Abstract
Objectives
Relative importance of multiple indoor and outdoor venues on personal exposure concentrations to pro-carcinogenic polycyclic aromatic hydrocarbons (c-PAHs) remains poorly understood. This is particularly challenging because many c-PAHs share sources and occur as a complex mixture. Accurate and precise apportionment of personal exposure according to exposure venues could aid in understanding of human health effects due to given source. Here, we partitioned indoor and personal exposure concentrations to seven c-PAHs and pyrene according to the indoor- and outdoor- origins.
Methods
A simultaneous, integrated monitoring of personal, indoor and outdoor concentrations of nine PAHs was conducted in 75 homes for a consecutive 48-hour period across a two-year period in Kraków, Poland. Due to few known indoor sources for chrysene, we used this PAH species as a tracer for infiltration of outdoor PAHs. Personal and indoor concentrations of seven c-PAHs and pyrene were apportioned to home indoor, non-home indoor and outdoor origin.
Results
Using Chrysenein / Chryseneout as proxy for an infiltration factor, Finf, infiltrated PAHs of outdoor origin are overall higher in concentration than those emitted from the indoor origin. Average contribution by the outdoor sources on B[a]A, B[b]F, and B[k]F were 92%, 79%, and 78% across all seasons. In contrast, in homes where a household members smoked, average contribution by the outdoor sources on B[ghi]P, B[a]P, D[ah]A, and IP were lower (i.e., 67%, 65%, 67%, and 66%, respectively). Season-averaged contribution by the outdoor sources on personal exposure to B[a]A, B[b]F, and B[k]F were 92%, 74%, and 77%, respectively. On the other hand, season-averaged home indoor source contribution on personal exposure to B[a]A, B[b]F, and B[k]F were estimated at 6%, 15%, and 19%, respectively. Similar contributions by season-averaged home indoor sources on personal exposure were estimated at 28% for B[ghi]P, 31% for B[a]P, 25% for D[ah]A, and 28% for IP.
Conclusion
Of the seven c-PAHs, B[a]A, B[b]F, and B[k]F are enriched in indoor and personal exposure concentrations from the outdoor coal-combustion. B[ghi]P, B[a]P, D[a,h]A, and IP, PAHs with some of the highest carcinogenic and mutagenic potencies, are considerably enriched by cigarette smoke in addition to the outdoor sources.
Keywords: secondhand smoke, tobacco, polycyclic aromatic hydrocarbons, coal combustion, indoor pollution, benzo[a]pyrene
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) constitute particle-bound or gaseous component of indoor smoke with carcinogenic and a number of other effects on human health (WHO 2000a; WHO 2000b). Yet, understanding the health risks from indoor exposure to airborne PAHs face a set of unique challenges. Such challenges include scarcity of information regarding the indoor and outdoor sources as well as the conditions which mediate human exposure (e.g. fuel type, housing age, housing structural material, ventilation quality, and food preparation method) (Smith and Mehta 2003). Specifically, variable rates of environmental photo-degradation of the PAHs, human behavior (e.g., cooking, cigarette smoking, cleaning), weather conditions (e.g., temperature, relative humidity, wind speed), characteristics of the residential building (Spengler et al., 1996), and indoor environment conditions (e.g., ventilation, temperature, ultraviolet ray, humidity) could modify the primarily generated PAHs through removal or secondary generation (Schauer et al., 2003). Apart from such chemical interactions, the toxicity of PAH mixture within in vitro models has been shown to be synergistic or antagonistic compared to those of component individual compounds (Tarantini et al., 2011).
To date, the extent to which multiple indoor and outdoor sources contribute to personal PAH exposure concentration remains very poorly understood. Popular approach to estimating personal exposure includes using centrally located ambient monitor. One of the essential assumptions of such approach is that the ambient pollutant concentration is highly correlated with outdoor-originating portion of the personal exposure (Wallace and Williams 2005). Implicitly, such assumption postulates that personal exposure to outdoor originating PAHs significantly contributes to health outcomes. However, as adults spend an estimated 80 to ≥ 90% of daily hours within indoor microenvironments (Brunekreef et al., 2005; Samet and Spengler 2003), health consequences from personal exposure to indoor-originating PAHs may often be underestimated. For example, the PAH mixture emitted from cigarette smoke is estimated to possess higher carcinogenic potencies compared to that emitted from diesel engine exhaust (Valberg and Watson 1999). Furthermore, reliance on coal-burning for home heating during early life is associated with impaired skeletal growth at 36th month of age (Ghosh et al., 2011).
Based on such evidence, estimating personal exposure contribution by outdoor- originating PAHs represents an important research need. In this investigation, we follow our previous inquiry (Choi et al., 2008a), to quantify the extent to which indoor and outdoor sources influence the indoor and personal exposure concentrations of eight PAHs. Earlier source apportionment and economic analyses have shown that airborne PAHs in Krakow are predominantly generated from coal-burning in low-efficiency residential stoves and boilers (Junninen et al., 2009; Lvovsky et al., 2000). In addition, we showed that pregnant women are exposed to a sharp seasonal trend in infiltrated PAHs (Choi et al., 2008a). Based our earlier recognition of outdoor-originating PAHs and secondhand cigarette smoke as two important sources (Choi et al., 2008a), here, we quantify relative contributions of such sources on personal exposure.
We conducted such analyses by adapting the Sulfur tracer method (Sarnat et al., 2009; Wallace and Williams 2005) and enrichment factor to meet following specific aims: 1) partition the indoor PAH concentration according to the indoor and outdoor sources; 2) estimate the contribution of the outdoor versus indoor-based PAHs in personal exposure concentration; 3) explore the utility of enrichment factor as predictive marker of the proximity effect in personal exposure cloud.
METHODS
Details on the subject enrollment and air monitoring methods have been published (Choi et al., 2006; Jedrychowski et al., 2004; Jedrychowski et al., 2006) and briefly summarized below.
Study Site Characterization
The city of Kraków in south of Poland represents one of the areas in Europe with historically intensive coal-burning power generation (Junninen et al., 2009). Additional sources of local air pollution include commercial activities with high automobile traffic within the Kraków city center , and coal–burning for home heating (Junninen et al., 2009). Women in the cohort study live in the urbanized area of Kraków (Jedrychowski et al., 2004). The easternmost district of Kraków, Nowa Huta, encompasses several steel mills, including an iron ore sinter plant, blast furnace, coke, gas and coal combustion power plant, natural gas–fired steel production plant, and an oxygen furnace steel plant. The same district also contains a coal– fired cement kiln, and a coal–fired power plant (Junninen et al., 2009).
Subject enrollment
Briefly, we recruited young (age 18–35), non–smoking pregnant women with no known pre–existing risks of adverse birth outcomes from the prenatal care clinics throughout the seasons (23% December–February, 27% March–May, 27% June–August, and 24% September–November) in Kraków (n = 344). During the late 2nd trimester, a research questionnaire was administered and collected information on demographic and socioeconomic status as well as description of the surrounding outdoor environment. The questionnaire inquired about indoor features, active and passive smoking, dietary intake of PAH–containing foods, as well as other daily activities. Passive smoking was self-reported in terms of duration (hours/day) and intensity (number smoked/day). The institutional review board of Columbia Presbyterian Medical Center approved the study, and informed consent was obtained from all study participants.
