating load, i.e. whether 1,2, or 3 digits were being updated, did not reveal any parametric effects in terms of RT or Accuracy (p<.25), hence, this factor was not considered in any of the following fMRI analyses.
3.2 fMRI Results
3.2.1 Main Effect of Task Conditions
T-tests for each condition were performed across the whole brain to investigate if distinct networks of activity underlay each experimental condition (Table 1; p<0.001, uncorrected). The maintenance (MAI) condition evoked activation in the occipital cortices as well as the superior, medial, middle, pre-central, para-central, and post-central frontal gyri. The overwriting (OVR) condition, in which participants had to non-selectively remove old information and encode new information into working memory content, evoked activation in the occipital gyri, the precuneus, the superior and inferior parietal cortices, as well as the inferior, medial, middle, and superior frontal gyri. The updating (UPD) condition, in which participants had to selectively remove and update information into working memory content, evoked activation in the dorsal striatum, the midbrain region encompassing the SN/VTA, the occipital gyri, the precuneus, the superior and inferior parietal cortices, as well as the inferior, medial, middle, and superior frontal gyri. Importantly, activations in the midbrain, and striatum were unique to the UPD condition.
Table 1.
Local maxima of brain regions showing significant activations (p=0.001, uncorrected) during experimental phases compared to baseline, respectively.
| Label | hemi | k | x | y | z | Z |
|---|---|---|---|---|---|---|
| Maintenance | ||||||
| BA 17/18 | L | 10 | −19 | −94 | −15 | 4.78 |
| R | 15 | 22 | −94 | −11 | 4.73 | |
| BA 6 | L/R | 35 | 8 | −4 | 68 | 4.30 |
| BA4/6 | R | 26 | 52 | −4 | 49 | 3.94 |
| BA 5/6 | R | 18 | 8 | −30 | 64 | 3.67 |
| Overwriting | ||||||
| BA 17/18 | L | 155 | −18 | −94 | −15 | 7.27 |
| R | 124 | 22 | −98 | −11 | 7.59 | |
| BA 6 | L | 276 | −4 | 0 | 64 | 7.09 |
| L | 208 | −52 | 0 | 52 | 6.49 | |
| BA 6/9 | R | 135 | 49 | 4 | 30 | 4.93 |
| BA 9/10 | R | 54 | 38 | 41 | 30 | 4.29 |
| BA 7/31 | L | 20 | −26 | −68 | 26 | 4.26 |
| BA 7/39/40 | R | 25 | 30 | −60 | 49 | 3.95 |
| Updating | ||||||
| BA 7/39/40 | L | 505 | −19 | −71 | 49 | 18.11 |
| R | 419 | 49 | −41 | 45 | 13.62 | |
| BA 7/28/29 | L | 371 | −16 | −71 | 30 | 15.2 |
| R | 353 | 26 | −71 | 41 | 17.71 | |
| BA 6/9 | L | 763 | −49 | 8 | 26 | 14.08 |
| R | 497 | 52 | 8 | 26 | 7.75 | |
| BA 6 | L/R | 448 | −4 | 4 | 60 | 17.21 |
| L | 294 | −26 | 0 | 60 | 13.28 | |
| R | 321 | 26 | 4 | 60 | 12.49 | |
| Midbrain | L/R | 203 | −4 | −26 | −11 | 8.75 |
| Striatum | L | 190 | −15 | 11 | −4 | 7.58 |
| R | 142 | 15 | 11 | 8 | 6.29 | |
3.2.2 Whole Brain Conjunction Analysis of UPD > OVR ∪ UPD > MAI
To investigate brain regions selectively recruited during UPD compared to OVR and MAI, a whole-brain conjunction analysis of the contrasts UPD > OVR and UPD > MAI was performed (p<0.05, FWE-corrected) (Table 2) using an ANCOVA. Significant activations were seen bilaterally in the caudate, inferior parietal cortex, superior parietal cortex, occipital cortex, cerebellum, and the left DLPFC, pre-motor cortex, and midbrain region encompassing the SN/VTA (small-volume corrected; Fig. 2). Conjunction analyses of other conditions (i.e. MAI>UPD ∪ MAI > OVR, OVR>UPD ∪ OVR>MAI) did not show any significant effects.
