Abstract
Background
[Arg8]-vasopressin (AVP) activates 3 G-protein coupled receptors: V1A, V2 and V1B. The AVP-V2 receptor is the primary AVP receptor in the heart; however, its role in cardiac homeostasis is controversial. To better understand AVP-mediated signaling in the heart, we created a transgenic mouse with controlled over-expression of the V1A receptor.
Methods and Results
The V1A receptor transgene was placed under the control of the tetracycline-regulated, cardiac-specific alpha-myosin heavy chain promoter (V1A-TG). V1A-TG mice had a normal cardiac function phenotype at 10 weeks of age. However, by 24 weeks of age, tTA/V1A-TG mouse hearts had reduced cardiac function, cardiac hypertrophy and dilatation of the ventricular cavity. Contractile dysfunction was also observed in isolated adult cardiac myocytes. When V1A receptor transgene was induced to express in adult mice (V1A-TGInd), left ventricular dysfunction and dilation were also seen, albeit at a later time point. Since V1A receptor mediates cell signaling through Gαq protein, we blocked Gαq signaling by crossing tTA/V1A mice with transgenic mice that expressed a small inhibitory peptide against Gαq (Gq-I inhibitor peptide). Gαq blockade abrogated the development of the heart failure phenotype in tTA/V1A TG mice. The heart failure phenotype could be reversed by administration of doxycyline.
Conclusion
Our results demonstrate a role for V1A-mediated signaling in the development of heart failure and support a role for V1A blockade in the treatment of patients with elevated levels of vasopressin.
Keywords: Vasopressin receptor, V1A receptor, signal transduction, heart failure, hypertrophic cardiomyopathy
Introduction
Over 40 years ago it was first reported that levels of the neurohypophyseal hormone arginine vasopressin (AVP) were elevated in patients with congestive heart failure.1 This finding was subsequently confirmed in humans 2, 3 and in animal models of chronic heart failure. 4, 5 AVP elicits a wide range of physiologic effects that are mediated by three known G protein-coupled seven transmembrane spanning vasopressin receptor subtypes: V1A, V1B, and V2. The V1A receptor is expressed in both neuronal and non-neuronal tissues including the heart and elicits a variety of physiological effects including cell contraction and proliferation, stimulation of hepatic glycogenolysis, platelet aggregation and coagulation factor release. 6, 7 The V1B receptor subtype is found predominantly in the pituitary gland where it stimulates adrenocorticotropic hormone release 8, 9. Both the V1A and V1B AVP receptors act through a G protein a-subunit of the Gαq family (αq, q11, q14, α15/16) to activate phospholipase C-β 10, 11,12, 13, and, thus enhance cellular IP3 and calcium levels. 10, 14 By contrast, the V2 receptor subtype is localized predominantly to the kidney where it mediates the anti-diuretic effects of AVP through the heterotrimeric G protein Gs and activation of adenylyl cyclase. 15-17 Activation of adenylyl cyclase results in increased production of cyclic AMP, activation of protein kinase A and subsequent redistribution of specific water channels called aquaporin-2 from intracellular vesicles to the apical plasma membrane of cells of the renal collecting ducts. 18
Although the pathways responsible for AVP signaling have been described, the role of AVP in the heart remains unclear. Physiologically relevant concentrations of AVP depressed cardiac function in conscious dogs, 19 elicited a biphasic hemodynamic response in isolated Langendorf-perfused rat hearts 20 and reduced the weight of the right ventricle in an aortocaval fistula model of heart failure, but V1 antagonism did not have any effects. 21 The administration of a V1A receptor antagonist had no effect on contractility in pigs with pacing induced heart failure, 22 whereas the chronic administration of a V1A antagonist prevented the development of heart failure but not the development of left ventricular hypertrophy in a rodent model of heart failure post-myocardial infarction. 23 Low dose infusion of AVP during ischemia-reperfusion in mice increased mortality and significantly depressed myocardial function. 24 Administration of AVP to neonatal mouse cardiomyocytes elicited an increase in cell hypertrophy but not in mice in which the V1A receptor had been ablated. 25 The V1A knockout mice have a normal cardiac phenotype 26, but develop less hypertrophy after trans-aortic constriction (TAC) then do wild type controls. 25 The disparate affects of exogenously administered AVP on the heart is due in large part to the confounding effects of AVP on the coronary and peripheral vasculature.
It has become increasingly important to understand the effects of AVP on the cardiac myocyte because of the development and the approval of both selective (V2) and non-selective (V1/V2) vasopressin antagonists for the treatment of patients with euvolemic and hypervolemic hyponatremia. We therefore created transgenic mice with controlled over-expression of the human V1A receptor. This allowed us to identify the effects of V1A activation in vivo without the confounding effects on the coronary or peripheral vasculature or on hepatic metabolism. Mice with cardiac-restricted and either constitutive or controlled over-expression of the V1A receptor demonstrated left ventricular hypertrophy, dilatation and diminished contractile performance and “reprogramming” of gene expression. The myocardial effects of V1A over-expression were abrogated by “turning off” the V1A transgene shortly after birth or after the development of the heart failure phenotype and by genetic inhibition of Gαq/11 signaling. Mice over-expressing the V1A receptor provide a novel model in which to study left ventricular dysfunction.
Methods
V1A-R Transgenic Mouse Generation
The human AVP V1A receptor cDNA was cloned into a cardiac-specific and inducible controlled vector (TREMHC) composed of a modified mouse α-myosin heavy chain minimal promoter fused with nucleotide binding sites for tetracycline transactivating factor (tTA)27. To induce transgene expression, V1A receptor transgenic (V1A-TG) mice in FVB background were crossed with mice that expressed tTA in the heart (MHC-tTA) (Fig. 1A). Since crossings of double or triple transgenes were required for gene expression, we used multiple control mice for our experiments. Where possible, these consisted of non-transgenic littermates. In the case of multiple crosses using homozygous parental lines (eg. tTA+/+ crossing V1A+/+), we assessed the phenotype in age matched and co-habitating mice expressing the parental single transgene as seen in Table 1, 2 and 3. Detailed methods are located in the Supplemental Section.
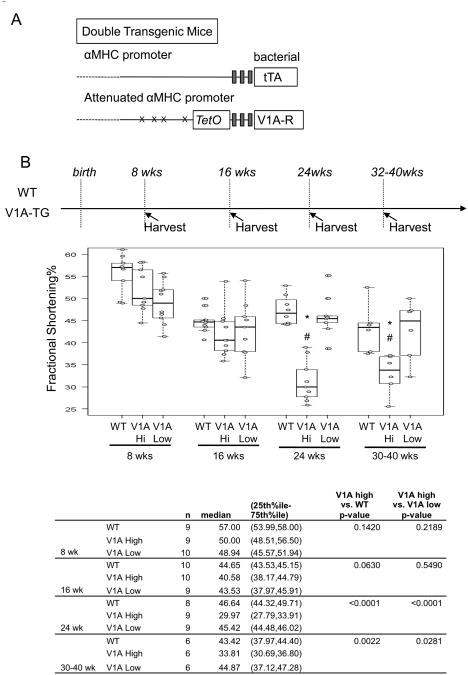
Figure 1.