Indoor and Outdoor Air Monitoring
As described elsewhere (Choi et al., 2008a; Choi et al., 2008b), we conducted the home indoor and the home outdoor PAH monitoring simultaneously for a 48–hour period using two identical monitors. Briefly, we fitted a backpack with the URG– 2000–25 Personal Air Sampler (URG, Chapel Hill, NC, USA). The impactor inlet was fastened to the top of the shoulder strap, to collect air sample near the woman’s breathing zone. For the indoor measurement, we placed an identical backpack in a room where the woman spent most of her time at home (i.e. living room, bedroom or near the kitchen). The sampler was placed atop furniture 0.5–2 m above the floor away from the heating source or the window. For the outdoor measurement, we secured an identical monitor at a window height usually in the balcony, about one meter away from the wall of the home or the apartment. The sampling pump (BGI, Waltham, MA, USA) with the split flow inlet drew in the air continuously at 2 liters per minute. The pump flow was split two ways to simultaneously collect particles ≤ 2.5 μm in aerodynamic diameter (PM2.5) and PAHs. PAHs were collected on a quartz microfiber filter (Palliflex Tissuquartz 2500 QAS, 25 mm in diameter) and semi–volatile vapors and aerosols were collected on a polyurethane foam (PUF) plug backup (Kinney et al., 2002). Personal air monitoring data were given a Quality Assurance (QA) score (0–3) for flow rate, flow time, and completeness of documentation (Kinney et al., 2002). Most samples were shipped to the laboratory within 60 days of sample collection, and were extracted within 14 days after arrival.
Statistical Analyses
We limited our analysis to the indoor and outdoor air samples with a high/good quality assurance (QA) score (0 or 1), which resulted in 76 (100%) personal, 76 (97%) indoor, and 76 (91%) outdoor samples. The indoor/outdoor ratios of the nine PAHs were calculated for 75 households with simultaneous measurements. The indoor and outdoor exposure levels of nine individual PAHs were skewed (all p-values for Kolmogorov–Smirnov test < 0.001). After natural log (ln) transformation, the distribution of the indoor and outdoor measurements conformed to normal distribution. There were no PAH concentrations below the detection limit. Season of PAH morning is defined as summer (June—August), transition (March—June and September—November), and winter (December –February).
In our earlier analyses, an indicator of secondhand smoke at home was the only significant indoor source of personal exposure (Choi et al., 2008a). We also validated secondhand smoke exposure using cotinine (Choi et al., 2006). That is, both maternal and newborn levels were within the range expected for the secondhand smoke exposure (Choi et al., 2006).
Here, we analyzed secondhand smoke as hours/day of exposure as well as the number smoked in presence of the pregnant women.
Contribution of indoor PAHs by Indoor and Outdoor Sources
The indoor concentrations of the eight PAHs were estimated by adapting a sulfur tracer method (Sarnat et al., 2002; Sarnat et al., 2009; Wallace and Williams 2005). Since we did not measure the factors such as particle infiltration, exfiltration, deposition, air exchange rate, the corresponding gas-particle partition kinetics, particle penetration, particle deposition, we investigated chrysene as a tracer PAH compound to estimate infiltration factor (Finf). Robust body of earlier investigations showed that a simplified version of Finf and the associated uncertainty can be estimated for each home under three assumptions (Sarnat et al., 2002; Sarnat et al., 2009; Wallace and Williams 2005). These include 1) absence of indoor source; 2) equal infiltration rate of the nine PAHs and PM2.5; 3) all homes in this study as a well-mixed zone (Sarnat et al., 2002; Sarnat et al., 2009; Wallace and Williams 2005). The first assumption was tested by examining the distribution of Chrysenein / Chryseneout according to possible indoor sources (i.e., candle- and incense- burning, and secondhand smoke exposure) and season (Table S1). Among the 75 homes, secondhand smoke was a possible source of chrysene for two homes (i.e. ratio >1). However, other building characteristics (i.e. apartment height and city heating), seasonal factors (i.e. indoor heating degree days (HDD)) and mean wind speed in city’s commercial hub), or proximal outdoor sources (i.e. car repair shop, dry-cleaning shop, photo developing booth, industrial plant, bus depot, street intersection, incinerator, or gas station), home location within city’s commercial hub, and outdoor diesel-vehicle traffic intensity) were not associated with a linear trend in the same ratio using an ordinary least squares regression model. HDD refers to mean ambient temperature (18 °C) under which household heating is required for thermal comfort, and was calculated by subtracting averaged ambient temperature of given day from 18 °C (Silverberg et al., 2013). Regarding our second assumption of equal infiltration rate of the nine PAHs and PM2.5, our earlier analysis showed that PM2.5 is likely to be a carrier for chrysene (Pearson’s rho correlation coefficient > 99%) (Jedrychowski et al., 2007). Furthermore, high mutual correlations of the eight c-PAHs (all Pearson’s rho correlation coefficient > 97%) suggest that the remaining eight particle-bound PAHs in this analysis are also carried by PM2.5 (Choi et al., 2008a). Our third assumption of the homes as a single well-mixed zone is reasonable based on relatively small size of most homes (88% of women live in a flat with ≤ 3 rooms, excluding the kitchen and the bathroom. In addition, we expect the indoor air to mix during our 48-hour monitoring period.
Based on our observation, secondhand cigarette smoke is the only known indoor sources for chrysene (Figure 1). Thus, within non-smoking households (n = 46), chemical mass balanced Finf is simplified to
| (1) |
in which Chrysenein and Chryseneout are indoor and outdoor concentrations. We assumed that the Chrysenein / Chryseneout ratio reflects infiltration of particle bound-PAHs similar to the behavior of chrysene-bound PAHs, regarding penetration and deposition (Sarnat et al., 2009). Our study is not designed to assess of the penetration and deposition rates of particulate matter with aerodynamic diameter < 2.5 μm, and such information is not available to us. However, we simultaneously measured eight particle-bound PAHs and PM2.5 in all of our air samples. Mutual correlations among the PAHs and PM2.5 were ranged 0.97—0.99, supporting the validity of chrysene ratio as Finf marker.
Figure 1.
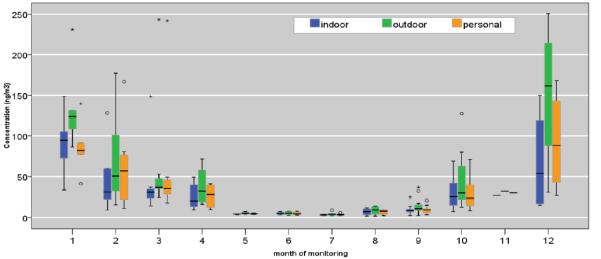
Distribution of personal, indoor, and outdoor monitored levels of Σ8 c-PAHs (ng/m3). Boxes show 25th, 50th and 75th percentile; the whiskers show 5th and the 95th percentiles. The symbol (ο) represents measurements that are > 1.5- fold of the interquartile range.
Since indoor concentration of PAHs (PAHIN) can have both indoor and outdoor sources,
| (2) |
PAHIS represents the indoor-generated and PAHOS the infiltrated concentration. The Chrysenein / Chryseneout was then used to calculate PAHOS as follows:
| (3) |
Where PAHout is the outdoor concentration of PAH species X. PAHIS was then determined as the difference between PAH in and PAHOS, according to eq. (2).
In addition, we calculated enrichment factor (EFx) as a surrogate for indoor generated PAHs (Habre et al., 2013). EF is estimated by standardizing the PAH I/O ratios to Chrysene I/O ratio. Thus, a median EF > 1 emphasizes species with indoor concentrations beyond those expected from outdoor infiltration only.
| (4) |
Estimation of Personal Exposure Concentration from Indoor and Outdoor Sources
Personal exposure E is the sum of exposure from outdoor and non-outdoor sources
| (5) |
In which Eo and Eno represent exposure from outdoor and nonoutdoor sources, respectively. Nonoutdoor sources include those within home, work, commute, as well “personal cloud”. Wallace and Williams (2005) populate that personal exposure represents a sum of Eno and a product term of personal outdoor exposure factor (Fpex) and outdoor concentration (PAHout):
| (6) |
Wallace and Williams (2005) postulate that under the assumption of no nonoutdoor sources of Chrysene other than cigarette smoke, Fpex can be simplified as
| (7) |
The term, Fpex×PAHout, represents personal exposure to outdoor-originating PAH in indoor or outdoor setting. Eno is obtained by subtracting (Fpex×PAHout) from E. We postulated that Eno is a linear function of following two terms,
| (8) |
The personal exposure at home, fhome(PAHIS × EFx), is a product of fhome, daily home hour fraction, EFx, the enrichment factor from home indoor sources, and PAHIS, the PAH concentration from the indoor sources. We calculated, fhome, was calculated as (24 hrs – (summed daily hours at work, commute, and other non-specified microenvironments))/24 hrs. The term, fnon-home was calculated as 1 – fhome. The “other” exposure includes those at work, commute, as well as the proximity effect within the indoor setting (McBride et al., 1999). We conducted the statistical analyses in SAS version 9.1 (SAS Institute Inc., Cary, NC, USA), and figures were created using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The majority (79%) of the 75 women continued to work during pregnancy, in which all of the reported occupations were those within the indoor setting. On average, the women spent 5± 3 hrs/day at work, 1±0 hrs/day in commute, and 9±4 hrs/day in total time outside of home. We estimated total time at home as 24 hrs – (Hrs at work, commute, and other non-specified microenvironments). We used an estimated value (15 ± 3 hrs/day) rather than self-reported total time at home (14 ± 4 hrs/day) in all of our subsequent analyses. The estimated information was more complete and highly correlated with self-reported total time at home (intraclass correlation coefficient, 0.98, 95% CI, 0.98—0.99). The estimated time at home and non-home setting respectively corresponded to 61 ± 15 % (range, 33–92 %), and 39 ± 15 % (range, 8–67 %).