Table 2.
Local maxima of brain regions showing significant activations (p=0.05, corrected) in a conjunction analysis of updating > maintenance and updating>overwriting.
| Label | hemi | k | x | y | z | Z |
|---|---|---|---|---|---|---|
| BA 7/40 | L | 353 | −11 | −71 | 56 | 7.40 |
| R | 352 | 11 | −68 | 56 | 6.34 | |
| Cerebellum | L | 67 | −30 | −52 | −38 | 6.23 |
| R | 151 | 34 | −52 | −45 | 6.85 | |
| Caudate* | L | 111 | −15 | 19 | −4 | 6.25 |
| R | 113 | 19 | 19 | 0 | 5.67 | |
| BA 19 | L | 17 | −34 | −86 | 22 | 5.90 |
| R | 32 | 38 | −82 | 19 | 5.69 | |
| BA 6 | L | 28 | −30 | 0 | 68 | 5.10 |
| BA 46/45/9 | L | 16 | −52 | 26 | 26 | 4.71 |
| BA 37/19/20 | L | 9 | −52 | −60 | −15 | 4.68 |
| BA 32/6/8/9 | R | 9 | 4 | 22 | 38 | 4.62 |
| SN/VTA* | L | 4 | −11 | −19 | −15 | 2.99 |
| R | 3 | 11 | −8 | −15 | 3.25 |
indicate small volume correction.
Figure 2.
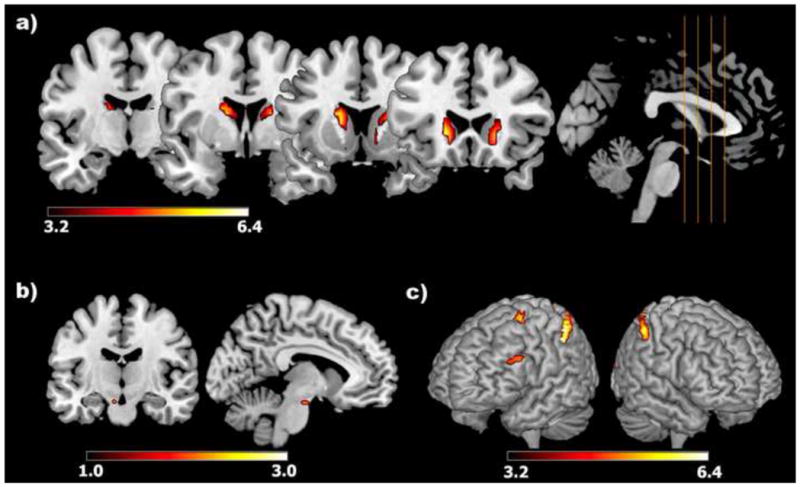
Significant activations within an anatomical mask of (A) the caudate and the (B) SN/VTA as well as (C) a rendered view of cortical activations, including the dorsolateral, pre-motor, and parietal cortices, during updating relative to maintenance and overwriting conditions (results from conjunction analysis of updating > maintenance and updating>overwriting contrasts). Color bars reflect t-scores.
3.2.3 ROI Analysis of the Caudate and VTA/SN Across Conditions
To look at differences across conditions within anatomical ROIs, ANOVAs were performed across conditions on beta-parameters derived from the caudate and the VTA/SN. The main effect of condition was significant bilaterally in the caudate [Left: F(2) = 22.997, p < 0.001; Right: F(2) = 17.607, p < 0.001] with significantly greater activation during UPD compared to OVR and MAI (p < 0.001), as well as during MAI compared to OVR (p < 0.001) (Fig. 3). The main effect of condition was significant in the midbrain [F (2) = 13.99, p < 0.001] with significantly greater activation during UPD compared to MAI condition (p<0.001) and qualitatively greater activation during UPD > OVR (p=0.15), with no significant differences between the MAI and OVR conditions (p>0.40,Fig. 3).
Figure 3.
