Abstract
Hypo-glycosylated hFSH21/18 (possesses FSHβ21 and FSH18 bands) was isolated from hLH preparations by immunoaffinity chromatography followed by gel filtration. Fully-glycosylated hFSH24 was prepared by combining the fully-glycosylated FSHβ24 variant with hCGα and isolating the heterodimer. The hFSH21/18 glycoform preparation was significantly smaller than the hFSH24 preparation and possessed 60% oligomannose glycans, which is unusual for hFSH. Hypo-glycosylated hFSH21/18 was 9- to 26-fold more active than fully-glycosylated hFSH24 in FSH radioligand assays. Significantly greater binding of 125I-hFSH21/18 tracer than hFSH24 tracer was observed in all competitive binding assays. In addition, higher binding of hFSH21/18 was noted in association and saturation binding assays, in which twice as much hFSH21/18 was bound as hFSH24. This suggests that more ligand binding sites are available to hFSH21/18 in FSHR than to hFSH24. Hypo-glycosylated hFSH21/18 also bound rat FSHRs more rapidly, exhibiting almost no lag in binding, whereas hFSH24 specific binding proceeded very slowly for almost the first hour of incubation.
1. Introduction
Carbohydrate heterogeneity results in FSH preparations composed of populations of acidic isoforms (Ulloa-Aguirre and Chappel, 1982, Ulloa-Aguirre, Cravioto, Damian-Matsumura et al., 1992, Ulloa-Aguirre, Damian-Matsumura, Jimenez et al., 1992, Wide, 1985, Wide, 1987). The ratios of these isoforms have been shown to vary under physiological conditions, with the less acidic more abundant in young women and at mid-menstrual cycle, while more acidic isoforms are more abundant in older women and in men (Wide, 1982, Wide, 1985, Wide and Bakos, 1993, Zambrano, Olivares, Mendez et al., 1995).
Partial N-glycosylation of hFSHβ produces two hFSH glycoforms, the classical fully-glycosylated hFSH, which possesses all four N-glycans, and a hypo-glycosylated hFSH, which lacks either one or both β subunit N-glycans (Walton, Nguyen, Butnev et al., 2001). These glycoforms are most readily evaluated by FSHβ Western blotting, which reveals two bands, a 24,000 Mr band, that represents the fully-glycosylated form of the subunit (FSHβ24), and a 21,000 Mr band, that represents a hypo-glycosylated subunit (FSHβ21). In principle, hFSH21 should represent a less acidic hFSH isoform. Indeed, we have reported that above pI 5.4 only hFSH21 was found in chromatofocusing fractions (Walton et al., 2001). However, hFSH21 was also found in the less than pI 4.0 fractions and in all others in between, along with hFSH24 (Bousfield, Butnev, Bidart et al., 2008, Walton et al., 2001).
As the activities of hFSH isoforms possessing more 21,000 Mr than 24,000 Mr FSHβ were greater than those possessing more 24,000 Mr FSHβ, we proposed the hypothesis that hypo-glycosylation of hFSHβ increased FSH biological activity (Walton et al., 2001). Analysis of hFSH derived from individual human pituitaries revealed an age-related decreased abundance of hFSHβ21 indicating the loss of a potentially more active FSH variant. Western blots of virtually all pituitary and urinary hFSH preparations exhibit both hFSHβ subunit variants, regardless of purity (Bousfield, Butnev, Walton et al., 2007, Walton et al., 2001). Exceptions included less acidic FSH isoform fractions that possessed only the 21,000 Mr hFSHβ variant mentioned above.
In chromatofocusing experiments, the least acidic hFSH isoform fractions consisted of hFSH21, but were heavily contaminated with hLH (Walton et al., 2001). We speculated that the 1–2% FSH activity associated with purified pituitary hLH preparations might consist of the hypo-glycosylated hFSH21 glycoform, and captured it with an FSH-specific antibody column. Since reverse-phase HPLC is known to produce the 24,000 Mr hFSHβ variant in high purity (Walton et al., 2001), we isolated hFSHβ24 and combined it with hCGα to produce a semi-synthetic, fully-glycosylated hFSH preparation that would enable us to compare the biological activities of both glycoforms.
2. Materials and Methods
2.1 Hormone preparations
Three purified hLH preparations were obtained from Anne Hartree following her retirement from Cambridge University (Walton et al., 2001). An additional hLH preparation was obtained from Dr. A.F. Parlow and the National Hormone and Pituitary Program (Ward, Glenn, Nahm et al., 1986), along with the hFSH reference preparation, AFP7298A (8560 IU/mg). Recombinant hFSH was purified from a stable, transformed GH3 cell line, which was the generous gift of Dr. Irving Boime, Washington University, St. Louis, MO. Details of its isolation and characterization will be described in a separate publication. Mutant recombinant hFSHβ T26A, expressed in insect cells, was provided by Dr. James A. Dias, Wadsworth Center, Albany, NY (Fox, Dias and Van Roey, 2001). Purification of hFSH glycoforms is described in the supplement. Equine FSH was isolated from horse pituitaries in our laboratory, following our usual procedures (Bousfield and Ward, 1984). The hCGα preparation was purified as previously reported (Bousfield, Liu and Ward, 1985). HiTrap-NHS columns and Superdex 75 gel filtration columns were obtained from GE Healthcare, Piscataway, NJ.
2.2 Antibodies
A monoclonal antibody 46.3H6.B7, which recognized hFSH and hFSHβ, was also provided by Dr. Dias (Bousfield et al., 2007). This antibody was coupled to 1-ml HiTrap-NHS columns following the manufacturer’s instructions. The anti-hFSHβ monoclonal antibody RFSH20 (recognizes both FSHβ and FSH), and anti-α subunit antibody HT13 (recognizes all human α-subunits and glycoprotein hormones), were provided by Dr. Jean-Michel Bidart, Institut de Cancérologie Gustave-Roussy, Paris, France (Walton et al., 2001).
2.3 Glycoform nomenclature
The hFSH glycoforms are differentiated by the relative molecular weights of the FSHβ subunit Western blot bands, as indicated by a trailing superscript. Thus, pituitary and urinary hFSH preparations possessing both 21,000 and 24,000 Mr hFSHβ variants are designated as hFSH24/21, where the first number indicates the more abundant form. FSH glycoform preparations are designated as either fully-glycosylated hFSH24 (formerly tetra-glycosylated hFSH) and hypo-glycosylated hFSH21 (formerly di-glycosylated hFSH), or hFSH21/18. The latter is the variant characterized in the present study.
2.4 FSH glycoform characterization
Western blotting was carried out as described previously (Bousfield et al., 2007). Primary antibodies typically employed were anti-hFSHβ monoclonal antibody, RFSH20, anti-α subunit monoclonal antibody, HT13. Oligosaccharides released from hFSH samples by PNGaseF digestion were analyzed by nano-electrospray mass spectrometry (Harvey, Royle, Radcliffe et al., 2008). FSH iodination and radioligand assay, association and saturation binding assays were performed as described previously (Bousfield and Ward, 1984, Liu, Yang, Nakagawa et al., 1974, Liu, Young and Ward, 1984). FSH and FSH subunit carbohydrate analyses were carried out as reported earlier (Bousfield, Baker, Gotschall et al., 2000) except that a Dionex ICS-5000 carbohydrate analyzer was employed. Detailed methods can be found in the supplement.
2.5 Edman degradation
Protein sequencing was carried out with an Applied Biosystems (Foster City, CA), Procise, model 494, automated sequencer, following the manufacturer’s directions. Automated Edman degradation was carried out on 2 μg samples applied to Biobrene-coated glass fiber disks that were precycled in the sequencer prior to sample application. A typical experiment consisted of 10 Edman degradation cycles.