No one owned an air-conditioner, and almost all women (99 %) kept the windows open at all times during warmer months. Consistent with our earlier analysis (Choi et al., 2008a), building structural factors (e.g., single family vs. other types; communal kitchen use within school dormitories), fuel usage (e.g. coal- or wood-burning stove in the basement; gas, electric, or city central heating), and other occupant behavioral traits within their home (e.g., keeping the windows open; routinely using an exhaust fan during food preparation) were not associated with marked difference in the indoor concentrations or PE to individual PAHs. Other outdoor factors such as living in the commercial center, vertical height of the apartment, home adjacency to an industrial plant; a bus depot, cross-road; the subject’s job type or the commute duration were not associated with a personal, indoor, or the outdoor Σ8 c-PAH levels (all P’s > 0.05). On the other hand, self-reported intensity of bus and truck traffic outside of the home or apartment where the woman spent most time was weakly associated with an elevated concentration of all nine PAHs.
Figure 1 demonstrates the distribution of concurrent personal, indoor and outdoor measurements. Considering the indoor measurement as a proxy for personal exposure, the difference in [personal – indoor] concentrations is associated with an interquartile range, −0.42 to 1.18 ng/m3 during April – September (i.e. non-heating season), and −0.30 to 12.47 ng/m3 during October – March period (i.e. heating season). During the same season, the median [personal – indoor] difference of 5.42 ng/m3 suggests that the indoor measurement underestimates the personal exposure. On the other hand, [personal – outdoor] concentration differences is associated with the interquartile range of −5.36 to 0.07 ng/m3 during April – September, and −39.44 to −4.06 ng/m3 during October – March period (Figure 1, Supporting Documents). Similarly, the median [personal – outdoor] concentration differences is −9.57 ng/m3 respectively, indicating that for half of the subset the outdoor concentration overestimates the personal exposure by ≥ 10 ng/m3. Regardless of the season, [personal – indoor] concentration difference was always less than [personal – outdoor] concentration differences (Figure S1, Supporting Documents).
Among the secondhand smoke exposed women, the duration of exposure (hrs/day) was significantly correlated with the number of cigarettes smoked in the women’s presence (Table 1). Within the subset, those who reported ≥ 5 hrs/day of cigarette smoke exposure (n = 4) reported a mean of 24 cigarettes/day smoked in their presence (ASD, 11, range, 15 – 40). We used daily number of cigarette exposure as a marker of ETS, because this represented a finer scale of exposure than hourly exposure as the marker. Within the households with ≥5 daily hours of SHS, indoor Σ 8 c-PAH concentration was 24 ng/m3 higher than the outdoor concentration (95% CI, −50.92 to 98.75 ng/m3). This difference contrasts to the non-smoking households, for whom the [indoor – outdoor] concentration difference of Σ 8 c-PAHs was 13.38 ng/m3 (95% CI, 7.85 – 18.91 ng/m3).
Table 1.
Concentration Ratios of PAHs (ng/m3) in concurrent personal-indoor-outdoor samples (n=75). I/O, P/O, and P/I denote indoor/outdoor, personal/outdoor, and personal/indoor concentration ratios, respectively.
| Mean | SD | Minimum | 5th | 25th | Median | 75th | 95th | Maximum | |
|---|---|---|---|---|---|---|---|---|---|
| B[a]A I/O | 0.71 | 0.29 | 0.07 | 0.32 | 0.51 | 0.71 | 0.87 | 1.10 | 2.08 |
| B[a]A P/O | 0.77 | 0.29 | 0.28 | 0.32 | 0.56 | 0.78 | 0.96 | 1.24 | 1.65 |
| B[a]A P/I | 1.19 | 0.75 | 0.54 | 0.62 | 0.97 | 1.09 | 1.25 | 1.80 | 7.16 |
| B[b]F I/O | 0.70 | 0.23 | 0.10 | 0.43 | 0.57 | 0.67 | 0.84 | 1.01 | 1.85 |
| B[b]F P/O | 0.76 | 0.23 | 0.33 | 0.41 | 0.64 | 0.72 | 0.86 | 1.24 | 1.67 |
| B[b]F P/I | 1.19 | 0.79 | 0.50 | 0.66 | 0.95 | 1.06 | 1.28 | 1.72 | 7.46 |
| B[k]F I/O | 0.84 | 0.66 | 0.10 | 0.40 | 0.60 | 0.67 | 0.89 | 1.84 | 5.44 |
| B[k]F P/O | 0.88 | 0.55 | 0.40 | 0.42 | 0.63 | 0.77 | 0.95 | 1.71 | 4.17 |
| B[k]F P/I | 1.20 | 0.79 | 0.39 | 0.67 | 0.90 | 1.05 | 1.30 | 2.01 | 7.06 |
| B[ghi]P I/O | 0.86 | 0.31 | 0.10 | 0.53 | 0.72 | 0.79 | 0.91 | 1.24 | 2.53 |
| B[ghi]P P/O | 0.95 | 0.30 | 0.33 | 0.54 | 0.79 | 0.91 | 1.04 | 1.41 | 2.26 |
| B[ghi]P P/I | 1.22 | 0.82 | 0.43 | 0.72 | 0.97 | 1.11 | 1.31 | 1.79 | 7.88 |
| B[a]P I/O | 0.89 | 0.36 | 0.08 | 0.51 | 0.74 | 0.85 | 0.93 | 1.46 | 3.01 |
| B[a]P P/O | 0.97 | 0.37 | 0.35 | 0.48 | 0.76 | 0.92 | 1.09 | 1.98 | 2.32 |
| B[a]P P/I | 1.22 | 1.01 | 0.44 | 0.64 | 0.93 | 1.08 | 1.31 | 1.73 | 9.47 |
| Chrysene I/O | 0.59 | 0.27 | 0.07 | 0.30 | 0.45 | 0.54 | 0.70 | 0.91 | 2.24 |
| Chrysene P/O | 0.64 | 0.24 | 0.23 | 0.30 | 0.51 | 0.61 | 0.75 | 1.08 | 1.61 |
| Chrysene P/I | 1.21 | 0.76 | 0.48 | 0.61 | 0.95 | 1.10 | 1.34 | 1.91 | 7.13 |
| IP I/O | 0.85 | 0.31 | 0.09 | 0.50 | 0.71 | 0.80 | 0.96 | 1.23 | 2.71 |
| IP P/O | 0.92 | 0.28 | 0.34 | 0.56 | 0.74 | 0.89 | 1.02 | 1.44 | 2.21 |
| IP P/I | 1.19 | 0.83 | 0.46 | 0.68 | 0.96 | 1.12 | 1.25 | 1.58 | 8.00 |
| D[ah]A I/O | 0.85 | 0.31 | 0.08 | 0.44 | 0.71 | 0.81 | 1.00 | 1.39 | 2.18 |
| D[ah]A P/O | 0.89 | 0.31 | 0.28 | 0.44 | 0.72 | 0.87 | 1.00 | 1.36 | 2.48 |
| D[ah]A P/I | 1.19 | 1.08 | 0.33 | 0.59 | 0.93 | 1.02 | 1.23 | 1.64 | 10.02 |
| Pyrene I/O | 0.56 | 0.23 | 0.08 | 0.25 | 0.41 | 0.54 | 0.67 | 1.07 | 1.20 |
| Pyrene P/O | 0.78 | 1.04 | 0.24 | 0.28 | 0.44 | 0.58 | 0.79 | 1.36 | 9.02 |
| Pyrene P/I | 1.45 | 1.76 | 0.57 | 0.72 | 0.97 | 1.14 | 1.31 | 2.67 | 14.72 |
Table 2 shows the distributions of indoor/outdoor, personal/outdoor, and personal/indoor concentrations of all available concurrently monitored samples. Absence of indoor source for chrysene is shown by a small number of I/O values > 1. When we further stratified the I/O concentration distribution according to the indoor source, building characteristics, seasonal contributions, SHS was the only significant indoor source for the eight c-PAHs (p-value for nine Mann-Whitney’s U tests < 0.05).