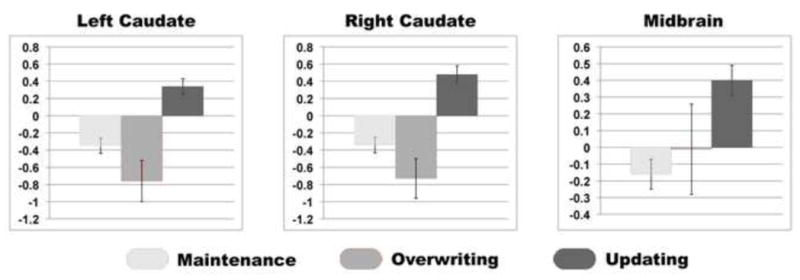
Extracted beta-parameters from the caudate and midbrain across all conditions during the experimental phase.
3.2.4 Mesocorticostriatal Network Activity during Updating
Midbrain, caudate, and DLPFC during selective updating operated as a concerted, functional network. Individual subjects’ beta-parameters during the UPD condition were extracted from the midbrain, caudate, and dlPFC, and correlated with each other across participants. Significant correlations existed between the midbrain and the left and right caudate, respectively (r = 0.57, r = 0.52, p < 0.001; Fig. 4.a). Furthermore, there was a trend towards significant correlation between the midbrain and left middle frontal gyrus/DLPFC (r = 0.27, p =0.08), however, across all subjects there was no correlation between either the left or right caudate and the left DLPFC (p>0.25).
Figure 4.
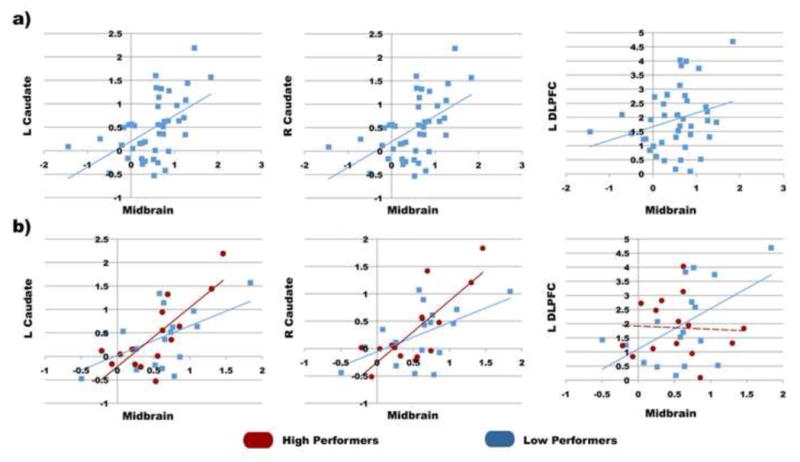
(A) Significant covaration of updating beta-parameters between the midbrain and the caudate and DLPFC across all individual participants. (B) Significant covaration of updating beta-parameters between the midbrain and the caudate and DLPFC for high performers (red circles) and low performers (blue squares). Solid lines signify significant and trending correlations (p<0.05) and dashed lines signify non-significant correlations.
3.2.5 Performance Based Differences in Correlated Networks during Updating
To look at the relationship between performance on updating and correlated network activations, high and low performers on the updating task were compared. Participants were divided in to two groups based on whether their updating performance accuracy was either above (high performers, UPD: Accuracy ± SD = 92.80 ± 3.0) or below (UPD: Accuracy ± SD = 74.50 ± 7.51) median accuracy. This analysis revealed significant correlations between the midbrain and the left and right caudate across both high performer (HPs) and low performer (LPs) groups, with no difference in correlations across the groups (table 3, Fig. 4.b,c). However, there was also a significant correlation between the midbrain and the left DLPFC in the LPs (r = 0.532, p=0.028), but not in high performers (p>.86, table 3, Fig. 4.c,d). Interestingly, no differences were seen between high and low performing groups in isolated activations (BOLD signal change) in either the midbrain, caudate, or left DLPFC (p>0.50).
Table 3.