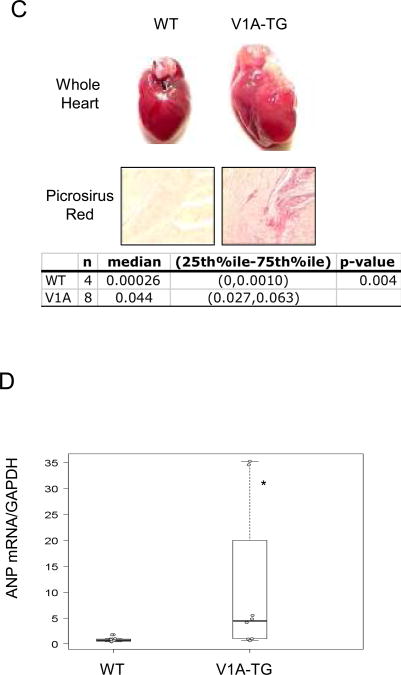
(A) Schematic depiction of the two component transgenic system to induce cardiac V1A receptor expression. (B) Echocardiography was performed on high expression V1A-TG line (S13, V1A-high), low expression line (S10, V1A-low) and FVB-WT mice at indicated ages. Fractional shortening percentage was shown. Samples were analyzed by exact Wilcoxon test with results shown in the table. *adjusted p<0.001 vs WT, #adjusted P<0.05 vs V1A-low. (C) Whole heart images and Picrosirius Red staining of wild-type and V1A-TG mouse hearts at 24-week of age. 5um paraffin-embedded heart cross sections were stained with Picrosirius Red and digitally quantified. Representative of 200× magnified fields were shown. 5 images from two WT mice and 8 images from three V1A-TG mice were quantified. Samples were analyzed by exact Wilcoxon test and shown in the table. (D) ANP gene expression in the hearts of WT and V1A-TG mice. Total ventricular mRNA extracts from 24-week-old male mice were used for real-time PCR and signals were normalized to GAPDH expression in WT. *p<0.001 vs WT. Table of median, IQR and P values are included in the Supplemental Section.
Table 1. A. 24 Week-Old WT and V1A-TG Mouse Echocardiographic Phenotype.
| FVB | V1A | tTA | tTA/V1A | tTA vs FVB p-values | V1A vs FVB p-values | tTA/V1A vs FVB p-values | ||
|---|---|---|---|---|---|---|---|---|
| n | 16 | 6 | 6 | 14 | ||||
| FS% | median | 45.3 | 41.6 | 47.5 | 32.6 | 0.9714 | 0.1664 | <0.0001 |
| (25th%ile, 75th%ile) | (43.0, 54.0) | (37.7, 45.0) | (46.4, 49.3) | (29.8, 34.6) | ||||
| LVEDD (mm) | median | 2.8 | 2.9 | 2.9 | 4.2 | 0.7606 | 0.7606 | <0.0001 |
| (25th%ile, 75th%ile) | (2.6, 3.2) | (2.8, 3.0) | (2.8, 3.1) | (3.9, 4.5) | ||||
| LVESD (mm) | median | 1.4 | 1.7 | 1.5 | 2.8 | 0.9144 | 0.5876 | <0.0001 |
| (25th%ile, 75th%ile) | (1.3, 1.8) | (1.6, 1.9) | (1.5, 1.6) | (2.5, 3.2) | ||||
| Heart Rate | median | 486.5 | 478.5 | 441.0 | 414.0 | 0.5529 | 0.5529 | 0.5529 |
| (25th%ile, 75th%ile) | (311.5, 500.0) | (348.0, 485.0) | (399.0, 459.0) | (400.0, 457.0) | ||||
| Echocardiography of V1A TG mice and control mice at 24 weeks of age. FS%, percent fractional shortening; LVEDD, LV end-diastolic dimension in mm; LVESD, LV end-systolic dimension in mm. | ||||||||
| B. 24 Week-Old WT and V1A-TG Mouse Molecular Phenotype | |||||||
|---|---|---|---|---|---|---|---|
| WT | V1A | ||||||
| n | median | (25th%ile, 75th%ile) | n | median | (25th%ile, 75th%ile) | p-value | |
| ANP | 10 | 0.7 | (0.5, 0.9) | 8 | 4.5 | (1.0, 20.1) | 0.0028 |
| BNP | 10 | 0.7 | (0.6, 0.9) | 8 | 2.6 | (1.9, 6.0) | <0.0001 |
| bMHC | 10 | 1.2 | (0.7, 1.5) | 8 | 1.7 | (0.7, 4.8) | 0.3951 |
| NCX | 3 | 1 | (0.5, 1.6) | 3 | 1 | (0.7, 1.5) | 1 |
| SERCA | 9 | 1.5 | (1.0, 2.1) | 9 | 0.9 | (0.4, 1.5) | 0.0893 |
Relative gene expression in ventricular tissue measured by real-time PCR.
Table 2. Invasive hemodynamics of tTA/V1A vs tTA/V1A/GqI at 24 weeks of age.
| WT | tTA/V1A | tTA/V1A/GqI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | median | 25th%ile 75%ile | n | median | 25th%ile 75%ile | n | median | 25th%ile 75%ile | p-value (tTA/V1A vs WT) | p-value (tTA/V1A/GqI vs WT) | ||
| (+) dP/dt | base | 1 1 |
7429.85 | 6792.09 8035.71 |
8 | 6942.86 | 5887.76 7669.01 |
7 | 7798.35 | 6632.22 7936.42 |
0.6396 | 0.9100 |
| iso | 1 1 |
13065.0 5 |
12468.11 13488.52 |
7 | 9757.65 | 7270.41 11001.2 8 |
7 | 10276.7 7 |
9938.26 12786.3 5 |
<0.0001 | 0.253 | |
| (-) dP/dt | base | 1 1 |
- 6983.42 |
-7079.08 -6444.33 |
7 | - 5484.69 |
- 6696.43 - 4719.39 |
7 | - 6931.50 |
- 7157.34 - 6733.31 |
0.0150 | 0.9120 |
| iso | 1 1 |
- 9470.66 |
- 11065.0 5 -9215.56 |
7 | - 6473.21 |
- 8896.68 - 6250.00 |
7 | - 7942.56 |
- 9885.45 - 7122.54 |
0.0003 | 0.0249 | |
| LVESP | base | 1 1 |
104.24 | 94.36 122.26 |
7 | 96.91 | 88.78 103.13 |
7 | 100.10 | 98.23 107.60 |
0.4117 | 0.4117 |
| iso | 1 1 |
123.21 | 121.14 142.83 |
7 | 110.3 | 105.84 132.78 |
8 | 130.50 | 113.64 150.91 |
0.1324 | 0.8869 | |
| LVEDP | base | 1 1 |
4.10 | 1.77 4.89 |
7 | 9.93 | 6.15 13.20 |
7 | 6.33 | 4.78 9.35 |
0.0004 | 0.0041 |
| iso | 1 1 |
2.11 | 1.25 3.50 |
7 | 8.35 | 6.32 15.00 |
7 | 4.77 | 3.71 6.01 |
0.0088 | 0.0088 | |
Baseline and isoproterenol (10ng ISO) stimulated cardiac hemodynamic functions were recorded in 24 week-old mice using a Millar catheter. +dP/dt and −dP/dt (mm Hg/dt): maximal 1st time derivatives of left ventricular (LV) pressure rise and fall, respectively; LVESP and LVEDP (mm Hg): LV end-systolic pressure and -diastolic pressure, respectively.