Table 2.
Distribution of Chrysene IN/Chrysene OUT Among Non-Smokers.
| SHS exposure at home | N | Mean | S.D. | Minimum | Median | Maximum | |
|---|---|---|---|---|---|---|---|
| non-smoker homes | summer | 13 | 0.72 | 0.14 | 0.40 | 0.73 | 0.93 |
| transition | 28 | 0.51 | 0.16 | 0.28 | 0.46 | 0.82 | |
| winter | 12 | 0.45 | 0.13 | 0.07 | 0.47 | 0.58 | |
| 1-9 cigarette/day | summer | 4 | 0.72 | 0.11 | 0.59 | 0.72 | 0.85 |
| transition | 8 | 0.60 | 0.25 | 0.40 | 0.54 | 1.19 | |
| winter | 3 | 0.39 | 0.09 | 0.30 | 0.39 | 0.48 | |
| 10-40 cigarette/day | summer | 1 | 2.24 | 2.24 | 2.24 | 2.24 | |
| transition | 5 | 0.58 | 0.12 | 0.45 | 0.64 | 0.70 | |
| winter | 1 | 0.91 | 0.91 | 0.91 | 0.91 | ||
|
| |||||||
| Overall | summer | 18 | 0.81 | 0.38 | 0.40 | 0.73 | 2.24 |
| transition | 41 | 0.54 | 0.17 | 0.28 | 0.48 | 1.19 | |
| winter | 16 | 0.47 | 0.17 | 0.07 | 0.47 | 0.91 | |
I. Chrysene IN/Chrysene OUT as tracer of FINF
As shown in Figure 2 and Table 3, self-reported SHS is not associated with I/O values > 1 in all homes, except for one outlier and one extreme value homes. For example, during the transition season, the median Chrysenein / Chryseneout ratios were 0.46, 0.54, and 0.64 for non-smoker, low SHS exposure group, and high SHS exposure group, respectively. During the transition season, 5th and 95th percentile values of Chrysenein / Chrysene out ratios remained < 1, except for one outlier value (Figure 3). Furthermore, we regressed the outdoor concentration of Chrysene on the indoor concentration of the same compound among non-smoking households (n=53). One ln-unit increase in the outdoor concentration was associated with 83% ± 4% increase in corresponding indoor concentration (p-value < 0.001). The negative value of y-intercept (–47 ± 6%) suggests absence of indoor source for chrysene in non-smoking households.
Figure 2.
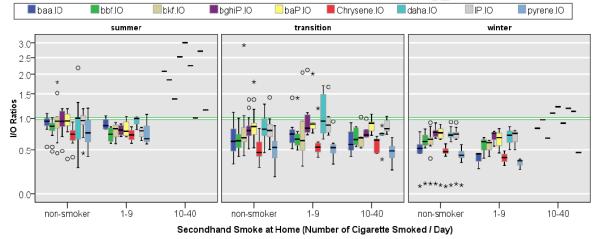
Indoor / Outdoor PAH Concentration Ratios. Box show 25th, 50th, and 75th percentile; the whiskers show 5th and the 95th percentiles. The symbols, Ο and *, represent measurements that are >1.5– and >3–fold of the interquartile range.
Table 3.
Distribution of indoor concentrations of PAHs (ng/m3) from indoor and outdoor sources using Chrysene I/O ratio as a tracer.
| Summer (N=18) | Transition (N=41) | winter (N=16) | Total (N=75) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Indoor | Indoor | Indoor | Indoor | |||||||||||||
| Concentration | Concentration | Concentration | Concentration | |||||||||||||
| from | from | from | from | from | from | from | from | |||||||||
| Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | |||||||||
| Sources | Sources | Sources | Sources | Sources | Sources | Sources | Sources | |||||||||
| (ng/m3) | ||||||||||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| B[a]A | 0.51 | 0.07 | 0.12 | 0.02 | 2.77 | 0.63 | 0.33 | 0.09 | 10.12 | 1.91 | 0.56 | 0.51 | 3.80 | 0.66 | 0.33 | 0.12 |
| B[b]F | 1.06 | 0.15 | 0.11 | 0.06 | 3.72 | 0.51 | 0.95 | 0.18 | 11.25 | 2.07 | 3.34 | 0.65 | 4.69 | 0.66 | 1.26 | 0.21 |
| B[k]F | 0.36 | 0.05 | 0.06 | 0.03 | 1.07 | 0.16 | 0.38 | 0.06 | 3.50 | 0.66 | 0.91 | 0.37 | 1.42 | 0.21 | 0.41 | 0.09 |
| B[ghi]P | 0.60 | 0.06 | 0.16 | 0.03 | 1.96 | 0.34 | 1.11 | 0.20 | 4.78 | 0.98 | 2.23 | 0.34 | 2.24 | 0.32 | 1.12 | 0.15 |
| B[a]P | 0.46 | 0.07 | 0.10 | 0.02 | 2.04 | 0.36 | 1.37 | 0.26 | 7.16 | 1.46 | 3.30 | 0.59 | 2.75 | 0.46 | 1.48 | 0.23 |
| D[ah]A | 0.14 | 0.02 | 0.03 | 0.02 | 0.47 | 0.09 | 0.26 | 0.06 | 1.22 | 0.25 | 0.57 | 0.09 | 0.55 | 0.09 | 0.27 | 0.04 |
| IP | 0.70 | 0.08 | 0.16 | 0.04 | 2.47 | 0.40 | 1.62 | 0.46 | 6.23 | 1.25 | 2.72 | 0.38 | 2.85 | 0.40 | 1.50 | 0.28 |
| pyrene | 1.99 | 0.19 | −0.03 | 0.13 | 4.92 | 0.90 | −0.32 | 0.22 | 15.70 | 3.33 | −3.05 | 1.22 | 6.52 | 1.02 | −0.83 | 0.31 |
Figure 3.
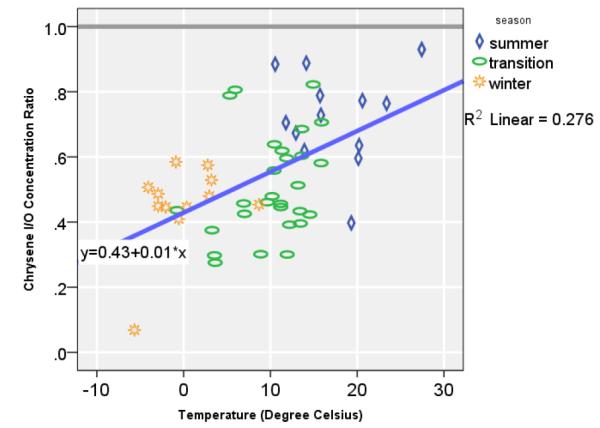
Karakow ambient Mean Temperature (°C) and Chrysene I/O Ratio among Non-Smoker Households (n=53).