Correlations of beta-parameters across participants/network analysis in the SN/VTA, caudate, and DLPFC during updating.
| Low Performers | High Performers | |||
|---|---|---|---|---|
|
| ||||
| Regions | R | p-value* | R | p-value* |
| Midbrain – L Caudate | 0.532 | 0.03 | 0.792 | 0.001 |
| Midbrain – R Caudate0 | 0.542 | 0.03 | 0.782 | 0.001 |
| Midbrain – L DLPFC | 0.532 | 0.03 | 0.010 | 0.71 |
Bold font indicates significant correlations
3.2.6 Mesocorticostriatal Functional Connectivity
To investigate which regions are functionally connected with the dorsal stratium, specifically the caudate, during UPD, single-trial analysis was performed during the UPD condition with seeds independently placed in the left and right caudate. More specifically, single-trial beta parameter values from the left and right caudate were correlated across the whole brain during the UPD condition. This analysis showed significant functional connectivity between the left caudate and bilateral SN/VTA (x=−9,y=−12,z=−15, k=64, p<0.05, FWE-SVC corrected).
4.0 DISCUSSION
We report significant engagement of a meso-cortico-striatal network during the updating of working memory content. In the context of this study, updating refers to the selective overwriting of some elements of working memory content while maintaining the rest, a process distinct from the pure maintenance of information as well as overwriting of all information in working memory to encode new information. First, a whole brain analysis of functional imaging data revealed that the selective updating of information selectively engages the caudate, DLPFC, and midbrain region encompassing the SN/VTA as well as parietal, occipito-temporal, cerebellar, and cingulate regions when compared to maintaining or overwriting working memory content. These results were reconfirmed using a ROI-based approach focusing on the bilateral caudate and SN/VTA regions, which revealed the predominant engagement of these regions during updating when compared to overwriting and maintenance conditions. Finally a network analysis looking at co-activation patterns between the midbrain, caudate, and DLPFC revealed significant co-variations between the midbrain and caudate, as well as the midbrain and left DLPFC during working memory updating. While similar co-activation was seen between the midbrain and the caudate regardless of performance, correlated engagement of the midbrain and the DLPFC was greater in low performing individuals.
The task that we used has several novel aspects. It demands selective updating of information content that is being maintained in working memory. In both behavioral as well as neurophysiological terms, our analyses revealed that selective updating can be clearly distinguished from the maintenance of information or nonselective overwriting of information. Further, the conjunction analyses, comparing updating with maintenance and overwriting, allowed us to control for differences in other basic working memory elements such as delay and interference (both visual and simple proactive). Although these basic elements were controlled for in our study, other elements such as dual-task performance and task switching were greater during the updating condition. However, we believe that these component processes are necessary for selective updating. Other neuroimaging studies have investigated updating processes, however, these studies have not studied the selective updating of information within working memory content (Lenartowicz et al., 2010; Roth et al., 2006; Sorqvist and Saetrevik, 2010; Takahama et al., 2010) but rather have investigated the updating of task instructions (Montojo and Courtney, 2008; Roth and Courtney, 2007) or attentional focus within working memory content (Bledowski et al., 2010; Bledowski et al., 2009).
Converging evidence from animal and computational studies supports a model that involves selective engagement of the VTA/SN, caudate, and DLPFC during the updating of working memory content (Frank et al., 2001; Gruber et al., 2006; Hazy et al., 2006; O'Reilly and Frank, 2006). Evidence from animal studies indicates that the caudate plays a unique role in gating information processing in the DLPFC, an area that subserves working memory (Goldman-Rakic, 1990, 1995). This gating by the caudate, a region significantly modulated by dopaminergic projections from the SN/VTA, would provide a unique system to mediate the updating of working memory that requires the selective maintenance of some information while replacing other information. In fact, computational models of dopamine and cortico-striatal interactions suggest that neuromodulatory projections from the SN/VTA are responsible for the ability to maintain goal-relevant information within the PFC in the face of constant interference, a situation very similar to the UPD condition in our task (Braver et al., 1999; Braver and Cohen, 1999). Further, the models proposed by O’Reilly and colleagues have been able to successfully model updating behavior (Gruber et al., 2006; Hazy et al., 2006). Our results are consistent with these models which predict greater engagement of the striatal system (including the caudate) during working memory updating compared to pure maintenance. Further, simulations from these models have shown that changing the gating mechanisms over the PFC disrupts working memory performance in a manner that is consistent with disrupting selective updating processes (Braver and Cohen, 1999), and that the mechanisms by which this operates is via transient potentiation of both excitatory afferents and local inhibitory inputs in the PFC (Braver et al., 1999; Braver and Cohen, 1999), both of which would be reflected in greater BOLD signal within the DLPFC. Given these models, it would be predicted that a network consisting of the dopaminergic neurons in the midbrain region as well as the caudate and DLPFC, which receive dopaminergic inputs from the SN/VTA, would be engaged during the selective updating of working memory content, as demonstrated by the above findings.