3. Results
3.1 FSH glycoform isolation from hLH preparations
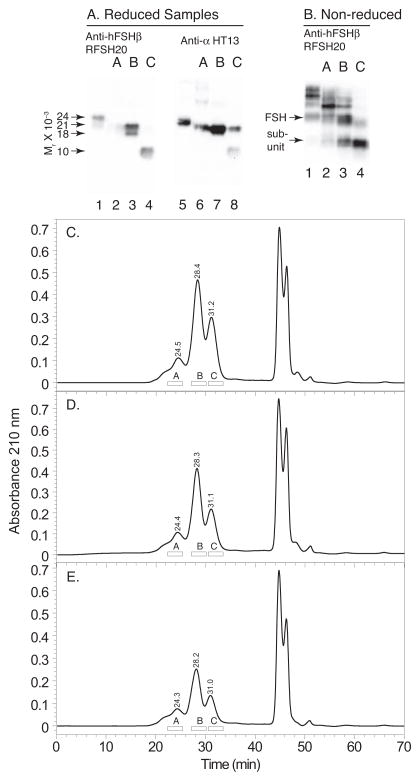
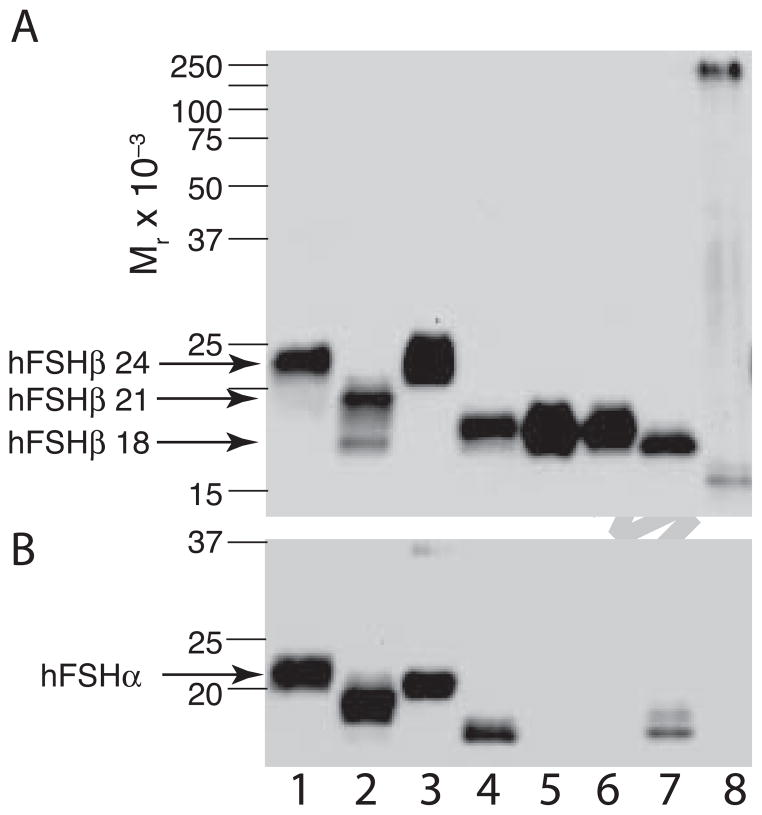
FSH was extracted from 100 mg batches of purified hLH three times using 46.3H6.B7 antibody columns. The average total hypo-glycosylated hFSH21/18 recovery, based on extracting ten hLH batches three times each, was 44.2 ± 4.9 μg (mean ± standard deviation) per batch. FSH immunoactivity emerged from a 10 X 300 mm Superdex 75 column in three peaks (Fig. 1C–1E). The first of these, with retention times of 24.3–24.5 min., consisted of aggregated FSH. Hormone aggregation was confirmed by Western blotting following electrophoresis of reduced samples probed with anti-α monoclonal antibody, HT13 (Fig. 1A, lane 6), and non-reduced hFSH isoform samples probed with anti-hFSHβ monoclonal antibody RFSH20 (Fig. 1B, lane 2). Both blots showed the presence of high MW immunoreactive bands indicating aggregation. The hFSHβ immunoactivity in the aggregated FSH fraction A was below the limits of detection in the reduced hFSH Western blot (Fig. 1A, lane 2). The second Superdex 75 peak, fraction B retention times 28.2–28.4 min.), possessed the hFSH heterodimer fraction. Based on peak areas, hFSH recoveries from 30 Superdex 75 chromatograms ranged from 6.1 to 27.2 μg, and the average recovery was 13.5 ± 6.3 μg per chromatogram. Western blot analysis revealed the presence of a second hFSHβ band at Mr 18,000, as well as the anticipated, 21,000 Mr band (Fig. 1A, lane 3). Because there was more of the latter, we designate this hFSH variant as hFSH21/18. Fraction C, derived from the third Superdex 75 peak (retention times 31.0–31.2 min.), possessed the subunit fraction. Western blot analysis detected both α and FSHβ subunit immunoactivities, although most of the latter migrated as a 10,000 Mr band, suggesting proteolytic nicking (Fig. 1A, lanes 4 and 8).
Figure 1. Hypo-glycosylated hFSH isolation from hLH preparations.
FSH bound to anti-hFSHβ immunoaffinity columns was applied to a 10 X 300 mm Superdex 75 column and the chromatogram developed as described under Methods. A. FSHβ and FSHα Western blots of reduced samples from the chromatogram in panel C. Anti-FSHβ: Lane 1, 1 μg hFSH (AFP7298A); lane 2, 1 μg fraction A; lane 3, 1 μg fraction B; lane 4, 1 μg fraction C. Anti-α: Lane 5, 1 μg hFSH (AFP7298); lane 2, 1 μg fraction A; lane 3, 1 μg fraction B; lane 4, 1 μg fraction C. B. FSHβ Western blot of non-reduced samples from the chromatogram in panel C. Lane 1, 1 μg hFSH (AFP7298A); lane 2, 1 μg fraction A; lane 3, 1 μg fraction B; lane 4, 1 μg fraction C. C. First round of hFSH isolation. D. Second round of hFSH isolation. E. Third round of hFSH isolation. The open bars show portions of each chromatogram containing aggregated hFSH (A) hFSH heterodimer (B), and subunits (C).