The Chrysenein / Chryseneout demonstrated a strong co-linearity with the seasons, in which the ratio was lowest in the winter (median, 0.47), followed by the transitional season (median, 0.48), and summer (median, 0.73) (Figure 3). Based on a linear regression, one degree Celsius increase in heating demand was associated with 1.3% lower indoor chrysene concentration relative to the outdoor concentration (95% CI, 0.6—2%) in non-smoker households (R2 = 0.237, n=41). For example, at 0 °C, chrysene indoor concentration was 0.43 of the corresponding outdoor concentration (95% CI, 0.38—0.56; P< 0.001). To preclude any contribution of SHS in the indoor concentration of Chrysene, we considered the Chrysenein / Chryseneout values of the non-smoking households only in all subsequent analysis.
II. Indoor and Outdoor Source Contribution on the Indoor Concentration
The contribution of indoor PAH concentrations by the indoor and outdoor sources are shown for eight PAH species according to the season (Table 3). Season-averaged indoor concentrations of the PAH species, according to their indoor and outdoor origins is shown in Figure 4. For the seven c-PAHs and pyrene, infiltrated PAHs of outdoor origin are overall higher in concentration than those emitted from the indoor origin. In particular, infiltrated concentration of the outdoor origin were of predominant importance for B[a]A, B[b]F, and B[k]F. That is, average contribution by the outdoor sources on B[a]A, B[b]F, and B[k]F were 92%, 79%, and 78% across all seasons. The infiltrated concentrations from the outdoor sources were 3 to 12-times greater than that from the indoor source. In contrast, the indoor concentrations of B[ghi]P, B[a]P, D[ah]A, and IP were contributed by both indoor and outdoor sources. Average contribution by the outdoor sources on B[ghi]P, B[a]P, D[ah]A, and IP were 67%, 65%, 67%, and 66%. For these four c-PAHs, the infiltrated concentrations were, on the average, twice that of the indoor-generated PAHs.
Figure 4.
Chrysene I/O Ratios.
III. Contribution of Personal Exposure Concentration by Indoor and Outdoor Sources
Table 4 shows the personal exposure concentrations of the seven c-PAHs and pyrene by outdoor, home indoor, and other microenvironmental sources according to seasons. As shown in Figure 6, season-averaged contributions by the outdoor sources were markedly larger than those by the home or other microenvironmental contributions. Season-averaged outdoor source contribution on the personal exposure concentrations to B[a]A, B[b]F, and B[k]F were 92%, 74%, and 77%, respectively. During the summer, the outdoor contribution on the personal exposure to B[a]A, B[b]F, and B[k]F were 73%, 105%, and 119% respectively. During the transition season, the outdoor contribution on the personal exposure ranged between 90—95% for B[a]A, B[b]F, and B[k]F.
Table 4.
Contributions of Personal exposure concentration to seven c-PAHs and pyrene by outdoor, home indoor and other microenvironmental sources.
| Summer (n=18) |
Transition (n=41) |
Winter (n=16) |
Overall (n=75) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| B[a]A | Outdoor sources | 0.52 | 0.06 | 3.40 | 1.05 | 11.51 | 1.88 | 4.44 | 0.83 |
| Home indoor sources | 0.10 | 0.02 | 0.31 | 0.07 | 0.41 | 0.35 | 0.28 | 0.08 | |
| Other indoor sources | 0.09 | 0.03 | −0.12 | 0.19 | 0.64 | 0.90 | 0.09 | 0.22 | |
| B[b]F | Outdoor sources | 1.10 | 0.14 | 4.27 | 0.75 | 12.90 | 2.01 | 5.35 | 0.76 |
| Home indoor sources | 0.08 | 0.04 | 0.80 | 0.18 | 2.76 | 0.57 | 1.05 | 0.19 | |
| Other indoor sources | −0.13 | 0.10 | −0.33 | 0.73 | 4.68 | 3.74 | 0.79 | 0.90 | |
| B[k]F | Outdoor sources | 0.38 | 0.05 | 1.25 | 0.25 | 3.96 | 0.62 | 1.62 | 0.24 |
| Home indoor sources | 0.07 | 0.03 | 0.37 | 0.07 | 0.84 | 0.27 | 0.40 | 0.08 | |
| Other indoor sources | −0.13 | 0.06 | −0.29 | 0.29 | 1.33 | 0.82 | 0.09 | 0.25 | |
| B[ghi]P | Outdoor sources | 0.62 | 0.06 | 2.34 | 0.56 | 5.48 | 0.98 | 2.60 | 0.41 |
| Home indoor sources | 0.13 | 0.03 | 1.09 | 0.18 | 2.06 | 0.30 | 1.07 | 0.14 | |
| Other indoor sources | 0.11 | 0.11 | −0.18 | 0.54 | 1.34 | 2.02 | 0.21 | 0.52 | |
| B[a]P | Outdoor sources | 0.46 | 0.07 | 2.41 | 0.57 | 8.12 | 1.48 | 3.16 | 0.54 |
| Home indoor sources | 0.08 | 0.02 | 1.44 | 0.25 | 2.93 | 0.49 | 1.43 | 0.20 | |
| Other indoor sources | 0.06 | 0.06 | −0.87 | 0.83 | 2.22 | 2.63 | 0.01 | 0.72 | |
| D[ah]A | Outdoor sources | 0.14 | 0.02 | 0.57 | 0.16 | 1.40 | 0.26 | 0.65 | 0.11 |
| Home indoor sources | 0.05 | 0.03 | 0.25 | 0.04 | 0.51 | 0.08 | 0.26 | 0.03 | |
| Other indoor sources | −0.10 | 0.05 | 0.01 | 0.10 | 0.60 | 0.59 | 0.11 | 0.14 | |
| IP | Outdoor sources | 0.72 | 0.08 | 2.91 | 0.62 | 7.17 | 1.29 | 3.29 | 0.50 |
| Home indoor sources | 0.14 | 0.04 | 1.62 | 0.45 | 2.45 | 0.34 | 1.44 | 0.27 | |
| Other indoor sources | 0.00 | 0.10 | 0.08 | 0.70 | 1.72 | 2.32 | 0.41 | 0.62 | |
| pyrene | Outdoor sources | 2.07 | 0.19 | 5.91 | 1.49 | 17.10 | 2.77 | 7.37 | 1.17 |
| Home indoor sources | 0.08 | 0.07 | −0.04 | 0.11 | −1.20 | 0.40 | −0.26 | 0.12 | |
| Other indoor sources | 2.84 | 2.40 | −2.08 | 0.95 | −2.25 | 1.80 | −0.94 | 0.89 | |
Figure 6.
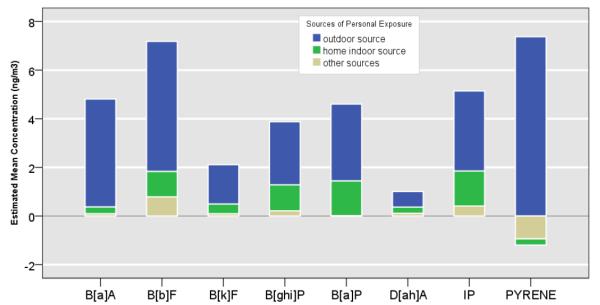
Mean Personal Exposure concentrations of PAHs (ng/m3) shown according to outdoor (blue), home indoor (green) and other microenvironmental (tan) contributions.
On the other hand, season-averaged home indoor source contribution on personal exposure to B[a]A, B[b]F, and B[k]F were estimated at 6%, 15%, and 19%, respectively. Similar contributions by season-averaged home indoor sources on personal exposure were estimated at 28% for B[ghi]P, 31% for B[a]P, 25% for D[ah]A, and 28% for IP. Corresponding contributions by the outdoor sources on the on personal exposure concentrations to B[ghi]P, B[a]P, D[ah]A, and IP were 67%, 69%, 64%, and 64%, respectively.