The results from our study, using a novel behavioral paradigm, further contributes to the literature of the neurophysiology underlying working memory not only by demonstrating fronto-striatal engagement during selective working memory updating, but also by providing evidence for the functional engagement of the midbrain region during this process. Although dopaminergic tone cannot be directly assayed with fMRI, prior fMRI studies have shown reliable engagement of these regions during other tasks known to engage the dopaminergic system (Adcock et al., 2006; D'Ardenne et al., 2008; Duzel et al., 2009; Shohamy and Wagner, 2008). These studies have also demonstrated that activity in this region is modulated by genetic variation in the dopamine transporter gene DAT1 VNTR, a genetic variation thought to affect the clearance of midbrain and striatal dopamine (Schott et al., 2006). Furthermore, functional neuroimaging studies of dopaminergic genes, studies in patients with disorders affecting the dopaminergic system, and dopaminergic drug challenge studies in humans have demonstrated that cortico-striatal activations are sensitive to modulations in dopaminergic tone, with decreased striatal activity in hypodominergic states (Clatworthy et al., 2009; Wolf et al., 2008; Zhang et al., 2007). More specific to the our task, a recent study (Dodds et al., 2009) demonstrated that D2 receptor antagonist sulpiride specifically modulated working memory updating performance without affecting working memory maintenance, and also demonstrated dose-dependent decreases during updating between striatal activity and sulpiride serum levels. Given this, the DLPFC and caudate should be engaged with the VTA/SN during the updating of working memory in a correlated manner, which we demonstrated in our network analysis between the midbrain, DLPFC, and caudate.
Finally, our analysis also revealed that the midbrain-caudate and midbrain-DLPFC network related connectivity was related to individual differences in performance during working memory updating. In an analysis that split high performing and low performing subjects, both groups showed network connectivity between the midbrain and the caudate, but only the low performing group showed network connections between the SN/VTA and the DLPFC. Interestingly, both groups engaged the left DLPFC selectively during the updating condition compared to maintenance and overwriting conditions, but co-activations with the midbrain were only seen in low-performing participants. Since this analysis was based on using correct trials only, it is plausible that individuals having difficulty with selective updating engage the dopaminergic meso-striato-cortical loop to a greater extent as a compensatory mechanism in an attempt to keep up with the updating process. In fact, behavioral modeling studies have shown that individuals with the worst working memory performance show the greatest enhancement in working memory updating performance in response to a dopaminergic agonist (Hazy et al., 2006), a pharmacological challenge that theoretically should enhance subcortical gating of the DLPFC. Similar compensatory mechanisms in the DLPFC have been demonstrated during a variety of memory tasks (Cabeza, 2002; Callicott et al., 2003; Mattay et al., 2006). Using genetic assays, individuals with lower dopaminergic tone showed not only performance deficits during executive tasks (Goldberg and Weinberger, 2004), but have also shown greater DLPFC activation during working memory manipulation (Tan et al., 2007), a process very similar to the updating condition in our task. In fact, a study combining genetics, pharmacological challenges, and functional imaging demonstrated that increased dopaminergic tone by amphetamine administration caused performance deficits that tracked onto a U-shaped curve during an n-back task that involved working memory updating, in that excessive increases in dopaminergic tone reduced working memory performance (Mattay et al., 2003). Alternately, another interpretation could posit that correlated activity between the midbrain and DLPFC is detrimental to performance. The computational models of working memory discussed above have suggested that over-engagement of the midbrain system during these processes is detrimental for the flexible updating of information into working memory (Braver et al., 1999; Braver and Cohen, 1999), as it disrupts the balance of selectively maintaining information while attending to goal-relevant shifts in contextual demands. However, only correct trials were analyzed, so in this case synchronized activity between the midbrain and DLFPC supports better performance. Given that this correlated activity is seen only in low-performing participants when they are successful in their working memory updating, our findings are more supportive of compensatory DLPFC mechanisms. Alternatively, performance differences could be attributed to differences in arousal, attention, or visuo-spatial strategy use. Future studies are warranted to further elucidate the role of these other cognitive mechanisms in modulating selective updating performance.