Table 3.
Echocardiography of tTA/V1A/GqI triple transgenic mice at 24 weeks of age.
| FS% | LVEDD | LVESD | Heart Rate | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | median | (25th%ile, 75th%ile) | median | (25th%ile, 75th%ile) | median | (25th%ile, 75th%ile) | median | (25th%ile, 75th%ile) | |
| FVB | 8 | 45.2 | (43.2, 48.3) | 3.5 | (2.9, 3.5) | 1.8 | (1.6, 2.0) | 486.0 | (429.0, 491.0) |
| tTA | 8 | 44.8 | (36.7, 47.4) | 3.2 | (2.7, 3.5) | 1.9 | (1.6, 1.9) | 456.0 | (378.5, 472.0) |
| GqI | 8 | 49.2 | (44.8, 50.5) | 2.9 | (2.8, 3.1) | 1.5 | (1.4, 1.8) | 457.0 | (375.0, 472.0) |
| V1A | 8 | 41.3 | (39.1, 43.6) | 3.1 | (2.2, 3.6) | 1.8 | (1.2, 2.2) | 439.5 | (355.0, 468.5) |
| GqI/V1A | 8 | 49.0 | (44.3, 49.3) | 2.9 | (2.8, 3.5) | 1.7 | (1.4, 1.9) | 451.0 | (378.5, 472.0) |
| tTA/GqI | 8 | 44.7 | (39.2, 47.4) | 3.2 | (2.8, 3.8) | 1.8 | (1.5, 2.2) | 466.5 | (441.5, 481.5) |
| tTA/V1A | 8 | 32.0 | (30.4, 34.0) | 4.3 | (4.1, 4.5) | 2.9 | (2.7, 3.2) | 412.5 | (406.0, 418.5) |
| tTa/V1A/GqI | 8 | 45.6 | (41.6, 47.3) | 3.0 | (2.8, 3.2) | 1.7 | (1.5, 1.8) | 451.0 | (409.0, 463.5) |
Echocardiography of V1A TG mice and control mice at 24 weeks of age. FS%, percent fractional shortening; LVEDD, LV end-diastolic dimension in mm; LVESD, LV end-systolic dimension in mm. tTA/V1A was tested against GqI/V1A, tTa/GqI, and tTA/V1A/GqI combined. P-values for FS%, LVEDD, LVESD, and Heart Rate were <0.0001, <0.0001, <0.0001, and 0.1703, respectively.
Generation and maintenance of tTA/V1A-R transgenic mouse lines that co-expresses Gαq inhibitor, Gq-I
Transgenic mice that overexpressed a peptide derived from a carboxyl-terminal peptide of the α subunit Gαq (Gq-I TG) were described previously28. The tTA/V1A/Gq-I triple transgenic mice and their littermates in 75%FVB/25%C57/B6 strain background were used for analysis. All protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. Detailed methods are located in the Supplemental Section.
Echocardiography Echocardiography studies were performed using an ultrasonographic system (ACUSON Sequoia C256). More detailed methods are located in the Supplemental Section.
Real-Time Quantitative PCR Real-time quantitative polymerase chain reaction analysis determined gene expression. Briefly, reverse-transcribed cDNA from myocardial RNA was used to determine gene expression. Real time PCR was performed in a 50 μl reaction (5 μl cDNA or 40ng of genomic DNA; 250 nM each primer; 1× SYBRE Green Master Mix). Each experimental group was performed in triplicate. The Δ CT method using GAPDH as reference gene was used to quantify the results and perform statistic analysis. Detailed methods are located in the Supplemental Section.
Histopathology of Myocardium
Detailed methods are located in the Supplemental Section.
Hemodynamic Analysis of Cardiac Function
Detailed methods are located in the Supplemental Section.
Isolation of Adult Murine Cardiac Myocytes
Cardiac myocytes were isolated from the septum and LV free wall of WT and V1A-TG mice. Detailed methods are located in the Supplemental Section.
Contraction and [Ca2+]i Transient Measurements
Detailed methods are located in the Supplemental Section.
Action Potential Measurements
Action potentials were recorded using current-clamp configuration at 1.5x threshold stimulus and 4-ms duration. Detailed methods are located in the Supplemental Section.
Immunoblots
Immunoblotting of ventricular protein extracts was digitally detected as described29. More detailed methods are located in the Supplemental Section.
D-myo-inositol 1,4,5 trisphosphate (IP3) surrogate, IP1, in adult myocytes
Stable accumulation of IP1 in cells in the presence of LiCl is a surrogate measure of IP3 induction by Gαq coupled receptors, including vasopressin V1A and V1B receptors. Detailed methods are located in the Supplemental Section.
Statistical Methods
For experiments with small sample sizes, exact nonparametric tests were used. For these experiments, summary statistics reported include median and inter-quartile range (IQR), which represents the 25th to 75th percentiles. Graphically, box plots are employed to visualize the range of data, and are overlaid with individual points. For uptake experiments, sufficient sample size allows for a two-way anova, followed by post-hoc group comparisons within each level of concentration. Thus, uptake experiments are reported as least square mean and standard error. For remaining analyses, the Wilcoxon exact test or Kruskal-Wallis exact tests are employed, with adjustment for multiple comparisons based on the Hochberg method, when applicable. Two-sided tests are reported, with p≥0.05 considered statistically significant. Analysis and graphs were completed using StatXact v 8.0, SAS v9.2, and R v.2.11.1.
Results
We generated human V1A receptor transgenic (V1A-TG) mice controlled by an inducible cardiac-specific promoter with binding sites for the tetracycline-transactivating factor (tTA). Gene expression was initiated by crossing five founder V1A-TG lines with mice that expressed tTA in the heart (MHC-tTA) (Fig. 1A). Using real-time PCR, we found two of the founder lines having 60× more V1A-R mRNA expression than WT FVB mice (Lines S11 and S13, Sup. Fig. 1 and data not shown) and three of the founder lines expressed V1A-R mRNA at levels similar to WT FVB (S1, S10, S12, data not shown). We followed one high expression line (S13) and one low expression line (S10) for over 30 weeks by echocardiography and found at eight weeks-of-age, both V1A-TG mouse lines did not display changes in cardiac function when compared to wild-type (WT) control mice (Fig. 1B and Table 1). By 24 week-of-age, the high expression V1A-TG line, but not the low expression line, had decreased cardiac function, increased heart weight/body weight ratio, increased fibrosis and increased ANP mRNA (Fig. 1B, 1C, 1D and Table 1). Similarly, the other high expression line, S11, also developed cardiomyopathy by 24 weeks of age (Data not shown). We used the S13 line for the remaining studies.