When the data were stratified according to the season, the outdoor contribution on personal exposure to B[a]A, B[ghi]P, B[a]P, D[ah]A, and IP were 73%, 72%, 77%, and 84%. During the transition season, similar contribution for B[ghi]P, B[a]P, D[ah]A, and IP ranged between 63—81%. During winter, the similar contribution on the personal exposure ranged between 56—63%.
DISCUSSION
Chronic exposure in humans to complex PAH mixture and its effect on human health morbidity and mortality is not well understood (WHO 2010). Source apportionment of airborne PAHs are particularly challenging because generation, persistence, and degradation of PAHs are influenced by multiple source, human behavior, and environmental conditions. Here, we measured and studied the source contributions for eight c-PAHs and pyrene, which are known to be generated by numerous sources, including coal combustion, industrial plant activity, diesel and gasoline combustion (Bostrom et al., 2002), various cooking methods (Gunter et al., 2005; Shen et al., 2008; Wang et al., 1996), and other indoor behavior, such as cleaning and cigarette smoking (Semple et al., 2012). Distinguishing the relative importance of given source on the personal exposure has critical implications on human health outcome assessment and managing risks.
We used Chrysene I/O ratio as a tracer compound for infiltrated portion of the outdoor originating PAHs, based on our earlier observation of Pearson’s rho correlation coefficient > 99% between summed total of eight c-PAHs with PM2.5 (Jedrychowski et al., 2007), as well as similarly high mutual correlations of the eight c-PAHs (all Pearson’s rho correlation coefficient > 97%)(Choi et al., 2008a). Such high correlation supports the use of Chrysene I/O ratio as a marker of infiltration. Also, to preclude the contributions of the indoor sources on the indoor concentration, we restricted the application of Chrysene I/O ratio to the non-smoking households.
Consistent with our earlier observation (Choi et al., 2008a), infiltrated PAHs of outdoor origin constitute predominant contributor to the indoor PAH concentrations. Based on this observation, the present analysis quantifies for the first time, mean contributions by the outdoor- based, home indoor-originating, and other microenvironment-generated PAHs contributions on the personal exposure concentration. In particular, the outdoor source contributions were particularly predominant for the personal and indoor concentrations of B[a]A, B[b]F, and B[k]F. In contrast, home indoor sources contributed an estimated 25—31% for B[ghi]P, B[a]P, D[ah]A, and IP across the seasons.
Such estimates of indoor –sourced PAHs were most highly correlated with cigarette smoke. In particular, contributions of indoor-generated B[ghi]P (34%), B[a]P (48%), D[ah]A (30%), and IP (35%) on the corresponding personal exposures were highest during the transition season, compared to the levels during other seasons. This is consistent with our earlier analysis, which has shown that an hour-unit of SHS exposure is associated with a corresponding increase in personal exposure to B[b]F by 8% (95% CI, 1-15%), 9% for B[ghi]P (95% CI, 1-15 %), and 9% for IP (95% CI, 3-16 %) only during the period of relatively low concentration (April – September) (Choi et al., 2008a).
In the present analyses, the observed I/O ratios for the intensive SHS exposed households during the heating season are considerably lower than the estimated I/O ratios of 5.4 in the smoker’s homes in Germany (Fromme et al., 2001) or I/O ratio of 5.8 in households near an aluminum smelter plant in Canada (Sanderson and Farant 2004). This might be due to > 10-fold increase ambient PAH concentrations in Kraków during the coal-burning season, compared to the summer (Junninen et al., 2009). In urban locations in Germany and rural Canada, sources of ambient PAHs have been noted to be more diverse (Fromme et al., 2001).
Strengths of the present analysis include development of novel and feasible method to attribute relative importance of the outdoor- versus indoor-generated portions of personal exposure concentration. To date, popular approaches for source apportionment of personal exposure concentrations include chemical mass balance models or various matrix factorization analyses (Gokhale et al., 2008; Wei et al., 2010). However, such approaches may suffer from severe limitations in source apportionment of personal exposure to PAHs. First, the underlying assumption of the chemical mass balance models (i.e. sources do not interact to cause mass generation or removal) is likely to be too strong, considering numerous observation of secondary PAH interactions in the literature (Baek et al., 1991; Fromme et al., 1998; Lau et al., 1997; Li and Ro 2000; Lung et al., 2003; Zhu et al., 1997). Second, the approaches mentioned above require comprehensive information on all sources. Third, even when prominent sources and indoor concentrations are measured, unexplained inconsistencies are noted (e.g. ‘personal cloud’ effect). Currently, establishing a simple source marker is problematic for PAHs (Bostrom et al. 2002). Some of the proposed indices, such as molecular diagnostic ratios (MDRs) demonstrated a limited utility due to their temporal and spatial variability (Brown and Brown 2012). In addition, most MDRs proposed in the literature ignore the relative importance of cigarette smoke apart from the ambient sources.
Also, this analysis supports the application of Chrysene I/O ratio as a proxy for infiltration factor, Finf, as well as the enrichment factor as tools to partition relative importance of multiple sources on indoor and personal exposure concentrations of PAHs. Absence of any observable indoor source for Chrysene in our data supports the reliability of Chrysene I/O as an estimate of Finf in each household. Consistent with other investigations using sulfur as a tracer compound (Sarnat et al., 2009), Chrysene I/O ratio was predominantly predicted by Heating Degree Days (i.e. demand for home heating). In addition, to rule out cigarette smoke or other unidentified indoor source(s) for Chrysene, we restricted estimated Finf in non-smoking households only.
At the same time, several limitations of the analyses warrant confirmation of our observation through replication. First, pyrene concentration could not be successfully estimated using chrysene I/O method. Pyrene has greater volatility than other particle-bound PAHs in this analysis. Our analysis demonstrates that Chrysene I/O ratio may not be an appropriate infiltration factor proxy for pyrene. Second, a gold-standard for PAH Finf needs to be developed and by considering, at minimum, infiltration, exfiltration, deposition, and indoor sources for chemical mass-balanced model of the concentration. Third, we could not estimate the source contributions on the indoor and personal exposure to Chrysene. Fourth, we lack the data on exact time-activity diary as well as the venue-apportioned measurement of the personal exposure. Such data would have helped us to estimate the contributions from work and commute setting. Finally, we only have a single Chrysene I/O ratio measurement per household as a proxy for Finf. Considering strong seasonal variability in infiltration efficiency, our application of single Finf might have overestimated the contribution of the outdoor PAHs during the summer.
Present analysis shows a unique exposure experience of the population in Kraków. In our present investigation, we carefully selected healthy, young, and non-smoking women into the study, and validated their self-reported smoking status with cotinine analysis in maternal venous and newborn’s cord blood. Accordingly, secondhand smoke exposure is relatively rare (~20%) in the present cohort. In the general Polish population, approximately 50% of men and 33% of women are estimated to be active smokers (Wojtyla et al., 2012). Such high prevalence of active cigarette smoking indicates that a substantial proportion of the population, including children, newborns, and unborn, are exposed to SHS-emitted PAHs indoors. The species enriched in cigarette smoke – B[ghi]P, B[a]P, D[a,h]A, and IP – are potent pro–carcinogenic PAHs (Valberg and Watson 1999). It should be noted that B[a]P is an established carcinogen (WHO 2000b) with a reference toxic equivalency factor (RTEF) of 1 (Bostrom et al., 2002). In particular, toxic equivalency factor (TEF) of D[ah]A ranges between 0.7– to 5–times that of B[a]P (Bostrom et al., 2002; Valberg and Watson 1999). TEF of B[ghi]P ranges 0.01 to 0.03 times that of B[a]P (Bostrom et al., 2002). The TEF for IP ranges between 0.02 to 0.2 (Bostrom et al., 2002). As a result, estimation of health effects based on routine ambient monitoring of PAHs as proxy measurements of personal exposure might underestimate the true risk from B[ghi]P, B[a]P, D[a,h]A, and IP for SHS exposed individuals. Furthermore, in vitro models suggest that toxicity of co-exposure to multiple PAH compounds depends on constituent PAHs (Binkova et al., 2007; Mahadevan et al., 2005a; Mahadevan et al., 2005b; Staal et al., 2007). For example, the PAH mixture of cigarette smoke is believed to have higher carcinogenic potencies compared to that emitted from diesel engine exhaust (Valberg and Watson 1999). Considering the complexity of exposure scenario and overall high toxicity of many PAH species, indoor and personal monitoring represents appropriate data for an accurate understanding of PAH effects on human health outcomes.