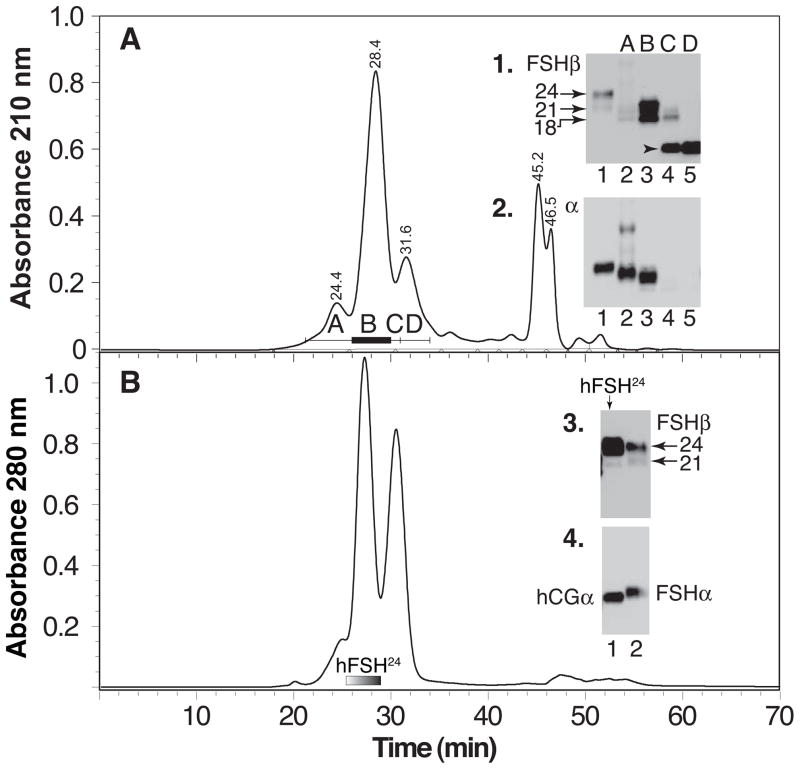
The hFSH21/18 heterodimer fraction isolated from a second hLH preparation was relatively more abundant than the aggregated FSH and subunit fractions (Fig. 2A). Western blotting of reduced samples from fraction A in this chromatogram, using both anti-hFSHβ (RFSH20) and anti-human α-subunit (HT13) antibodies, revealed high MW immunoreactive bands, consistent with aggregation, (Fig. 2A, lane 2 in insets 1 and 2). Both 18,000 and 21,000 Mr bands were observed in the FSHβ Western blot for the heterodimer faction B (Fig. 2A, inset 1, lane 3). As Edman degradation revealed only FSH α and β N-terminal sequences (supplement Table 1), the 18,000 Mr band appeared to represent another variant of intact hFSHβ rather than a subunit fragment. In fraction C, the subunit Western blot, only hFSHβ immunoactivity was detectable (Fig. 2A, insets 1 & 2, lane 5). However, Edman degradation revealed the presence of both α and FSHβ subunit amino acid sequences (supplement Table 2), consistent with the presence of both subunits in Western blot analysis of other hypo-glycosylated hFSH21/18 preparations (Fig. 1A).
Figure 2. Hypo- and fully-glycosylated hFSH isolation.
A. Immunopurified hFSH fractionated by Superdex 75 chromatography and fractions analyzed by Western blotting. Fractions indicated by bars and numbers. Insets 1 & 2. Western blot analysis of 1 μg samples of reduced Superdex 75 fractions with FSHβ- and FSHα-specific antibodies RFSH20 and HT13, respectively. Lane 1, hFSH AFP7298A; lane 2, fraction A; lane 3, fraction B; lane 4, fraction C; lane 5, fraction D. The arrows show the positions of the hFSHβ21,000 and 24,000 Mr bands, as indicated. B. The 24,000 Mr hFSHβ was combined with hCGα and incubated for 72 hr at 37°C. The mixture was reduced to <200 μl and applied to a Superdex 75 column as described under Methods. Insets 3 and 4, FSHβ and FSHα Western blot analysis of reduced Superdex 75 fractions, respectively. Lane 1, hFSH24; lane 2, pituitary hFSH (AFP7298A).
3.2 Preparation of fully-glycosylated hFSH24
Reassociation of 874 μg HPLC-purified, 24,000 Mr hFSHβ (Walton et al., 2001) with 2640 μg hCGα, followed by gel filtration over Superdex 75, produced 834 μg fully-glycosylated hFSH in the heterodimer fraction (Fig. 2B, bar). The heterodimer fraction possessed both hFSHβ and hCGα subunit immunoactivities according to Western blots with RFSH20 and HT13 monoclonal antibodies, respectively (Fig 2B, insets 3 and 4, lane 1). A < 21,000 Mr band was detected in the hFSHβ Western blot that represented < 2% of the immunoactivity (the 24,000 Mr band signal was saturated), much less than the 20% typically encountered in pituitary hFSH preparations (Fig. 2B, inset 3, lane 2).
3.3 FSH Glycoform receptor-binding competition assays
The availability of hFSH glycoform preparations permitted us to test the hypothesis, based on previous studies, that the presence of the carbohydrate-deficient hFSHβ21 provides hFSH with greater FSH receptor-binding activity than hFSH variants possessing largely or exclusively the fully-glycosylated 24,000 Mr FSHβ24 subunit (Walton et al., 2001). Both hFSH glycoform preparations were compared with two other FSH preparations: a highly active, hypo-glycosylated eFSH preparation, purified in this laboratory (Bousfield and Ward, 1984), that largely lacks βAsn7 glycans (Dalpathado, Irungu, Go et al., 2006), and a highly purified 70/30 mixture of hFSH24 and hFSH21 glycoforms, obtained from the National Hormone and Pituitary Program (AFP7298A). For reasons of clarity, we will limit labeling of β subunit relative molecular weight to hFSH preparations. All seven eFSH preparations purified in our laboratory were 90% hypo-glycosylated, possessing the 18,000 Mr eFSHβ band, while fully-glycosylated eFSHβ had a relative molecular weight of 21,000, reflecting a combination of largely biantennary instead of tri- and tetra-antennary glycans attached to eFSH, with a significant percentage of these terminated with sulfate rather than sialic acid (Bousfield, Butnev, Butnev et al., 2004, Dalpathado et al., 2006).
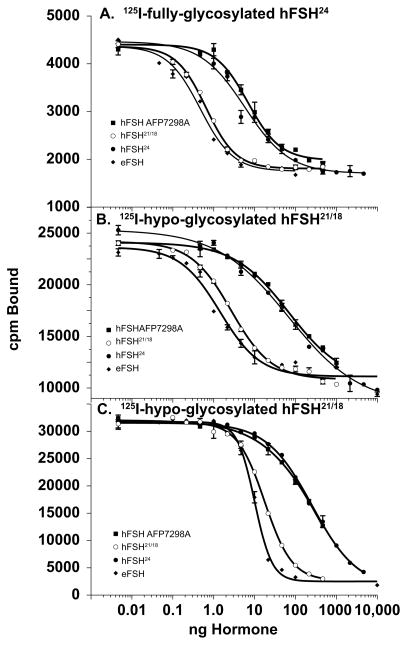
When rat testis FSH receptors were employed (Fig. 3A and Table 1) and 125I-hFSH24 was used as tracer, cold hypo-glycosylated hFSH21/18 was a highly significant, 9-fold more active than cold fully-glycosylated hFSH24 (p < 0.0001). Despite its high activity relative to highly purified pituitary hFSH24/21, the hypo-glycosylated hFSH21/18 preparation was significantly less active than the eFSH preparation (p = 0.023). The activity of fully-glycosylated hFSH24 was not significantly different from pituitary hFSH24/21 (p = 0.132).
Figure 3. FSH receptor-binding assays of fully- and hypo-glycosylated hFSH preparations.
A. 125I-fully-glycosylated hFSH24 tracer and rat testis homogenate. B. 125I-hypo-glycosylated hFSH21/18 tracer with rat testis homogenate. C. 125I-hypo-glycosylated hFSH21/18 tracer with CHO cells expressing hFSHR. Receptor-binding assays carried out as described under Methods. Quantitative results are shown in Table 1.
Table 1.
FSH receptor binding assay comparison of hFSH glycoform preparations.
| Receptor Prep. | Rat testis homogenate | Rat testis homogenate | CHOhFSHR cells | |||
|---|---|---|---|---|---|---|
| Tracer | 125I-hFSH21/18 | 125I-hFSH24 | 125I-hFSH21/18 | |||
| FSH Preparation | ID50 (ng) | Relative Potency (IU/mg) | ID50 (ng) | Relative Potency (IU/mg) | ID50 (ng) | Relative Potency (IU/mg) |
| a Pit-hFSH24/21 | 7.85 | 8,560 | 63.7 | 8,560 | 554.0 | 8,560 |
| hFSH24 | 5.83 | 11,526 | 69.6 | 7,834 | 253.1 | 18,737 |
| hFSH21/18 | 0.646 | 104,019 | 2.67 | 204,221 | 17.6 | 269,445 |
| eFSH | 0.468 | 143,581 | 1.43 | 381,309 | 10.4 | 455,985 |
| hFSH21/18:hFSH24 | 9 | 26 | 14 | |||
Pituitary hFSH preparation AFP7298A, 8560 IU/mg.