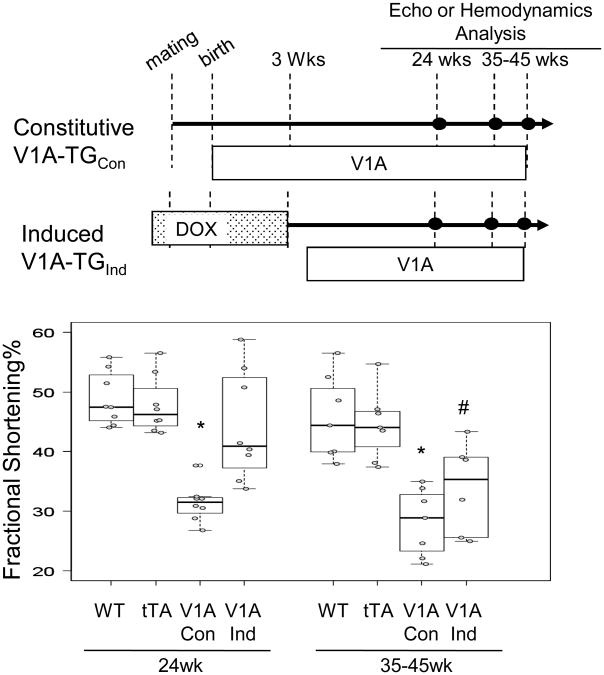
In a separate group of experiments, female mice were treated with doxycycline throughout pregnancy and during the post-partum period to ensure that transgene expression were suppressed during the neonatal period. At three to four weeks of age, doxycycline was removed from transgenic pups to induce V1A expression. Mature mice that were induced to express the V1A transgene (V1A-TGind) still demonstrated a significant decrease in fractional shortening (Fig. 2) when compared to control mice. However, the diminution in ventricular functions in V1A-TGind mice were less robust than that seen in the mice with constitutive overexpression of the transgene (V1A-TG) and was evident at a later point in time (35 weeks of age). For that reason, we used mice with constitutive overexpression in our subsequent experiments.
Figure 2.
Echocardiography was performed on FVB-WT mice, tTA expressing mice, constitutively expresssed V1A-TG line, S13 (V1A-TGCon) and mice with induced expression of V1A receptor transgene at three weeks of age (V1A-TGInd). Graph of Fractional shortening percentage measured at indicated ages is shown. *adjusted p<0.001 vs WT 24wk, #adjusted P<0.05 vs WT 35-45wk.
Hemodynamic response in 24 week-old V1A-TG mice
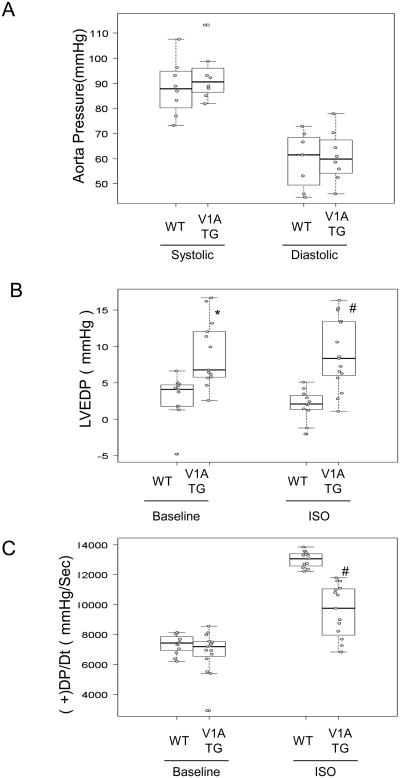
Since vasopressin is known to mediate vasoconstriction, we evaluated aortic blood pressure in 24 week-old V1A-TG mice using a 1.4 F micromanometer catheter and found that both WT and V1A-TG mice had similar systolic and diastolic aortic blood pressure (Fig. 3A), supporting our finding that V1A receptor overexpression did not occur in the vasculature. Catheter-based in vivo hemodynamic measurements showed that chronic overexpression of the V1A receptor in the heart significantly reduced hemodynamic response to the adrenergic receptor agonist, isoproterenol, when compared to WT mice (Fig. 3B, 3C). Complete cardiac hemodynamic parameters are shown in Table 2.
Figure 3.
Baseline and 10ng isoproterenol (Iso) stimulated cardiac hemodynamic functions were recorded in 24 week-old wild-type and V1A-TG mice. (A) Aorta Pressure. (B) LVEDP: LV end-diastolic pressure. *p<0.001 vs WT Systolic, #P<0.001 vs WT Diastolic. (C) +dP/dt and –dP/dt: maximal 1st time derivatives of left ventricular (LV) pressure rise. #P<0.001 vs WT ISO. Tables of median, IQR and P values are included in the Supplemental Section.
Effects of induced V1A receptor overexpression on myocyte contraction, [Ca2+]i transients and action potential
In both WT and V1A myocytes, elevating extracellular calcium ([Ca2+]o) resulted in the expected increase in contraction amplitudes. However, compared to WT myocytes, SR Ca2+ uptake appears to be slower in V1A-TG myocytes. Also, action potential amplitude was significantly lower in V1A-TG myocytes (P<0.037). The most dramatic finding is a 3.5-fold prolongation of action potential duration at 90% repolarization (APD90) in V1A myocytes (P<0.0001). A complete description of these findings is found in the Supplemental Section and Supplemental Figures 2 and 3.
Blocking V1A receptor expression reversed V1A-TG cardiomyopathy
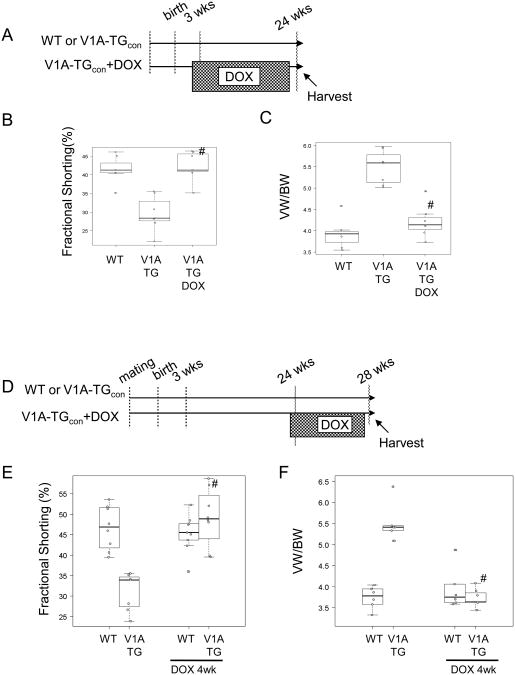
We previously determined that the tetracycline transactivating factor (tTA)-regulated promoter can be inhibited by the stable tetracycline analog, doxycycline, when used at 300mg/kg of mouse diet30. To determine whether the cardiomyopathy induced by V1A receptor overexpression was reversible, V1A-TG mice were fed with doxycycline diets at 3 weeks-of-age in order to inhibit V1A receptor transgene expression (Fig. 4A). Cardiac function was assessed when mice reached 24 weeks of age. The discontinuation of transgene expression at puberty was sufficient to prevent the development of left ventricular hypertrophy and failure (Fig. 4B and 4C). To determine whether the pathology induced by V1A receptor overexpression after 24 weeks of age could be reversed, we added doxycycline to the diets of V1A-TG mice and wild-type mice at 24 weeks of age (Fig. 4D). After only four weeks on doxycycline diets (to inhibit V1A-R transgene expression), V1A-TG mice (with confirmed reduced cardiac function at 24 weeks of age) demonstrated a significant improvement in left ventricular function and ventricular weight to body weight ratio (Fig. 4E and F). In addition, 4 weeks of doxycycline treatment also normalized the levels of expression of ANP (data not shown), indicating that the cardiomyopathy in this model was largely reversible.