Beyond the meso-cortico-striatal system, there was significant recruitment of the parietal cortex, premotor cortex, occipital cortex, and cerebellum during updating when compared to overwriting and maintenance trials. Although these regions are not explicitly discussed in striatal-gating models of working memory, many of these regions have been implicated in other cognitive processes that are relevant to selective updating during our task. A rich literature from both humans and non-human primates has implicated the parietal cortices in number processing (Nieder and Dehaene, 2009), suggesting that the recruitment of this region was specific to the stimuli used in our paradigm. However, the parietal regions seen in our task may not be domain specific, as human neuroimaging studies have implicated the parietal cortex in working memory tasks that manipulate spatial attention (Ikkai and Curtis, 2010; Silk et al., 2010), internal attention (Berryhill et al., 2011; Olson and Berryhill, 2009), and task difficulty (Callicott et al., 1999; Leung et al., 2007; Manoach et al., 1997; Nagel et al., 2010), all cognitive processes that could contribute to selective updating. In fact, an fMRI study demonstrated parietal activations during the updating of stimulus content, as opposed to the updating of tasks rules (Montojo and Courtney, 2008). Together, these findings suggest that the parietal activations seen in our task may have contributed to the attention processes necessary to selectively update working memory content. Selective updating also elicited activity in anterior regions of the cingulate cortex, a region reliably activated in paradigms that evoke cognitive conflict and interference (Carter and van Veen, 2007). Hence, cingulate activations during selective updating may be in response to the cognitive conflict that emerges when participants have to selectively maintain some digits in working memory while updating others. Further, although simple forms of interference, such as visual and proactive, were controlled by the overwriting condition, more complex forms of interference may have emerged from the dual task operations necessary to simultaneously maintain some information while encoding other. Finally, selective updating also elicited activations in the cerebellum. Although this region has typically been associated with motor function, recent evidence supports that cerebro-cerebellar connections may be critical in mediating many cognitive processes, including attention and working memory(Strick et al., 2009). Specifically, it has been proposed that the cerebellum is critical in coordinating the timing and execution of complex cognitive processes, which could include selective updating. Future studies, both neuroimaging and computational, will have to address how the above regions interact with the meso-cortico-striatlal system to facilitate selective working memory updating.
Although our study provided novel evidence about the neurophysiology underlying the selective updating of working memory content, some aspects of our current experimental design limit the scope and interpretation of our data. Firstly, the updating condition was contrasted against overwriting and maintenance conditions to isolate selective updating processes. However, some aspects of the design were not explicitly controlled across conditions, specifically, task difficulty and selective attention. Regarding task difficulty, participants had lower accuracy and longer RT in the UPD condition compared to the WM and MAI conditions. Although we limited our imaging analyses to trials in which participants performed accurately and included performance as a covariate, future studies will need to compare these cognitive processes in paradigms in which performance is matched or explicitly manipulated. Secondly, participant’s may have allocated a varying degree of vigor and/or attention during the experimental phase across task conditions. Our current design was not amenable to monitoring behavior during this experimental phase, and only assayed behavior during the response phase. Future studies will need to evaluate attention and effort during the performance of these cognitive processes, either through physiological monitoring (e.g. eye-tracking, skin-conductance response) or experimental design (e.g. probe trials), to fully assure that the reported activation differences remain when these baseline cognitive processes are are adequately controlled across the different conditions.
In summary, our findings suggest that the SN/VTA, caudate, and DLPFC as well as parietal and other pre-frontal regions are selectively engaged during working memory updating compared to the overwriting and maintenance of working memory content. Furthermore, it seems that the midbrain engages the DLPFC during updating to a greater degree in low performers than high performers. These findings of meso-cortico-striatal network engagement during updating support the notion of dopaminergic modulation of striatal gating of DLPFC function during the selective updating of working memory content, as suggested by computational models of working memory function. However, future studies using genetic assays and pharmacological manipulations to investigate this network will be needed to further support these claims.
RESEARCH HIGHLIGHTS.
Working memory consists of many subprocesses, including selective updating.
Selective updating engages the meso-cortical-striatal network.