When 125I-hFSH21/18 was employed as tracer with rat testis homogenate, a highly significant (p < 0.0001) 26-fold greater activity for hypo-glycosylated hFSH21/18 was observed as compared with fully-glycosylated hFSH24 (Fig. 3B and Table 1). The activities of fully-glycosylated hFSH24 and pituitary hFSH24/21 were not significantly different (p = 0.771), while those of hypo-glycosylated hFSH21/18 and eFSH were significantly different (p = 0.0028). A major difference between assays employing different hFSH glycoform tracers was in the magnitude of the specifically bound cpm, as the same amount of testicular homogenate, although prepared separately for each experiment, bound 14,733 cpm 125I-hFSH21/18, which was over 5 times as much as the 2621 cpm representing the specifically bound 125I-hFSH24 tracer. As will be shown below, the same phenomenon was observed when both tracers were simultaneously incubated with the same receptor preparation.
We also compared the four FSH preparations in a competitive human FSHR binding assay using intact CHO cells expressing the hFSHR and the better of the two tracers, 125I-hFSH21/18. Based on ID50 values, hypo-glycosylated hFSH21/18 was 14-fold more active (p < 0.0001) than fully-glycosylated hFSH24 (Fig. 3C and Table 1). Interestingly, the curves for the human glycoform preparations were not parallel, despite the use of homologous tracer and receptor preparation. The displacement curve for hypo-glycosylated hFSH21/18 appeared to be more parallel to the eFSH curve, which was the most active preparation in this assay, especially at higher concentrations. Based on ID50 values eFSH was significantly more active than hFSH21/18 (p < 0.0001). The least active preparations were fully-glycosylated hFSH24 and pituitary hFSH24/21, which did not differ significantly based on ID50 comparison (p = 0.368).
3.4 FSHR saturation binding studies
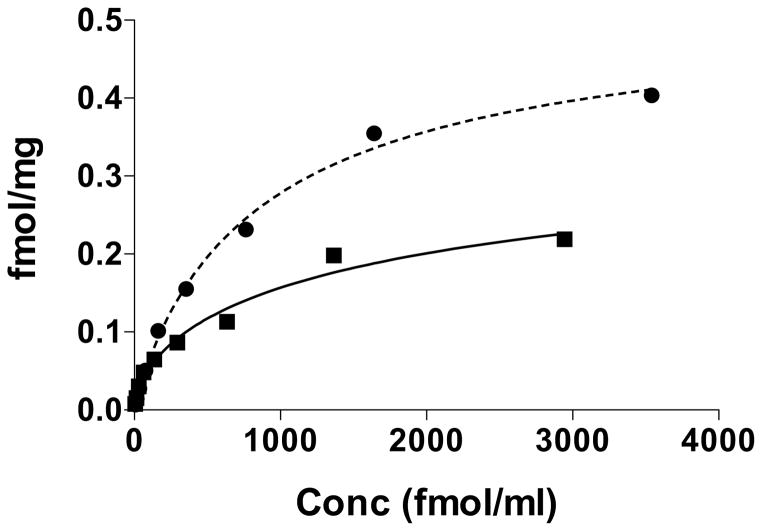
When the same rat testis FSH receptor preparation was exposed to increasing concentrations of both 125I-labeled hFSH glycoforms, in the presence or absence of 2000-fold excess cold eFSH, 2-fold greater specific binding was observed for 125I-hFSH21/18 than for 125I-hFSH24 (Fig. 4).
Figure 4. Saturation binding of hypo- and fully-glycosylated hFSH preparations to rat testis FSH receptors.
The same rat testis homogenate was used to measure specific binding of both glycoforms. Closed circles, saturation binding of 125I-hypo-glycosylated hFSH. Closed squares, saturation binding of 125I-fully-glycosylated hFSH. Specific binding (Bo – Bn) was determined using 2000-fold excess cold eFSH. Representative data from two experiments.
3.5 FSHR association studies
The radioligand assay procedure routinely employed in this laboratory was modified for association studies because samples in the latter studies were removed at 15-min intervals, while separation of bound from free hormone in the former procedure involved a 20-min centrifugation step. In the modified assay, tubes were incubated at 37°C for the indicated times, then placed in an ice bath until incubation of all the tubes was completed, when all assay tubes were centrifuged together. To prevent continued FSH binding during the ice bath step, 2000-fold excess eFSH was added to each tube and the binding compared with tubes lacking cold hormone. A small, but statistically non-significant difference was noted between the binding curves, indicating that binding did not continue at 4°C, nor was there significant dissociation from the receptor. In subsequent experiments, tubes removed at different times were stored at 4°C in the absence of cold hormone and all were centrifuged together following removal of the last tubes from the incubator. Moreover, when receptor binding was measured following 3 hr incubation at 4°C, no significant binding of 125I-hFSH21/18 or 125I-hFSH24 was observed. While 125I-eFSH binding to FSH receptors was observed in this experiment (data not shown), this tracer was not used in any studies reported herein.
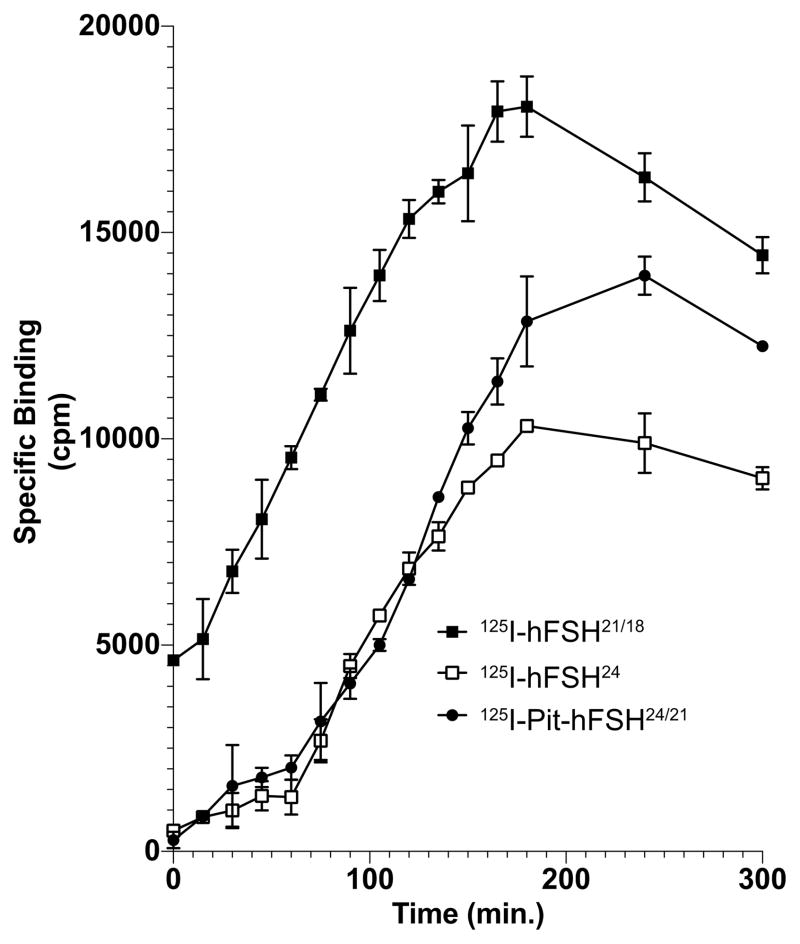
In the association study (Fig. 5), the 125I-hFSH24 tracer exhibited a similar binding pattern as 125I-pituitary hFSH24/21. The hypo-glycosylated hFSH21/18 binding was greater than either of the other tracers at all time points and this seemed at least partially due to the absence of lag during the first 30–50 min. Both 125I-hFSH tracer preparations possessing fully-glycosylated hFSH exhibited very limited receptor binding during this interval. Beginning with the 60 min time point, increased binding of both tracers commenced and progressed at the same rate as hypo-glycosylated hFSH21/18 binding, reaching a maximum at 3 hr. A lower maximum binding of hFSH24 tracer as compared with hypo-glycosylated hFSH21/18 was noted. In contrast, 125I-hFSH21/18 binding began with little or no lag and reached its maximum extent by 3 hr.