Figure 4.
DOX treatment reversed cardiomyopathy in V1A-TG mice. (A) Schematic diagram showing that at 3 weeks of age, V1A-TG mice were fed with DOX diets. (B) Assessment of cardiac function (Fractional Shortening %) at 24 weeks of age. #adjusted p<0.01 vs V1A-TG. (C) VW/BW ratio. #adjusted p<0.01 vs V1A-TG. (D) Schematic diagram showing that at 24 weeks of age, V1A-TG mice were fed with DOX diets for 4 weeks. (E) Assessment of cardiac function. #adjusted p<0.01 vs V1A-TG. (F) Assessment of VW/BW. #adjusted p<0.01 vs V1A-TG. Tables of median, IQR and P values are included in the Supplemental Section.
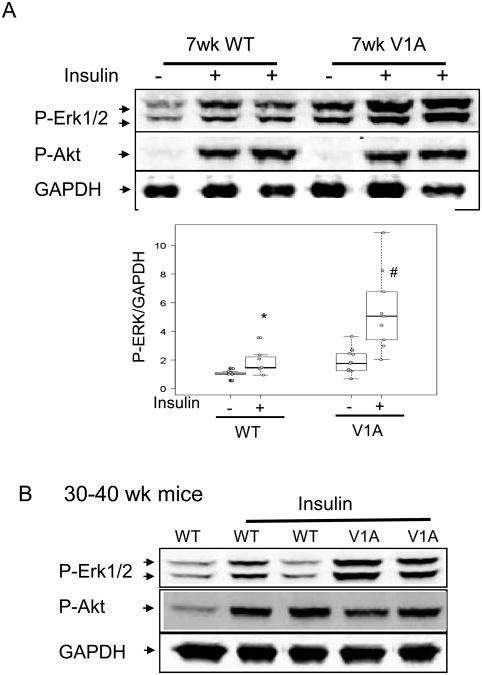
V1A receptor overexpression enhanced insulin-mediated Erk1/2 phosphorylation
Previous cell culture studies demonstrated that the V1A receptor signals, in part, through activation of map kinases, Erk1 and Erk2 25. To assess the effects of V1A receptor overexpression on Erk1/2 activation in vivo, 7 week-old V1A-TG (which had a normal cardiac phenotype) and wild type mice were deprived of food overnight to stabilize baseline Erk1/2 signaling. Subsequent analysis of cardiac extracts demonstrated a significant elevation in Erk1/2 phosphorylation (Fig. 5A). The effect of V1A receptor overexpression on Erk1/2 phosphorylation was kinase specific as V1A-TG mice did not change baseline Akt phosphorylation (Fig. 5A) or JNK phosphorylation (data not shown). To further confirm that V1A-TG mice did not alter Akt activation, cardiac Akt was activated by intraperitoneally injecting mice with insulin (0.4 mg/kg, 15 min). Fig, 5A shows that both WT amd V1A-TG mice activated Akt to a similar degree after insulin injection. We also measured insulin activation in older mice (30-40 weeks-of-age). Consistent with the results in younger mice, V1A receptor overexpression did not affect Akt phosphorylation (Fig. 5B).
Figure 5.
V1A receptor overexpression on ERK1/2 activation in the heart. (A) 7-wk-old male WT and V1A-TG mice were injected with insulin (0.4mg/kg body weight, 15 minutes) and ventricular extracts were prepared. Immunoblots show phosphorylated ERK1/2 (pERK1/2), phosphorylated Akt (p-Akt, Thr308) and GAPDH. Graph shows GAPDH normalized phospho-ERK1/2 level. *p<0.01 vs WT-baseline, #P<0.001 vs WT-insulin. Table of median, IQR and P values are included in the Supplemental Section. (B) 30-40 week-old WT and V1A-TG mice were simulated with insulin injection and ventricular extracts were probed for pErk1/2 and pAkt. Immunoblots of pAkt and GAPDH are shown.
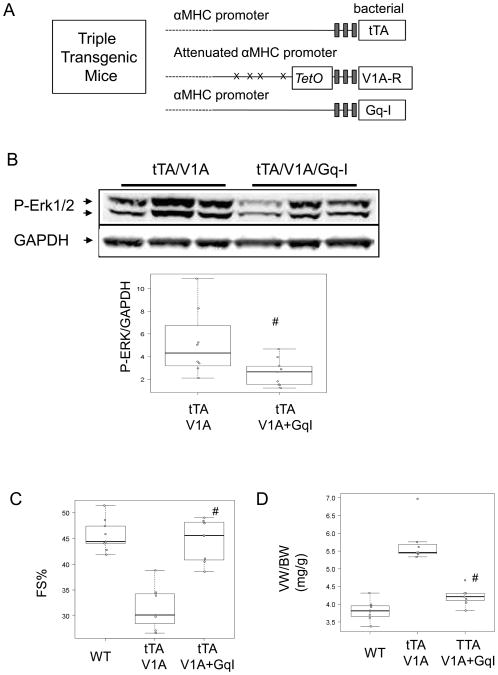
Gαq/11 is required for V1A receptor-mediated myopathy
The V1A receptor mediates at least some of its downstream effects by coupling with the Gαq proteins. 10, 14 To test whether V1A receptor mediated cardiomyopathy was dependent on Gαq signaling, we created transgenic mice overexpressing both the V1A receptor and a peptide derived from the carboxyl-terminal end of Gαq that inhibits Gαq/11 signaling (Gq-I) 28 (Fig. 6A). We assessed Erk1/2 activation in the triple transgenic mice and their littermates at 7 week-old of age. Fig. 6B shows that Gq-I co-expression reduced insulin-stimulated Erk1/2 phosphorylation. To determine phenotype, triple-transgenic mice and their littermates were studied at 24 weeks of age. As seen in Figure 6C and 6D and Table 2 and Table 3, mice expressing both V1A receptor and Gq-I transgenes had significantly better echocardiographic physiological parameters and hemodynamic responses than controls, suggesting that inhibition of cardiac Gαq signaling blocked V1A receptor-induced cardiomyopathy.
Figure 6.
Blocking Gαq reversed V1A-TG cardiomyopathy. (A) Schematic depiction of the three component transgenic system to induce cardiac tTA, V1A receptor and Gq-I expression, (B) 7 week-old V1A-TG and V1A/Gq-I TG mice were simulated with insulin injection and ventricular extracts were probed for pErk1/2. Immunoblots show pERK1/2 and GAPDH. Graph shows GAPDH normalized phospho-ERK1/2. #P<0.05 vs tTA/V1A. (C) Assessment of cardiac function of 24 week-old WT, V1A-TG and V1A/Gq-I TG mice. #adjusted p<0.001 vs V1A-TG. (D) Ventricular weight and body weight (VW/BW) ratio of 24 week-old WT, V1A-TG and V1A/Gq-I TG mice. #adjusted p<0.001 vs V1A-TG. Table of median, IQR and P values are included in the Supplemental Section.