CONCLUSION
In the present cohort, the outdoor-origination portions represent predominant contributors to the indoor and personal exposure concentration of B[a]A, B[b]F, and B[k]F. In contrast, indoor source, namely, cigarette smoke exposure at home, was associated with approximately 30% contribution in the personal exposure to more toxic PAHs, including, B[ghi]P, B[a]P, D[ah]A, and IP.
Supplementary Material
Highlights.
In Krakow, Poland, the outdoor-origination portions represent predominant contributors to the indoor and personal exposure concentration of B[a]A, B[b]F, and B[k]F. In contrast, indoor source, namely, cigarette smoke exposure at home, was associated with approximately 30% contribution in the personal exposure to more toxic PAHs, including, B[ghi]P, B[a]P, D[ah]A, and IP.
Figure 5.
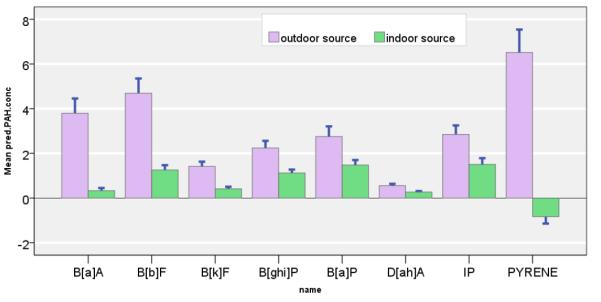
Mean indoor concentrations of PAHs (ng/m3) shown according to indoor (green) and outdoor (lavender) contribution. Bars represent SE of the estimated mean.
ACKNOWLEDGMENT
This work was supported by The National Institute of Environmental Health Sciences (NIEHS) (grant numbers 5 P01 ES009600, R01ES014939, 5 R01 ES008977, 5 R01ES11158, 5 R01ES012468, 5 R01ES10165, and ES00002), the U.S. Environmental Protection Agency (EPA) (grant numbers R827027, 82860901, RD832141), and the National Research Service Award (T32 ES 07069) and The Gladys and Roland Harriman Foundation. We thank Dr. Rima Habre for her helpful suggestions regarding the methods. We thank our colleagues who contributed to data collection and analysis, including Wieslaw Jedrychowski, Dorota Mrozek-Budzyn, Elzbieta Mroz, Elzbieta Flak, Anita Skarupa, Laura Mari Stighter, Jeffrey Jankowski, Maria Butscher, Elzbieta Mroz, Elzbieta Flak, and Irena Kaim. We also thank all mothers-to-be who participated in the present study.
ABBREVIATIONS
- PAHs
Polycyclic aromatic hydrocarbons
- c-PAHs
carcinogenic PAHs
- B[a]A
benz[a]anthracene
- B[a]P
benzo[a]pyrene
- B[b]F
benzo[b]fluoranthene
- B[k]F
benzo[k]fluoranthene
- B[ghi]P
benzo[ghi]perylene
- EF
Enrichment factor
- IP
indeno[123–cd]pyrene
- D[ah]A
dibenz[ah]anthracene
- I/O
Indoor/outdoor
- SHS
Secondhand smoke
- MDRs
Molecular diagnostic ratios
- HDD
Heating Degree Days
- RTEF
reference toxic equivalency factor
- TEF
toxic equivalency factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Baek SO, Field RA, Goldstone ME, Kirk PW, Lester JN, Perry R. A Review Of Atmospheric Polycyclic Aromatic-Hydrocarbons - Sources, Fate And Behavior. Water Air And Soil Pollution. 1991;60:279–300. [Google Scholar]
- Binkova B, Topinka J, Sram RJ, Sevastyanova O, Novakova Z, Schmuczerova J, Kalina I, Popov T, Farmer PB. In vitro genotoxicity of PAH mixtures and organic extract from urban air particles part I: acellular assay. Mutat Res. 2007;620:114–122. doi: 10.1016/j.mrfmmm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Brown RJC. Correlations in polycyclic aromatic hydrocarbon (PAH) concentrations in UK ambient air and implications for source apportionment. Journal of Environmental Monitoring. 2012 doi: 10.1039/c2em10963h. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Janssen NA, de Hartog JJ, Oldenwening M, Meliefste K, Hoek G, Lanki T, Timonen KL, Vallius M, Pekkanen J, Van Grieken R. Personal, indoor, and outdoor exposures to PM2.5 and its components for groups of cardiovascular patients in Amsterdam and Helsinki. Research Report - Health Effects Institute. 2005 1-70; discussion 71-79. [PubMed] [Google Scholar]
- Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, Tsai WY, Perera FP. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Perera F, Pac A, Wang L, Flak E, Mroz E, Jacek R, Chai-Onn T, Jedrychowski W, Masters M, Camann D, Spengler J. Estimating Individual-Level Exposure to Airborne Polycyclic Aromatic Hydrocarbons throughout the Gestational Period based on Personal, Indoor and Outdoor Monitoring. Environ Health Perspect. 2008a;116:1509–1518. doi: 10.1289/ehp.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008b;116:658–665. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Oddoy A, Piloty M, Krause M, Lahrz T. Polycyclic Aromatic Hydrocarbons (PAH) and diesel engine emission (elemental carbon) inside a car and a subway train. The Science of the Total Environment. 1998;217:165–173. doi: 10.1016/s0048-9697(98)00189-2. [DOI] [PubMed] [Google Scholar]
- Fromme H, Otto T, Pilz K. Polycyclic musk fragrances in fish samples from Berlin waterways, Germany. Food Additives & Contaminants. 2001;18:937–944. doi: 10.1080/02652030110063579. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Amirian E, Dostal M, Sram RJ, Hertz-Picciotto I. Indoor coal use and early childhood growth. Archives of Pediatrics & Adolescent Medicine. 2011;165:492–497. doi: 10.1001/archpediatrics.2010.294. [DOI] [PubMed] [Google Scholar]
- Gokhale S, Kohajda T, Schlink U. Source apportionment of human personal exposure to volatile organic compounds in homes, offices and outdoors by chemical mass balance and genetic algorithm receptor models. Science of The Total Environment. 2008;407:122–138. doi: 10.1016/j.scitotenv.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Gunter MJ, Probst-Hensch NM, Cortessis VK, Kulldorff M, Haile RW, Sinha R. Meat intake, cooking-related mutagens and risk of colorectal adenoma in a sigmoidoscopy-based case-control study. Carcinogenesis. 2005;26:637–642. doi: 10.1093/carcin/bgh350. [DOI] [PubMed] [Google Scholar]
- Habre R, Coull B, Moshier E, Godbold J, Grunin A, Nath A, Castro W, Schachter N, Rohr A, Kattan M, Spengler J, Koutrakis P. Sources of indoor air pollution in New York City residences of asthmatic children. J Expos Sci Environ Epidemiol. 2013 doi: 10.1038/jes.2013.74. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, Spengler JD, Camann D, Perera FP. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environ Health Perspect. 2004;112:1398–1402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Pac A, Choi H, Jacek R, Sochacka-Tatara E, Dumyahn T, Spengler J, Camman D, Perera FP. Personal exposure to fine particles and benzo[a]pyrene. Relation with indoor and outdoor concentrations of these pollutants in Krakow. International Journal of Occupational Medicine & Environmental Health. 2007;20:339–348. doi: 10.2478/v10001-007-0035-z. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Pac A, Jacek R, Whyatt RM, Spengler JD, Dumyahn TS, Sochacka-Tatara E. Variability of total exposure to PM2.5 related to indoor and outdoor pollution sources Krakow study in pregnant women. Sci Total Environ. 2006;366:47–54. doi: 10.1016/j.scitotenv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Junninen H, Mønster J, Rey M, Cancelinha J, Douglas K, Duane M, Forcina V, MuÌ^ller A, Lagler F, Marelli L, Borowiak A, Niedzialek J, Paradiz B, Mira-Salama D, Jimenez J, Hansen U, Astorga C, Stanczyk K, Viana M, Querol X, Duvall RM, Norris GA, Tsakovski S, Wåhlin P, Horák J, Larsen BR. Quantifying the Impact of Residential Heating on the Urban Air Quality in a Typical European Coal Combustion Region. Environmental Science & Technology. 2009;43:7964–7970. doi: 10.1021/es8032082. [DOI] [PubMed] [Google Scholar]
