Abstract
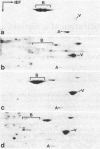
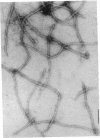
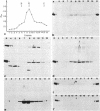
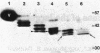
Intermediate-sized filaments (IF) are among the most insoluble intracellular protein polymer structures. We have analyzed the small amounts of soluble vimentin, an IF protein, present in cytosol fractions obtained from lysis of cultured cells [rat RVF-SM cells, simian virus 40-transformed human fibroblasts, and human rhabdomyosarcoma (RD line) cells]. The molecular form of this soluble vimentin was determined by sucrose density gradient centrifugation, using vimentin-specific antibodies for subsequent ELISA and immunoblotting analyses. The majority of the soluble vimentin appeared in a distinct form indistinguishable in its sedimentation behavior from reconstituted tetrameric subunits of purified vimentin arrested at low ionic strength. The tetrameric coiled-coil nature of the soluble form of vimentin was indicated by the digestion pattern with chymotrypsin and by chemical crosslinking with copper-1,10-phenanthroline and dimethylsuberimidate. The competence of this soluble vimentin to assemble into IF at higher salt concentrations was demonstrated by electron microscopy. Pulse-chase experiments showed that the soluble form was not an exclusively posttranslational intermediate. We propose that in the living cell a small pool of a distinct soluble tetrameric form of vimentin exists which may exchange with polymeric IF vimentin.
Full text
PDF


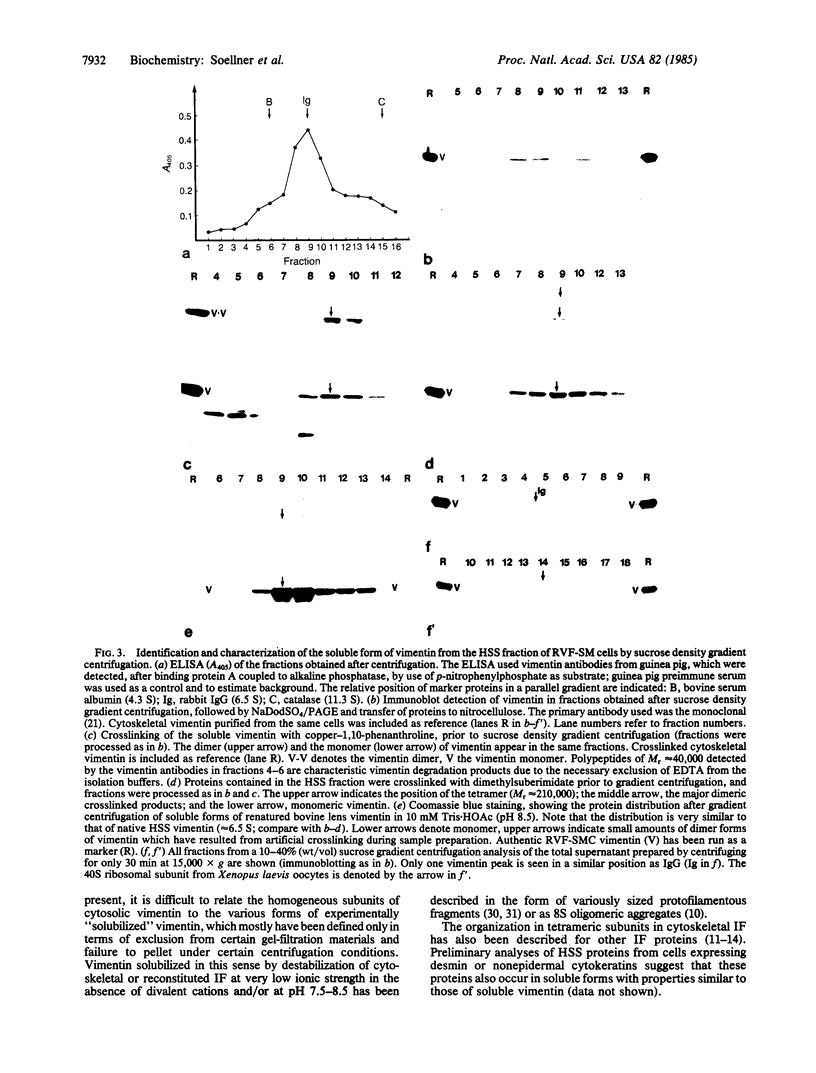

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Lazarides E. Vimentin filaments are assembled from a soluble precursor in avian erythroid cells. J Cell Biol. 1983 Jun;96(6):1803–1808. doi: 10.1083/jcb.96.6.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Vandekerckhove J., Weber K. Permanently proliferating rat vascular smooth muscle cell with maintained expression of smooth muscle characteristics, including actin of the vascular smooth muscle type. J Cell Biol. 1980 Dec;87(3 Pt 1):594–600. doi: 10.1083/jcb.87.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M. Many cytoskeletal proteins associate with the hela cytoskeleton during translation in vitro. Cell. 1983 Feb;32(2):619–625. doi: 10.1016/0092-8674(83)90481-6. [DOI] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Proteinchemical characterization of three structurally distinct domains along the protofilament unit of desmin 10 nm filaments. Cell. 1982 Aug;30(1):277–286. doi: 10.1016/0092-8674(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of polymerization-competent vimentin from porcine eye lens tissue. FEBS Lett. 1981 Mar 23;125(2):253–256. doi: 10.1016/0014-5793(81)80732-6. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen L. C., Woods E. F. Structural studies on the microfibrillar proteins of wool. Interaction between alpha-helical segments and reassembly of a four-chain structure. Biochem J. 1983 Mar 1;209(3):587–595. doi: 10.1042/bj2090587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huiatt T. W., Robson R. M., Arakawa N., Stromer M. H. Desmin from avian smooth muscle. Purification and partial characterization. J Biol Chem. 1980 Jul 25;255(14):6981–6989. [PubMed] [Google Scholar]
- Hügle B., Scheer U., Franke W. W. Ribocharin: a nuclear Mr 40,000 protein specific to precursor particles of the large ribosomal subunit. Cell. 1985 Jun;41(2):615–627. doi: 10.1016/s0092-8674(85)80034-9. [DOI] [PubMed] [Google Scholar]