Figure 5. Association kinetics for hypo- and fully-glycosylated hFSH binding to rat testis FSH receptors.
FSH glycoform tracers, in the presence and absence of 2000-fold cold eFSH, were incubated with rat testis FSH receptors at 37°C for the indicated times, then placed in an ice water bath. When all the tubes were incubated, they were centrifuged to separate bound from free hormone, the supernatants aspirated and 125I-hFSH glycoform bound to the pellets counted in a gamma counter. Specific binding is plotted against incubation time. Representative data from three experiments.
3.6 Comparison with recombinant insect cell hFSH in Western blots
Nano-electrospray mass spectrometry revealed that 60% of hFSH21/18 glycans were oligomannose glycans (Supplement Figs. 2–4 and Supplement Tables 3 & 4), which are rare in pituitary hFSH (Bousfield et al., 2008, Dalpathado et al., 2006, Green and Baenziger, 1988, Renwick, Mizuochi, Kochibe et al., 1987), but common in recombinant, insect cell-expressed glycoprotein preparations (Altmann, Staudacher, Wilson et al., 1999). We compared the pituitary hFSH glycoform subunit electrophoretic mobilities with those of wild type and glycosylation site-mutated, insect cell-expressed recombinant hFSH preparations in subunit-specific Western blots (Fig. 6). The hypo-glycosylated hFSHβ immunoactivity migrated as 3 bands at Mr 21,000, 20,000, and 18,0000 (Fig. 6A, lane 2). Most of the immunoactivity was associated with the 21,000 and 18,000 Mr bands (hFSHβ21 and hFSHβ18, respectively). The hFSHβ18 band migrated faster than the 19,000 Mr wild type recombinant insect hFSHβ band (compare lanes 2 and 4). It exhibited the same mobility as the 18,000 Mr βT26A-hFSH mutant band (lane 7), as if pituitary hFSHβ18 also possessed a single, oligomannose N-glycan. The hFSHβ21 migrated more slowly than the 19,000 Mr, fully-glycosylated recombinant insect hFSHβ subunits (compare lane 2 with lanes 4–6). A sample of bacterially expressed hFSHβ was largely aggregated as evidenced by the 167,000 Mr band near the top of the gel and the broad region of immunoreactivity ranging from 26,000 to 48,000 Mr. The fastest migrating form in this preparation appeared as a pair of bands at 15,000 and 16,000 Mr, which was similar to the pattern for PNGaseF-sensitive hFSHβ(see supplement Figs. 7 & 8). Thus, the mobility of even the 15,000 Mr bacterially expressed hFSHβ band was slower than expected for a protein with a formula weight of 12,485.
Figure 6. Comparison of recombinant insect hFSH subunit electrophoretic mobilities with those of pituitary FSH glycoforms.
A. FSHβ-Western blot comparing mobilities of hFSH glycoforms with those of recombinant insect cell hFSHβ subunit preparations. The primary antibody was RFSH20. B. FSHα-Western blot using HT13 primary antibody. The same preparations were compared in both blots. Lane 1, pituitary hFSH (AFP4161B); lane 2, hypo-glycosylated hFSH; lane 3, fully-glycosylated hFSH; lane 4, wt recombinant insect hFSH; lane 5, αT54A mutant recombinant insect hFSH; lane 6, αT80A mutant recombinant insect hFSH; lane 7, βT26A mutant recombinant insect hFSH; lane 8, recombinant bacterial hFSHβ. Pre-stained BioRad MW marker positions are indicated by lines.
While the α subunit band mobilities varied between hFSH preparations, no separate bands were observed (Fig. 6B). The relative molecular weight of pituitary hFSHα was 23,000 Mr, as it exhibited the slowest migration of the human FSH α-subunit preparations, the hypo-glycosylated hFSHα band was 20,000 Mr, and the slowest component migrated immediately ahead of the fastest element of the 23,000 Mr pituitary hFSHα band. The hCGα possessed by the fully-glycosylated hFSH24 preparation exhibited a band with intermediate relative molecular weight (21,000 Mr), probably because of the largely hybrid-type Asn52 glycans and biantennary Asn78 glycans (Weisshaar, Hiyama and Renwick, 1991), as compared with mostly tri- and tetra-antennary hFSH glycans presumably associated with the pituitary hFSHα preparation (Green and Baenziger, 1988, Renwick et al., 1987). Only the wt recombinant insect hFSH and βT26A mutant recombinant insect hFSH preparations possessed both α and FSHβ subunit bands indicating the presence of heterodimeric FSH. This was consistent with the observation of heterodimer peaks in the Superdex 75 gel filtration chromatograms for both the wt and βT26A preparations, while the α-subunit mutant chromatograms contained only the subunit peak (data not shown). It is possible the αT80A mutant α subunit was below the limits of detection because, the absence of Asn78 glycan affected HT13 antibody binding (supplement Figs. 7 & 8). In both wt and βT26A recombinant insect hFSH preparations, the α subunit band mobilities were greater than those for any pituitary hFSH preparation α subunit bands, including hypo-glycosylated hFSH21/18. If 21,000 Mr hFSHβ indicated the di-glycosylated hFSH preparation reported earlier (Bousfield et al., 2007), most of the glycans released by PNGaseF digestion should have come from the α subunit, yet its mobility was slower than α subunit associated with both heterodimeric insect hFSH preparations.
3.7 Hypo-glycosylated hFSHβ subunit characterization
Hypo-glycosylated hFSHα and β subunits were separated from pooled hypo-glycosylated hFSH subunit fractions by 46.3H6.B7 immunopurification followed by Superdex 75 gel filtration of the unbound and bound fractions, respectively (supplement Fig. 5). The yield of hypo-glycosylated hFSHβ appeared to be greater than that of hFSHα, consistent with the absence of detectable α subunit immunoactivity in some subunit fraction Western blots (Fig. 2A, inset). Automated Edman degradation revealed both hFSHβ subunit N-terminal and internal amino acid sequences for the hypo-glycosylated hFSHβ preparation (supplement Table 5), which was consistent with the appearance of fragment bands in Western blots of the subunit fraction (Figs. 1 and 2). Only the α subunit N-terminal sequence was observed during Edman degradation of the hFSHα preparation (supplement Table 6).
Neutral and amino sugar analysis of both α and β subunits revealed the presence of carbohydrate in both subunit preparations (Table 2). Mannose yields were highest for all the monosaccharide residues detected in both subunit hydrolysates, consistent with quantitative MS data that indicated 60% of N-glycans released from hypo-glycosylated hFSH21/18 were oligomannose, which possessed 3–9 mannose residues in addition to two GlcNAc residues (supplement Table 3). Relative mannose abundance data indicated hypo-glycosylated hFSH21/18 glycans possessed an average of 4.7 mol mannose/mol glycan. Using this value, we normalized the compositions for both the α and β subunits. The results indicated a lower GlcNAc content in α subunit glycans, suggesting enrichment of oligomannose glycans in this subunit. Indeed, we previously reported oligomannose glycans largely associated with αAsn56 (homologous to human αAsn52) in horse LH preparations (Bousfield et al., 2004).