Enhanced Gαq and IP3 signaling in adult myocytes overexpressing V1A receptor
Since the V1A receptor-Gαq complex activates phospholipase C and induces D-myo-inositol 1,4,5 trisphosphate (IP3) and calcium release, we isolated adult myocytes to determine functional coupling of V1A-TG myocytes to IP3 production. We used the D-myo-inositol 1 phosphate (IP1) surrogate assay to determine the regulation of IP3 production because IP3 production is highly transient, but its IP1 metabolite stably accumulates in LiCl-treated cells. Thus, IP1 measurement is a validated surrogate measure for ligand induced IP3 and calcium production in cell lines expressing V1A and V1B receptors. 31, 32
To determine IP1 production in cardiac myocytes, adult cardiac myocytes from WT mice were cultured in the presence of 50mM LiCl and stimulated with Insulin-Transferrin-Selenium (ITS, containing 10ug/ml insulin), 0.5 uM vasopressin or 1 uM isoproterenol for 30 minutes. Vasopressin induced a 15.3-fold increase in IP1 production when compared to unstimulated control myocytes. To determine IP1 production in V1A-TG myocytes, myocytes from 21-week-old V1A-TG mice were cultured in the presence of 50mM LiCl and stimulated without or with 0.5 uM vasopressin for 30 minutes. Myocytes from V1A-TG mice had a 2.2-fold increase in IP1 levels when compared to myocytes from wild-type control mice (p<0.001). Furthermore, while vasopressin stimulated IP1 level in WT myocytes by 15.3-fold, vasopressin could hyper-stimulate IP1 by 29.0-fold (p<0.001) in myocytes from V1A-TG mice, suggesting V1A-R overexpression in the heart enhanced IP3 signaling and that the protein expressed by the V1A transgene is physiologically linked to the endogenous V1A signaling pathway. See Sup. Fig. 4 and Sup. Fig. 5 in the supplemental materials.
Discussion
Mice with constitutive or controlled overexpression of the AVP V1A receptor in the heart showed a normal cardiac phenotype at 8-10 weeks of age. However, by 24 weeks of age, the hearts of mice constitutively overexpressing the V1A receptor were significantly dilated, hypertrophied and demonstrated reduced cardiac function. Mice with induced overexpression also developed LV dysfunction albeit at 35 weeks of age. In addition, cardiac gene expression was “reprogrammed” as evidenced by an increase in the expression of ANP and BNP. Adult cardiac myocytes isolated from these mice showed lower systolic [Ca2+]i, lower maximal contraction amplitudes, and reduced sarcoplasmic reticulum Ca2+-uptake activity consistent with earlier studies using AVP. The characteristic heart failure phenotype seen in the mice overexpressing the V1A receptor was elicited through activation of the Gαq/11-mediated signaling cascade as genetic ablation of the Gαq/11 signaling attenuated the myocardial effects of V1A receptor overexpression. In addition, the late development of the V1A-related heart failure phenotype could be abrogated by “turning off” the V1A receptor transgene shortly after birth while the phenotype could be “reversed” by “turning off” the transgene in 24 week-old mice that had already developed ventricular dilation, diminished left ventricular function and “reprogramming” of cardiac gene expression.
The use of a α-myosin heavy chain-driven and doxycycline-modulated transgene to increase V1A-dependent signaling in the heart provides several unique opportunities. Vasoconstriction is a common consequence of pharmacologic activation of AVP. Indeed, V1A receptors as well as a number of other Gαq-coupled receptors elicit vasoconstriction.33 Thus, driving V1A overexpression using the α-myosin heavy chain promoter facilitates the ability to assess the effects of V1A signaling in the heart without the vasoconstriction that occurs with the exogenous administration of vasopressin or V1A-selective agonists and also obviates the potential confounding effects of agonists or antagonists that do not have complete selectivity.
The bi-transgenic doxycycline-regulated system also provides the opportunity to “turn-off” transgene expression during pre- and peri-natal development as well as during postnatal over-expression. This is relevant to the study of cell surface receptors such as V1A that couple to Gαq. Previous studies have shown that constitutive overexpression of Gαq driven by the α-myosin heavy chain promoter results in the development of hypertrophy and many of the characteristics of pressue overload.34, 35 However, the effects of Gαq overexpression using an inducible bi-transgenic system in transgenic mice or adenoviral infection in cardiomyocytes are less clear. 36-38 Indeed, Dorn and co-workers were unable to identify a heart failure phenotype in mice with adult-onset over-expression of Gαq.38 By contrast, we found that induced overexpression begun at 3 weeks of age resulted in the development of cardiac hypertrophy, dilation and diminished left ventricular function similar to, but less than that seen with constitutive overexpression . We then asked a closely related but somewhat different question: could prenatal and early post-natal perinatal over-expression alone elicit the development of a heart failure phenotype? To address this question, we stopped V1A transgene expression at the conclusion of the peri-natal period. This experiment demonstrated that over-expression of the V1A receptor during the peri-natal period alone was not sufficient to elicit a change in the cardiac phenotype.
Finally, the use of the controlled transgene allowed us to evaluated the “reversibility” of the heart failure phenotype in 24 week-old mice, a time point at which the transgenic mice over-expressing the V1A transgene demonstrate left ventricular hypertrophy and dilation, decreased ventricular function and re-expression of the fetal gene program as evidenced by increased levels of ANF and BNP, but not a significant decrease in the expression of the Ca2+-ATPase. We found that discontinuation of V1A over-expression at 24 weeks resulted in a reversal in the abnormalities in fractional shortening and ventricular weight/body weight ratio as well as normalization of the levels of expression of ANP and BNP when measured four weeks after discontinuation of gene expression. A reversal of the heart failure phenotype has not been reported previously with other heart failure models secondary to over-expression of G protein-coupled seven transmembrane-spanning sarcolemmal receptors. This may be due more to the fact that until recently transgenic mouse models were constructed by constitutively driving the α-myosin heavy chain promoter rather than by using inducible bi-transgenic systems. Our finding that the heart failure phenotype can be reversed when transgene expression is interrupted is consistent with the finding that hypertrophy and left ventricular dysfunction found in mice with controlled over-expression of Gαq can be significantly improved after termination of the Gαq signal even in animals with overt heart failure 39. Thus, our results may be generalized to other heart failure models that are mediated through activation of G protein-coupled receptors.
The results of the present study are consistent with findings in Gαq transgenic mice which demonstrate hypertrophy, decreased ventricular function, loss of responsiveness to β-adrenergic receptor stimulation and induction of a classic hypertrophy gene expression profile.34 However, there are elements of V1A receptor over-expression that are unique. For example, Akt is thought to play an important role in the heart failure phenotype in some models of Gαq signaling. Over-expression of the wild type Gq results in an increase in the phosphorylation of Akt whereas over-expression of a constitutively active Gαq subunit markedly attenuates Akt phosphorylation and results in significant apoptosis and early mortality. 40, 41 Akt is also a major downstream target of the phosphatidylinositol 3-kinase involved in angiotensin II-induced proliferation of vascular smooth muscle. 42 By contrast, overexpression of V1A receptors did not influence Akt phosphorylation in the present experiments.
Another important difference between the V1A-TG mice and heart failure models created by overexpressing Gαq is the time course of the development of the heart failure phenotype. Constitutive 5-fold over-expression of Gαq effected the development of a heart failure phenotype by eight weeks of age in transgenic mouse models. 35, 43 However, over-expression of the V1A receptor took 24 weeks to illicit a change in the cardiac phenotype. This delay in the development of the heart failure phenotype was similar to that seen with over-expression of the β1-adrenergic receptor, albeit at a substantially lower level of V1A expression. 44 Comparison to signaling of angiotensin II type 1 receptor can be found in the supplemental materials.