- Ip W., Hartzer M. K., Pang Y. Y., Robson R. M. Assembly of vimentin in vitro and its implications concerning the structure of intermediate filaments. J Mol Biol. 1985 Jun 5;183(3):365–375. doi: 10.1016/0022-2836(85)90007-5. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Lazarides E. Canavanine inhibits vimentin assembly but not its synthesis in chicken embryo erythroid cells. J Cell Biol. 1983 Oct;97(4):1309–1314. doi: 10.1083/jcb.97.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Purification of the intermediate filament protein vimentin from Ehrlich ascites tumor cells. J Biol Chem. 1982 May 25;257(10):5536–5543. [PubMed] [Google Scholar]
- Nelson W. J., Vorgias C. E., Traub P. A rapid method for the large scale purification of the intermediate filament protein vimentin by single-stranded DNA-cellulose affinity chromatography. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1141–1147. doi: 10.1016/0006-291x(82)91231-1. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Quinlan R. A., Cohlberg J. A., Schiller D. L., Hatzfeld M., Franke W. W. Heterotypic tetramer (A2D2) complexes of non-epidermal keratins isolated from cytoskeletons of rat hepatocytes and hepatoma cells. J Mol Biol. 1984 Sep 15;178(2):365–388. doi: 10.1016/0022-2836(84)90149-9. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3452–3456. doi: 10.1073/pnas.79.11.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Molecular interactions in intermediate-sized filaments revealed by chemical cross-linking. Heteropolymers of vimentin and glial filament protein in cultured human glioma cells. Eur J Biochem. 1983 May 16;132(3):477–484. doi: 10.1111/j.1432-1033.1983.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Renner W., Franke W. W., Schmid E., Geisler N., Weber K., Mandelkow E. Reconstitution of intermediate-sized filaments from denatured monomeric vimentin. J Mol Biol. 1981 Jun 25;149(2):285–306. doi: 10.1016/0022-2836(81)90303-x. [DOI] [PubMed] [Google Scholar]
- Rueger D. C., Huston J. S., Dahl D., Bignami A. Formation of 100 A filaments from purified glial fibrillary acidic protein in vitro. J Mol Biol. 1979 Nov 25;135(1):53–68. doi: 10.1016/0022-2836(79)90340-1. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Stenbuck P., LeVine H., 3rd, Bronson D., Moncharmont B., Henderson C., Cuatrecasas P. Formation and identification of cytoskeletal components from liver cytosolic precursors. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7341–7345. doi: 10.1073/pnas.79.23.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Goldman R. D. Intermediate filaments of baby hamster kidney (BHK-21) cells and bovine epidermal keratinocytes have similar ultrastructures and subunit domain structures. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4534–4538. doi: 10.1073/pnas.77.8.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nelson W. J. Effect of the ionic environment on the incorporation of the intermediate-sized filament protein vimentin into residual cell structures upon treatment of Ehrlich ascites tumour cells with triton X-100. I. Biochemical analysis. J Cell Sci. 1982 Feb;53:49–76. doi: 10.1242/jcs.53.1.49. [DOI] [PubMed] [Google Scholar]
- Zackroff R. V., Goldman R. D. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6226–6230. doi: 10.1073/pnas.76.12.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]