Table 2.
Neutral and amino sugar composition of hypo-glycosylated hFSH21/18 subunit preparations.
| Subunit prep. | hFSH21/18α | hFSH21/18β |
|---|---|---|
|
| ||
| Monosaccharide | mol/4.7 mol Man | |
| Fucose | 0.1 | 0.5 |
| GalNAc | 0.2 | 0.1 |
| GlcNAc | 2.2 | 3.1 |
| Galactose | 0.7 | 0.7 |
| Mannose | 4.7 | 4.7 |
Hypo-glycosylated FSHβ subunit glycans possessed the typically higher fucose content as well as a higher GlcNAc content. The latter suggested more complex-type glycans were attached to this subunit than to the FSHα subunit. While the same relative amounts of galactose in both subunits suggested the same extent of branching in complex glycans, if FSHα in the subunit fraction represented free-α, rather than dissociated FSHα, O-glycosylation was possible. Bovine pituitary free α-subunit was reported to be O-glycosylated at Thr43 (Thr39 is the homologous human α-subunit residue), which is exposed in the free subunit, but not in the intact hormone (Parsons, Bloomfield and Pierce, 1983). Alternatively, the presence of complex glycans at one of the hFSH21/18 α subunit glycosylation sites might be responsible for the slower migration during electrophoresis than insect-expressed FSHα subunits, which possessed exclusively oligomannose glycans. Compositions for the latter included GlcNAc, Man, and Fuc, but no Gal (Table 3).
Table 3.
Carbohydrate compositions for FSHβ subunit samples derived from hypo-glycosylated pituitary, GH3-, and insect-cell hFSH preparations.
| Preparation | a hFSH21/18β | GH3-hFSHβ | βT26AFSHβ | αT54AFSHβ | αT80AFSHβ |
|---|---|---|---|---|---|
|
| |||||
| Monosaccharide Residue | mol/3 mol Man | ||||
| Fucose | 0.3 | 1.9 | 2.6 | 1.9 | 1.0 |
| Galactosamine | – | 0.2 | 0.1 | 0.1 | 0.1 |
| Glucosamine | 2.0 | 6.0 | 3.4 | 3.5 | 3.7 |
| Galactose | 0.4 | 1.9 | 0.2 | – | – |
| Mannose | 3 | 3 | 3 | 3 | 3 |
Data from Table 2 normalized to 3 mannose residues to facilitate comparison.
4. Discussion
4.1 Hypo- and fully-glycosylated hFSH preparation
The existence of two major hFSH glycoforms resulting from hFSHβ macroheterogeneity was inferred from subunit-specific Western blots, which revealed hFSHβ24 and FSHβ21 bands accompanied by a single hFSHα band (Bousfield et al., 2007, Walton et al., 2001). Confirmation of hypo-glycosylated hFSH glycoform existence required isolation of this variant. While evaluating potential sources of di-glycosylated hFSH (now designated hFSH21), the least acidic chromatofocusing fractions were considered because they possessed only the hFSHβ21 band in FSHβ-specific Western blots (Walton et al., 2001). However, these fractions possessed only a small percentage of the total hFSH recovered. Moreover, significant hLH contamination was present in these fractions, which suggested examining preparations of this hormone (Timossi, Barrios-de-Tomasi, Gonzalez-Suarez et al., 2000, Walton et al., 2001).
As 2.4 grams of hLH were available in the laboratory, we proceeded under the working hypothesis that the 1–2% FSH activity associated with most pituitary LH preparations represented hFSH21 and anticipated recovering as much as 24 mg of this variant. However, the receptor-binding activity measurements grossly overestimated the FSH mass because purified hFSH21/18 exhibited as much as 26-fold greater activity in rat FSH receptor-binding assays than the hFSH reference preparation used to quantify FSH (Fig. 3). Thus, the actual mass of hFSH present in hLH was less than 1 mg and only 500 μg of that was recovered, which limited biochemical and biological characterization. As all other hFSH isoform fractions possessed a mixture of both hFSH24 and hFSH21 and no purification method was available at that time, fully-glycosylated hFSH24 was reconstituted from HPLC-purified FSHβ24 combined with purified hCGα. The latter was employed to avoid FSHβ21 contamination associated with HPLC-purified FSHα preparations (Walton et al., 2001).
4.2 FSH receptor binding
Before discussing the increased FSHR binding activities associated with hFSH21/18, it is important to realize that hypo-glycosylated hFSH preparations have been shown to be active in animal studies conducted by our collaborator, Dr. T.R. Kumar, University of Kansas Medical Center, Kansas City, KS. In vivo studies comparing hFSH24 with hypo-glycosylated hFSH21/18 preparations show a similar pattern of rapid onset of stimulation and higher level of ovarian response in Fshb null mice to hFSH21/18. These unpublished studies firmly establish the physiological significance of the receptor-binding data reported herein (Kumar, T.R., et al., manuscript in preparation).
Comparison of glycoform ID50 values in FSH receptor-binding assays revealed a 9- to 26-fold higher apparent affinity for hFSH21/18 than for hFSH24. The latter was as active as purified pituitary hFSH24/21, indicating full recovery of biological activity in the heterodimer fraction following subunit reassociation. We have subsequently isolated pituitary hFSH24 preparations (unpublished data), which exhibited reduced activity compared with purified pituitary hFSH24/21, suggesting the activity of reconstituted hFSH24 represents the high end of the activity scale for pituitary hFSH24 preparations. The unexpectedly high receptor-binding activity of hFSH21/18 represented almost 10-fold greater activity than had been reported for other carbohydrate-deficient hFSH preparations.
Chemically deglycosylated hFSH was reported to be as active as, or up to 3-fold more active than native hFSH in porcine and bovine FSH receptor assays (Calvo, Keutmann, Bergert et al., 1986). Recombinant hFSH preparations produced mixed results when FSHβ glycosylation sites were mutated to prevent glycan addition. In one study, the activity of the recombinant, HEK293 cell-derived mutant hFSH preparation, βAsn7Gln, increased rat testis FSH receptor binding 2.5-fold, but decreased rat granulosa cell bioassay activity to 52% that of wild type recombinant hFSH (Flack, Froehlich, Bennet et al., 1994). The βAsn24Gln mutant exhibited 87% greater FSH receptor binding activity, but 59% reduced granulosa cell activity. Another laboratory reported no significant difference in FSH receptor binding activity between βAsn7Gln or βAsn24Gln mutant preparations and wild-type, CHO cell-expressed recombinant hFSH using rat testis membrane preparations (Bishop, Robertson, Cahir et al., 1994). In the Sertoli cell estradiol production assay, the biologic activity of both mutants was reduced by 31% or 25%, respectively. Mutating FSHβ glycosylation sites may affect hormone conformation and possibly heterodimer stability (Flack et al., 1994). The latter could reduce activity, as heterodimeric FSH structure is necessary for high affinity FSHR binding (Fan and Hendrickson, 2005).
Specific binding of 125I-hFSH24 tracer in the rat testis homogenate competition assays was 2%, which was much lower than the 8% observed for 125I-hFSH21/18 tracer. The specific activities for both tracers were very similar, 39 and 42 μCi/μg, respectively; indicating the difference in bound cpm represented a difference in the amount of FSH glycoform bound to the receptor. In the presence of excess rat testis homogenate, 2-fold more of 2.5 ng 125I-hFSH21/18 was bound than of the same amount of 125I-hFSH24. The difference in the maximum bindable fraction was difficult to rationalize, as iodine most likely modified either Tyr88 or Try89 in the α subunit C-terminus (Stanton and Hearn, 1987). Neither residue was close to the FSHβ N-glycans, and changes in FSH iodination in response to altered FSHβ glycosylation should have been attended by altered iodine incorporation, although a different distribution of iodine label was also possible. Saturation binding of both hFSH glycoforms confirmed the 2-fold difference in binding to rat testis FSH receptors (Fig. 4). If the difference in bindable fraction between both tracers was responsible for the two-fold greater uptake of 2.5 ng 125I-hFSH21/18, then at a concentration of 3000 fmol/ml, 125I-hFSH24 should have approached the same level of binding as 1500 fmol/ml 125I-hFSH21/18. Instead, the 2-fold difference was retained, suggesting that FSHβ glycosylation influenced the number of FSH binding sites available.