- Kinney PL, Chillrud SN, Ramstrom S, Ross J, Spengler JD. Exposures to multiple air toxics in New York City. Environ Health Perspect. 2002;110(Suppl 4):539–546. doi: 10.1289/ehp.02110s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Fiedler H, Hutzinger O, Schwind KH, Hosseinpour J. Levels of selected organic compounds in materials for candle production and human exposure to candle emissions. Chemosphere. 1997;34:1623–1630. doi: 10.1016/s0045-6535(97)00458-x. [DOI] [PubMed] [Google Scholar]
- Li CS, Ro YS. Indoor characteristics of polycyclic aromatic hydrocarbons in the urban atmosphere of Taipei. Atmospheric Environment. 2000;34:611–620. [Google Scholar]
- Lung SC, Kao MC, Hu SC. Contribution of incense burning to indoor PM10 and particle-bound polycyclic aromatic hydrocarbons under two ventilation conditions. Indoor Air. 2003;13:194–199. doi: 10.1034/j.1600-0668.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- Lvovsky K, Hughes G, Maddison D, Ostro B, Pearce D. Pollution Management Series. The World Bank Environment Department; Washington, D.C.: 2000. Environmental Costs of Fossil Fuels: A rapid assessment method with application to six cities. [Google Scholar]
- Mahadevan B, Keshava C, Musafia-Jeknic T, Pecaj A, Weston A, Baird WM. Altered gene expression patterns in MCF-7 cells induced by the urban dust particulate complex mixture standard reference material 1649a. Cancer Res. 2005a;65:1251–1258. doi: 10.1158/0008-5472.CAN-04-2357. [DOI] [PubMed] [Google Scholar]
- Mahadevan B, Marston CP, Dashwood WM, Li Y, Pereira C, Baird WM. Effect of a standardized complex mixture derived from coal tar on the metabolic activation of carcinogenic polycyclic aromatic hydrocarbons in human cells in culture. Chem Res Toxicol. 2005b;18:224–231. doi: 10.1021/tx0497604. [DOI] [PubMed] [Google Scholar]
- Samet JM, Spengler JD. Indoor environments and health: moving into the 21st century. Am J Public Health. 2003;93:1489–1493. doi: 10.2105/ajph.93.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson EG, Farant JP. Indoor and outdoor polycyclic aromatic hydrocarbons in residences surrounding a Soderberg aluminum smelter in Canada. Environ Sci Technol. 2004;38:5350–5356. doi: 10.1021/es030715c. [DOI] [PubMed] [Google Scholar]
- Sarnat J, Long C, Koutrakis P, Coull B, Schwartz J, Suh H. Using sulfur as a tracer of outdoor fine particulate matter. Environmental Science Technology. 2002;3:5305–5314. doi: 10.1021/es025796b. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Brown KW, Bartell SM, Sarnat SE, Wheeler AJ, Suh HH, Koutrakis P. The Relationship between Averaged Sulfate Exposures and Concentrations: Results from Exposure Assessment Panel Studies in Four U.S. Cities. Environmental Science & Technology. 2009;43:5028–5034. doi: 10.1021/es900419n. [DOI] [PubMed] [Google Scholar]
- Schauer C, Niessner R, Poschl U. Polycyclic aromatic hydrocarbons in urban air particulate matter: decadal and seasonal trends, chemical degradation, and sampling artifacts. Environmental Science & Technology. 2003;37:2861–2868. doi: 10.1021/es034059s. [DOI] [PubMed] [Google Scholar]
- Semple S, Garden C, Coggins M, Galea KS, Whelan P, Cowie H, Sanchez-Jimenez A, Thorne PS, Hurley JF, Ayres JG. Contribution of solid fuel, gas combustion, or tobacco smoke to indoor air pollutant concentrations in Irish and Scottish homes. Indoor air. 2012;22:212–223. doi: 10.1111/j.1600-0668.2011.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Chapman RS, He X, Liu LZ, Lai H, Chen W, Lan Q. Dietary factors, food contamination and lung cancer risk in Xuanwei, China. Lung Cancer. 2008;61:275–282. doi: 10.1016/j.lungcan.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752–1759. doi: 10.1038/jid.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Mehta S. The burden of disease from indoor air pollution in developing countries: comparison of estimates. Int J Hyg Environ Health. 2003;206:279–289. doi: 10.1078/1438-4639-00224. [DOI] [PubMed] [Google Scholar]
- Spengler JD, Schwab M, McDermott A, Lambert WE, Samet JM. Nitrogen dioxide and respiratory illness in children. Part IV: Effects of housing and meteorologic factors on indoor nitrogen dioxide concentrations. Res Rep Health Eff Inst. 1996 1-29; discussion 31-26. [PubMed] [Google Scholar]
- Staal YC, Hebels DG, van Herwijnen MH, Gottschalk RW, van Schooten FJ, van Delft JH. Binary PAH mixtures cause additive or antagonistic effects on gene expression but synergistic effects on DNA adduct formation. Carcinogenesis. 2007;28:2632–2640. doi: 10.1093/carcin/bgm182. [DOI] [PubMed] [Google Scholar]
- Tarantini A, Maître A, Lefèbvre E, Marques M, Rajhi A, Douki T. Polycyclic aromatic hydrocarbons in binary mixtures modulate the efficiency of benzo[a]pyrene to form DNA adducts in human cells. Toxicology. 2011;279:36–44. doi: 10.1016/j.tox.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Valberg PA, Watson AY. Comparative Mutagenic Dose Of Ambient Diesel Engine Exhaust. Inhalation Toxicology. 1999;11:215–228. doi: 10.1080/089583799197159. [DOI] [PubMed] [Google Scholar]
- Wallace L, Williams R. Use of Personal-Indoor-Outdoor Sulfur Concentrations to Estimate the Infiltration Factor and Outdoor Exposure Factor for Individual Homes and Persons. Environmental Science & Technology. 2005;39:1707–1714. doi: 10.1021/es049547u. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Zhou BS, Shi JP. Lung cancer in nonsmoking Chinese women: a case-control study. Lung Cancer. 1996;14:S93–S98. doi: 10.1016/s0169-5002(96)90214-7. [DOI] [PubMed] [Google Scholar]
- Wei Y, Han I-K, Hu M, Shao M, Zhang J, Tang X. Personal exposure to particulate PAHs and anthraquinone and oxidative DNA damages in humans. Chemosphere. 2010;81:1280–1285. doi: 10.1016/j.chemosphere.2010.08.055. [DOI] [PubMed] [Google Scholar]
- WHO . Organic Pollutants. WHO WHO Regional Office for Europe; Copenhanguen: 2000a. Air Quality Guidelines. Chapter 5. [Google Scholar]
- WHO . Polycyclic aromatic hydrocarbons. Copenhagen: WHO Regional Publications: 2000b. [Google Scholar]
- Wojtyla C, Gluszek L, Bilinski P, Paprzycki P, Warzocha K. Smoking during pregnancy--hematological observations in pregnant women and their newborns after delivery. Ann Agric Environ Med. 2012;19:836–841. [PubMed] [Google Scholar]
- Zhu LZ, Takahashi Y, Amagai T, Matsushita H. Highly sensitive automatic analysis of polycyclic aromatic hydrocarbons in indoor and outdoor air. Talanta. 1997;45:113–118. doi: 10.1016/s0039-9140(97)00109-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