Mice with gene-ablation of V1A receptor demonstrated altered cardiovascular hemodynamics and lower basal blood pressure.26, 45 AVP failed to induce vasoconstrictive responses in mice lacking V1A receptor. Also arterial baroreceptor reflexes were markedly impaired due to loss of V1A receptors in baroreflex neurons. Our finding that blood pressure was unchanged in mice over-expressing the V1A receptor was disparate from these earlier studies but reflected the fact that the V1A receptor was only over-expressed in cardiac muscle and not in the smooth muscle underlying the peripheral or coronary vasculature.
That overexpression of the V1A receptor in FVB mice is physiologically coupled to the downstream signaling pathways that are known to be activated by vasopressin is demonstrated by the fact that myocytes isolated from V1A-TG hearts showed significant activation of IP3 as measured by IP1 accumulation when compared with controls. IP3 is a well established primary signaling pathway for vasopressin. 10, 31, 32
The finding that chronic over-expression of the V1A receptor results in the development of cardiac hypertrophy, dilatation and diminished left ventricular function may have clinical relevance. The administration of V2-selective vasopressin antagonist tolvaptan increases serum [Na2+] in patients with hypervolemic hyponatremia secondary to congestive heart failure, but did not improve survival.46 Our results suggest that combining V1A and V2 receptor antagonists may have a more robust effect on myocardial function than the use of a V2 receptor antagonist alone and that V1A-selective vasopressin antagonists may be useful in the treatment of patients with heart failure and elevated levels of vasopressin.
Supplementary Material
Clinical Summary.
Heart failure is associated with an increase in circulating levels of arginine vasopressin (AVP). AVP activates vasopressin V2 receptors in the kidney resulting in redistribution of aquaporin-2 water channels and the re-absorption of free water. This results in the development of edema and hyponatremia. Selective antagonism of the V2-selective vasopressin receptor significantly increased serum sodium levels and removes free water in patients with heart failure. AVP also interacts with vasopressin V1A receptors in the heart; however, the effects of AVP on the heart muscle are not well understood. In the present study we found that cardiac-specific over-expression of the vasopressin V1A receptor causes the development of heart failure in transgenic mice. The vasopressin V1-A receptor-mediated development of heart failure was reversed by “turning-off” the over-expression of the transgene or by blocking a G protein-dependent signaling pathway, Gq, downstream of the V1A receptor. These results, albeit in a mouse model, suggest that a V1A receptor antagonist or inhibition of the Gq signaling pathway may be useful adjuncts to vasopressin V2 receptor antagonism in the treatment of patients with heart failure.
Acknowledgments
Funding Sources: This work was supported by the National Heart, Lung and Blood Institute grant HL 091799-01 (AMF), HL 061690, HL 085503, HL 075443 (WJK), HL 58672, HL 74854 (JYC), and NCI Support Grant P30CA56036 (TH).
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamane Y. Plasma ADH level in patients with chronic congestive heart failure. Jpn Circ J. 968:745–759. doi: 10.1253/jcj.32.745. [DOI] [PubMed] [Google Scholar]
- 2.Goldsmith SR, Francis GS, Levine TB, Cowley AW, Jr, Cohn JN. Impaired response of plasma vasopressin to orthostatic stress in patients with congestive heart failure. J Am Coll Cardiol. 1983;2:1080–1083. doi: 10.1016/s0735-1097(83)80333-7. [DOI] [PubMed] [Google Scholar]
- 3.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 4.Kim JK, Michel JB, Soubrier F, Durr J, Corvol P, Schrier RW. Arginine vasopressin gene expression in chronic cardiac failure in rats. Kidney Int. 1990;38:818–822. doi: 10.1038/ki.1990.276. [DOI] [PubMed] [Google Scholar]
- 5.Muders F, Riegger GA, Bahner U, Palkovits M. The central vasopressinergic system in experimental left ventricular hypertrophy and dysfunction. Prog Brain Res. 2002;139:275–279. doi: 10.1016/s0079-6123(02)39023-x. [DOI] [PubMed] [Google Scholar]
- 6.Howl J, Wheatley M. Molecular pharmacology of V1a vasopressin receptors. Gen Pharmacol. 1995;26:1143–1152. doi: 10.1016/0306-3623(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 7.Murasawa S, Matsubara H, Kijima K, Maruyama K, Mori Y, Inada M. Structure of the rat V1a vasopressin receptor gene and characterization of its promoter region and complete cDNA sequence of the 3'-end. J Biol Chem. 1995;270:20042–20050. doi: 10.1074/jbc.270.34.20042. [DOI] [PubMed] [Google Scholar]
- 8.Saito M, Sugimoto T, Tahara A, Kawashima H. Molecular cloning and characterization of rat V1b vasopressin receptor: evidence for its expression in extra-pituitary tissues. Biochem Biophys Res Commun. 1995;212:751–757. doi: 10.1006/bbrc.1995.2033. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto T, Saito M, Mochizuki S, Watanabe Y, Hashimoto S, Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994;269:27088–27092. [PubMed] [Google Scholar]
- 10.Abel A, Wittau N, Wieland T, Schultz G, Kalkbrenner F. Cell cycle-dependent coupling of the vasopressin V1a receptor to different G proteins. J Biol Chem. 2000;275:32543–32551. doi: 10.1074/jbc.M002171200. [DOI] [PubMed] [Google Scholar]
- 11.Exton JH. Cell signalling through guanine-nucleotide-binding regulatory proteins (G proteins) and phospholipases. Eur J Biochem. 1997;243(1-2):10–20. doi: 10.1111/j.1432-1033.1997.t01-1-00010.x. [DOI] [PubMed] [Google Scholar]
- 12.Levy A, Lightman SL, Hoyland J, Mason WT. Inositol phospholipid turnover and intracellular Ca2+ responses to thyrotrophin-releasing hormone, gonadotrophin-releasing hormone and arginine vasopressin in pituitary corticotroph and somatotroph adenomas. Clin Endocrinol (Oxf) 1990;33:73–79. doi: 10.1111/j.1365-2265.1990.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 13.Thibonnier M. Signal transduction of V1-vascular vasopressin receptors. Regul Pept. 1992;38:1–11. doi: 10.1016/0167-0115(92)90067-5. [DOI] [PubMed] [Google Scholar]
- 14.Schoneberg T, Kostenis E, Liu J, Gudermann T, Wess J. Molecular aspects of vasopressin receptor function. Adv Exp Med Biol. 1998;449:347–358. doi: 10.1007/978-1-4615-4871-3_44. [DOI] [PubMed] [Google Scholar]
- 15.Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- 16.Lolait SJ, O'Carroll AM, McBride OW, Konig M, Morel A, Brownstein MJ. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 17.Thibonnier M. Vasopressin and blood pressure. Kidney Int Suppl. 1988;25:S52–56. [PubMed] [Google Scholar]