As FSH receptors are now known to exist as at least dimers (Guan, Wu, Feng et al., 2010, Mazurkiewicz, Thomas, Nachamen et al., 2006, Urizar, Montanelli, Loy et al., 2005), the number of available binding sites may be affected by N-glycans of hFSH bound to one site affecting binding to a second FSH-binding site through an allosteric mechanism. However, both crystal structures of hFSH bound to the FSHR extracellular domain showed hFSHβ glycans oriented away from the hormone-receptor interface, where they should have no effect on receptor binding (Fan and Hendrickson, 2005, Jiang, Liu, Chen et al., 2012). The trimeric arrangement of enzymatically deglycosylated hFSH bound to the full-length extracellular domain led to a proposal that the non-reducing terminus of FSHαAsn52 glycan could occupy the binding site filled by the reducing terminal FSHαAsn52 glycan of a second hFSH ligand (Jiang, Dias and He, 2013). Evidence supporting this structure-based hypothesis came from modeling a biantennary glycan attached to αAsn52, which fit the notch between two extracellular domains normally occupied by the same glycan attached to a different FSH molecule, and from comparison of wt- and αAsn52 mutated hFSH binding. Three-fold more mutant hFSH was bound than wt-hFSH in saturation binding studies (Jiang et al., 2013). The hCGα glycans in the hFSH24 preparation could substitute for the biantennary glycan in the FSHR-ECD model because the complex branch possesses the same structure: Neu5Ac(α2–3)Gal(β1–4)GlcNAc(β1–2)Man(α1–3), which provides the extended branch used in the modeling exercise. The corresponding branch in largest oligomannose glycan associated with hFSH21/18, GlcNAc2Man9, is one residue shorter and the α-glycosidic bonds connecting the Man residues produce a helical rather than extended shape. However, the mobility of hFSH21/18 α was more similar to that of hCGα than to recombinant insect hFSHα. Since the hFSH21/18 α carbohydrate composition suggests a mixture of complex and oligomannose glycans, which is consistent with its intermediate electrophoretic mobility, it will be important to determine the αAsn52 glycan population in hypo-glycosylated hFSH glycoform preparations.
Association studies revealed another interesting difference between hFSH24 and hFSH21/18 binding to the FSH receptor. For the first 50 minutes of incubation at 37°C, specific binding of hFSH24 tracer proceeded very slowly, whereas hFSH21/18 binding commenced with almost no delay. At 60 min the rate of hFSH24 tracer binding increased and proceeded at the same rate as for hFSH21/18 (Fig. 5). Once again, the maximum binding of 125I-hFSH21/18 was 80% greater than that of 125I-hFSH24. Chemically deglycosylated oLH was reported to bind rat LH receptors faster than intact oLH (Liu et al., 1984), which could have either indicated a difference in how these related receptors interacted with their ligands or reflected removal of the inhibitory influence of oLHαAsn56 glycan (homologous to human αAsn52) on LH receptor binding (Butnev, Gotschall, Butnev et al., 1998).
When trying to rationalize the presence of hFSH21 in the most acidic hFSH isoform fractions, despite the absence of one or more N-glycans, as well as the lack of increased abundance of more negatively charged glycans in this fraction, we suggested dimerization of hFSH21 and hFSH24 with either glycoform. Dimerization combined two glycan populations and glycoforms with different overall net charges ended up in the same isoform fraction because of the average overall charge for two molecules rather than one (Bousfield et al., 2008). Supporting evidence consisted of a small increase in molecular size indicated by Superdex 75 gel filtration (Bousfield et al., 2008). In the present study we demonstrated that gel filtration over two Superdex 75 columns connected in series separated hFSH24 from hFSH21/18 (supplement Fig. 1). With the previous model of all-or-none glycosylation of hFSHβ (Walton et al., 2001), the rationale for the greater apparent molecular size for hFSH24 was the presence of hFSHβ24 N-glycans that roughly doubled the narrow diameter of the hormone as compared with hFSH21 lacking both hFSHβ glycans (Bousfield et al., 2008). Since evidence in the present study indicated that at least some of hFSHβ21 or hFSHβ18 bands represented partially glycosylated hFSHβ, an alternative explanation for the higher molecular weight of hFSH24 was that it existed as dimers of heterodimers. The orientation of hFSH heterodimers in the crystal structure of hFSH (Fox et al., 2001) was such that the receptor-binding surfaces interacted with each other, preventing receptor binding. If these FSH heterodimeric dimers existed in solution, then they would have to separate into monomers of FSH before binding the receptor. Dissociation might require 50–60-min, accounting for the lag in hFSH24 binding.
4.3 Hypo-glycosylated hFSH glycosylation
The higher apparent affinity, more rapid binding, and greater number of FSH receptor binding sites associated with hFSH21/18 indicated altered glycosylation exhibited a significant effect on hFSH glycoform biological activity. Glycosylation of the hFSH21/18 glycoform preparation isolated from hLH differed from that of pituitary hFSH24/21 used as a biological and biochemical reference preparation in our studies to a greater extent than was anticipated. While the hFSH21/18 lacked the hFSHβ24 band and possessed the hFSHβ21 band in FSHβ-specific Western blots, it also possessed an hFSHβ18 band that comprised 40% of the FSHβ immunoactivity. The hFSHβ18 band did not represent a fragment because automated Edman degradation detected only the hFSHβ amino terminal residues. Moreover, nano-ESI mass spectrometry revealed a glycan population dominated by oligomannose oligosaccharides, including several intermediates in the N-glycan processing pathway. Taken together with low abundance of this glycoform, the presence of oligomannose glycans suggested hFSH21/18 might have represented a biosynthetic intermediate of hFSH that is physiologically irrelevant. However, other evidence indicated this was unlikely, as complex N-glycans comprised over 30% of the hFSH21/18 oligosaccharide population and carbohydrate composition data provided evidence that both complex and oligomannose glycans were present in the FSH21/18 α subunit preparation. Moreover, hypo-glycosylated hFSH preparations are active in vivo.
Western blots revealed FSH21/18 α as a single band that exhibited an intermediate mobility between the complex glycan-dominated FSHα and hCGα subunit bands and the exclusively oligomannose glycan-decorated insect hFSHα band. Carbohydrate composition analysis of the FSHα fraction indicated a mixture of oligomannose and complex glycans due to the high ratio of Man and the presence of both Gal and GalNAc residues typical of complex N-glycan branches. The presence of two hFSHβ subunit bands in hFSH21/18 Western blots was also consistent with both types of glycans, but distinct mobilities were observed because only a single glycan was present, while the α subunit possessed two N-glycans and intermediate electrophoretic mobility was consistent with one of each glycan type residing in the same α subunit molecule. The co-migration of the hFSHβ18 band with the recombinant insect hFSHβ band suggested that it might possess a single oligomannose glycan. This might explain its apparent absence in other hFSH preparations. While it is possible that hFSHβ18 was below the detection limits of our Western blotting procedure, the glycan population of another hFSH21 preparation purified from pituitary hFSH fractions, was very similar to that of purified pituitary hFSH24/21 including the almost complete absence of oligomannose glycans (Bousfield and Harvey, unpublished).