Differential midbrain-pfc network connectivity separates high and low performers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Chein J, Olson IR. At the intersection of attention and memory: The mechanistic role of the posterior parietal lobe in working memory. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C, Kaiser J, Rahm B. Basic operations in working memory: contributions from functional imaging studies. Behav Brain Res. 2010;214:172–179. doi: 10.1016/j.bbr.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Rahm B, Rowe JB. What “works” in working memory? Separate systems for selection and updating of critical information. J Neurosci. 2009;29:13735–13741. doi: 10.1523/JNEUROSCI.2547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: the gating model. Prog Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, Robbins TW. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Marklund P, Eriksson E, Larsson A, Oman L, Annerbrink K, Backman L, Nilsson LG, Nyberg L. Influence of COMT Gene Polymorphism on fMRI-assessed Sustained and Transient Activity during a Working Memory Task. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21318. [DOI] [PubMed]
- Dodds CM, Clark L, Dove A, Regenthal R, Baumann F, Bullmore E, Robbins TW, Muller U. The dopamine D2 receptor antagonist sulpiride modulates striatal BOLD signal during the manipulation of information in working memory. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3(Suppl):1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Duzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion 335–326. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA. Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci. 2006;20:153–166. doi: 10.1007/s10827-005-5705-x. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O'Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139:105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Escobedo-Quiroz R, Cohen JD. Updating of context in working memory: an event-related potential study. Cogn Affect Behav Neurosci. 2010;10:298–315. doi: 10.3758/CABN.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Oh H, Ferri J, Yi Y. Load response functions in the human spatial working memory circuit during location memory updating. Neuroimage. 2007;35:368–377. doi: 10.1016/j.neuroimage.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Schlaug G, Siewert B, Darby DG, Bly BM, Benfield A, Edelman RR, Warach S. Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport. 1997;8:545–549. doi: 10.1097/00001756-199701200-00033. [DOI] [PubMed] [Google Scholar]
- Marklund P, Larsson A, Elgh E, Linder J, Riklund KA, Forsgren L, Nyberg L. Temporal dynamics of basal ganglia under-recruitment in Parkinson's disease: transient caudate abnormalities during updating of working memory. Brain. 2009;132:336–346. doi: 10.1093/brain/awn309. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Montojo CA, Courtney SM. Differential neural activation for updating rule versus stimulus information in working memory. Neuron. 2008;59:173–182. doi: 10.1016/j.neuron.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Backman L, Lindenberger U, Heekeren HR. Load Modulation of BOLD Response and Connectivity Predicts Working Memory Performance in Younger and Older Adults. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Nieder A, Dehaene S. Representation of number in the brain. Annu Rev Neurosci. 2009;32:185–208. doi: 10.1146/annurev.neuro.051508.135550. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem. 2009;91:155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Roth JK, Courtney SM. Neural system for updating object working memory from different sources: sensory stimuli or long-term memory. Neuroimage. 2007;38:617–630. doi: 10.1016/j.neuroimage.2007.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JK, Serences JT, Courtney SM. Neural system for controlling the contents of object working memory in humans. Cereb Cortex. 2006;16:1595–1603. doi: 10.1093/cercor/bhj096. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, Tischmeyer W, Gundelfinger ED, Heinze HJ, Duzel E. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Bellgrove MA, Wrafter P, Mattingley JB, Cunnington R. Spatial working memory and spatial attention rely on common neural processes in the intraparietal sulcus. Neuroimage. 2010;53:718–724. doi: 10.1016/j.neuroimage.2010.06.068. [DOI] [PubMed] [Google Scholar]
- Sorqvist P, Saetrevik B. The neural basis of updating: Distinguishing substitution processes from other concurrent processes. Scand J Psychol. 2010 doi: 10.1111/j.1467-9450.2010.00817.x. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Takahama S, Miyauchi S, Saiki J. Neural basis for dynamic updating of object representation in visual working memory. Neuroimage. 2010;49:3394–3403. doi: 10.1016/j.neuroimage.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schonfeldt-Lecuona C, Ecker D, Landwehrmeyer B. Altered frontostriatal coupling in pre-manifest Huntington's disease: effects of increasing cognitive load. Eur J Neurol. 2008;15:1180–1190. doi: 10.1111/j.1468-1331.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadee W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]