- 18.Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol. 1997;272:F3–12. doi: 10.1152/ajprenal.1997.272.1.F3. [DOI] [PubMed] [Google Scholar]
- 19.Cheng CP, Igarashi Y, Klopfenstein HS, Applegate RJ, Shihabi Z, Little WC. Effect of vasopressin on left ventricular performance. Am J Physiol. 1993;264:H53–60. doi: 10.1152/ajpheart.1993.264.1.H53. [DOI] [PubMed] [Google Scholar]
- 20.Walker BR, Childs ME, Adams EM. Direct cardiac effects of vasopressin: role of V1- and V2-vasopressinergic receptors. Am J Physiol. 1988;255:H261–265. doi: 10.1152/ajpheart.1988.255.2.H261. [DOI] [PubMed] [Google Scholar]
- 21.Nishikimi T, Kawano Y, Saito Y, Matsuoka H. Effect of long-term treatment with selective vasopressin V1 and V2 receptor antagonist on the development of heart failure in rats. J Cardiovasc Pharmacol. 1996;27:275–282. doi: 10.1097/00005344-199602000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Clair MJ, King MK, Goldberg AT, Hendrick JW, Nisato R, Gay DM, Morrison AE, McElmurray JH, 3rd, Krombach RS, Bond BR, Cazaubon C, Nisato D, Spinale FG. Selective vasopressin, angiotensin II, or dual receptor blockade with developing congestive heart failure. J Pharmacol Exp Ther. 2000;293:852–860. [PubMed] [Google Scholar]
- 23.Van Kerckhoven R, Lankhuizen I, van Veghel R, Saxena PR, Schoemaker RG. Chronic vasopressin V(1A) but not V(2) receptor antagonism prevents heart failure in chronically infarcted rats. Eur J Pharmacol. 2002;449:135–141. doi: 10.1016/s0014-2999(02)01972-6. [DOI] [PubMed] [Google Scholar]
- 24.Indrambarya T, Boyd JH, Wang Y, McConechy M, Walley KR. Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemia-reperfusion injury in mice. Crit Care. 2009;13:R98. doi: 10.1186/cc7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiroyama M, Wang S, Aoyagi T, Oikawa R, Sanbe A, Takeo S, Tanoue A. Vasopressin promotes cardiomyocyte hypertrophy via the vasopressin V1A receptor in neonatal mice. Eur J Pharmacol. 2007;559:89–97. doi: 10.1016/j.ejphar.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Koshimizu TA, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci U S A. 2006;103:7807–7812. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering Inducible Cardiac-Specific Transgenesis With an Attenuated Myosin Heavy Chain Promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 28.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 29.Chan TO, Funakoshi H, Song J, Zhang XQ, Wang J, Chung PH, DeGeorge BR, Jr, Li X, Zhang J, Herrmann DE, Diamond M, Hamad E, Houser SR, Koch WJ, Cheung JY, Feldman AM. Cardiac-restricted overexpression of the A(2A)-adenosine receptor in FVB mice transiently increases contractile performance and rescues the heart failure phenotype in mice overexpressing the A(1)-adenosine receptor. Clin Transl Sci. 2008;1:126–133. doi: 10.1111/j.1752-8062.2008.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funakoshi H, Chan TO, Good JC, Libonati JR, Piuhola J, Chen X, MacDonnell SM, Lee LL, Herrmann DE, Zhang J, Martini J, Palmer TM, Sanbe A, Robbins J, Houser SR, Koch WJ, Feldman AM. Regulated Overexpression of the A1-Adenosine Receptor in Mice Results in Adverse but Reversible Changes in Cardiac Morphology and Function. Circulation. 2006;114:2240–2250. doi: 10.1161/CIRCULATIONAHA.106.620211. [DOI] [PubMed] [Google Scholar]
- 31.Liu K, Titus S, Southall N, Zhu P, Inglese J, Austin CP, Zheng W. Comparison on functional assays for Gq-coupled GPCRs by measuring inositol monophospate-1 and intracellular calcium in 1536-well plate format. Curr Chem Genomics. 2008;1:70–78. doi: 10.2174/1875397300801010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinquet E, Fink M, Bazin H, Grillet F, Maurin F, Bourrier E, Ansanay H, Leroy C, Michaud A, Durroux T, Maurel D, Malhaire F, Goudet C, Pin JP, Naval M, Hernout O, Chretien Y, Mathis G. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal Biochem. 2006;358:126–135. doi: 10.1016/j.ab.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Holmes CL, Landry DW, Granton JT. Science review: Vasopressin and the cardiovascular system part 1--receptor physiology. Crit Care. 2003;7:427–434. doi: 10.1186/cc2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., 2nd Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakata Y, Hoit BD, Liggett SB, Walsh RA, Dorn GW., 3rd Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation. 1998;97:1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 36.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., 3rd Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan G, Jiang YP, Lu Z, Martin DW, Kelly DJ, Zuckerman JM, Ballou LM, Cohen IS, Lin RZ. A transgenic mouse model of heart failure using inducible Galpha q. J Biol Chem. 2005;280:40337–40346. doi: 10.1074/jbc.M506810200. [DOI] [PubMed] [Google Scholar]
- 38.Syed F, Odley A, Hahn HS, Brunskill EW, Lynch RA, Marreez Y, Sanbe A, Robbins J, Dorn GW., 3rd Physiological growth synergizes with pathological genes in experimental cardiomyopathy. Circ Res. 2004;95:1200–1206. doi: 10.1161/01.RES.0000150366.08972.7f. [DOI] [PubMed] [Google Scholar]
- 39.Jiang YP, Ballou LM, Lu Z, Li W, Kelly DJ, Cohen IS, Lin RZ. Reversible heart failure in G alpha(q) transgenic mice. J Biol Chem. 2006;281:29988–29992. doi: 10.1074/jbc.M604699200. [DOI] [PubMed] [Google Scholar]
- 40.Adams JW, Pagel AL, Means CK, Oksenberg D, Armstrong RC, Brown JH. Cardiomyocyte apoptosis induced by Galphaq signaling is mediated by permeability transition pore formation and activation of the mitochondrial death pathway. Circ Res. 2000;87:1180–1187. doi: 10.1161/01.res.87.12.1180. [DOI] [PubMed] [Google Scholar]
- 41.Howes AL, Arthur JF, Zhang T, Miyamoto S, Adams JW, Dorn GW., 2nd Akt-mediated cardiomyocyte survival pathways are compromised by G alpha q-induced phosphoinositide 4,5-bisphosphate depletion. J Biol Chem. 2003;278:40343–40351. doi: 10.1074/jbc.M305964200. [DOI] [PubMed] [Google Scholar]
- 42.Dugourd C, Gervais M, Corvol P, Monnot C. Akt is a major downstream target of PI3-kinase involved in angiotensin II-induced proliferation. Hypertension. 2003;41:882–890. doi: 10.1161/01.HYP.0000060821.62417.35. [DOI] [PubMed] [Google Scholar]
- 43.Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Galphaq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci U S A. 1998;95:13893–13898. doi: 10.1073/pnas.95.23.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, Communal C, Singh K, Colucci W, Bristow MR, Port DJ. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32:817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 45.Aoyagi T, Koshimizu TA, Tanoue A. Vasopressin regulation of blood pressure and volume: findings from V1a receptor-deficient mice. Kidney Int. 2009;76:1035–1039. doi: 10.1038/ki.2009.319. [DOI] [PubMed] [Google Scholar]
- 46.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.