4.4 Conclusion
Hypo-glycosylated hFSH represents a naturally occurring glycosylation variant that exhibits different structural and functional properties than the classical fully-glycosylated glycoform. When first discovered, we could only speculate that hypo-glycosylated hFSH action differed, perhaps by providing an episodic signal to the gonads, much like has been demonstrated for LH in peripheral circulation (Padmanabhan, McFadden, Mauger et al., 1997, Plant, Nakai, Berchetz et al., 1978, Reame, Sauder, Kelch et al., 1984). The present study shows that hypo-glycosylated hFSH binds the FSH receptor more rapidly and can access more binding sites than fully glycosylated hFSH. In sheep hypothalamo-hypophysial portal blood, episodic release of FSH was demonstrated that could not be detected in peripheral blood, at least in part due to a high background of tonic FSH secretion (Padmanabhan et al., 1997). Episodically released hypo-glycosylated hFSH might contribute to this pattern if it is cleared rapidly, as expected from recombinant hFSH glycosylation mutant clearance studies (Bishop, Nguyen and Schofield, 1995). Rapid clearance involves escape from capillaries, and in the gonads, this can mean rapid FSH delivery to target cells where hypo-glycosylated hFSH can quickly bind and activate FSH receptors. Thus, the apparent loss of hypo-glycosylated FSH as a function of age may contribute to reduced fertility associated with aging. The effects of reduced hFSH21 abundance would be manifested around the time of ovulation, when a recent report suggests such hypo-glycosylated hFSH concentrations are elevated in the circulation (Wide and Ericksson, 2013). Loss of this form could adversely affect follicle development (West, Carlson, Lee et al., 2002) and oocyte maturation (Andersen, Leonardsen, Ulloa-Aquirre et al., 2001), thereby contributing to infertility.
Supplementary Material
Highlights.
Hypo-glycosylated hFSH isolated from hLH preparations possessed the expected 21,000 Mr FSHβ subunit along with a novel 18,000 Mr band.
Hypo-glycosylated hFSH was decorated over 60% oligomannose glycans as well as complex glycans.
Hypo-glycosylated hFSH was 10-fold more active than fully-glycosylated hFSH in FSH receptor-binding assays.
Hypo-glycosylated hFSH bound more FSH receptors with no delay, while fully-glycosylated hFSH binding was slow for the first 50 minutes and only have as many binding sites were ultimately occupied.
Acknowledgments
This research was supported by NIH grants P01 AG-029531, G20 RR-031092, P20 RR-016475, and matching funds from Wichita State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate Journal. 1999;16:1091–1123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Leonardsen L, Ulloa-Aquirre A, Barrios-de-Tomasi J, Kristensen KS, Byskov AG. Effect of different FSH isoforms on cyclic-AMP production by mouse cumulus-oocyte complexes: a time course study. Molecular Human Reproduction. 2001;7:129–135. doi: 10.1093/molehr/7.2.129. [DOI] [PubMed] [Google Scholar]
- Bishop LA, Nguyen TV, Schofield PR. Both of the β-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potency. Endocrinology. 1995;136:2635–2640. doi: 10.1210/endo.136.6.7750487. [DOI] [PubMed] [Google Scholar]
- Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine–linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. J Mol Endocrinol. 1994;8:722–731. doi: 10.1210/mend.8.6.7935488. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Baker VL, Gotschall RR, Butnev VY, Butnev VY. Carbohydrate analysis of glycoprotein hormones. Methods. 2000;21:15–39. doi: 10.1006/meth.2000.0972. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Butnev VY, Bidart JM, Dalpathado D, Irungu J, Desaire H. Chromatofocusing fails to separate hFSH isoforms on the basis of glycan structure. Biochemistry. 2008;47:1708–1720. doi: 10.1021/bi701764w. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Butnev VY, Butnev VY, Nguyen VT, Gray CM, Dias JA, MacColl R, Eisele L, Harvey DJ. Differential Effects of α Asparagine56 oligosaccharide structure on equine Lutropin and Follitropin hybrid conformation and receptor-binding activity. Biochemistry. 2004;43:10817–10833. doi: 10.1021/bi049857p. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Butnev VY, Walton WJ, Nguyen VT, Singh V, Hueneidi J, Kolli K, Harvey DJ, Rance N. All or none N-glycosylation in primate follicle-stimulating hormone β subunits. Molec Cell Endocrinol. 2007;260–262:40–48. doi: 10.1016/j.mce.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Liu WK, Ward DN. Hybrids from equine LH: alpha enhances, beta diminishes activity, Mol. Cell Endocrinol. 1985;40:69–77. doi: 10.1016/0303-7207(85)90159-5. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Ward DN. Purification of lutropin and follitropin in high yield from horse pituitary glands. Journal of Biological Chemistry. 1984;259:1911–1921. [PubMed] [Google Scholar]
- Butnev VY, Gotschall RR, Butnev VY, Baker VL, Moore WT, Bousfield GR. Hormone-specific inhibitory influence of α-subunit Asn56 oligosaccharide on in vitro subunit association and FSH receptor binding of equine gonadotropins, Biol. Reprod. 1998;58:458–469. doi: 10.1095/biolreprod58.2.458. [DOI] [PubMed] [Google Scholar]
- Calvo FO, Keutmann HT, Bergert ER, Ryan RJ. Deglycosylated human follitropin: characterization and effects on adenosine cyclic 3′,5′-phosphate production in porcine granulosa cells. Biochemistry. 1986;25:3938–3943. doi: 10.1021/bi00361a030. [DOI] [PubMed] [Google Scholar]
- Dalpathado DS, Irungu JA, Go EP, Butnev VY, Norton K, Bousfield GR, Desaire H. Comparative glycomics of the glycoprotein hormone follicle-stimulating hormone (FSH): glycopeptide analysis of isolates from two mammalian species. Biochemistry. 2006;45:8665–8673. doi: 10.1021/bi060435k. [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack MR, Froehlich J, Bennet AP, Anasti J, Nisula BC. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J Biol Chem. 1994;269:14015–14020. [PubMed] [Google Scholar]
- Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Molecular Endocrinology. 2001;15:378–389. doi: 10.1210/mend.15.3.0603. [DOI] [PubMed] [Google Scholar]
- Green ED, Baenziger JU. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin II. distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. 1988;263:36–44. [PubMed] [Google Scholar]
- Guan R, Wu X, Feng X, Zhang M, Hébert TE, Segaloff DL. Structural determinants underlying constitutive dimerization of unoccupied human follitropin receptors. Cell Signal. 2010;22:247–256. doi: 10.1016/j.cellsig.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DJ, Royle L, Radcliffe CM, Rudd PM, Dwek RA. Structural and quantitative analysis of N-linked glycans by matrix-assisted laser desorption ionization and negative ion nanospray mass spectrometry, Anal. Biochem. 2008;376:44–60. doi: 10.1016/j.ab.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Jiang X, Dias JA, He X. Structural biology of glycoprotein hormones and their receptors: insights to signaling. Molec Cell Endocrinol ePUB. 2013:S0303–7207. doi: 10.1016/j.mce.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Jiang X, Liu H, Chen X, Chen PH, Fischer D, Sriraman V, Yu HN, Arkinstall S, He X. Structure of follicle-stimulating hormone in complex wiht the entire ectodomain of its receptor, Proc. Natl Acad Sci, USA. 2012;109:12491–12496. doi: 10.1073/pnas.1206643109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WK, Yang KP, Nakagawa Y, Ward DN. The role of the amino group in subunit association and receptor site interaction for ovine luteinizing hormone as studied by acylation. J Biol Chem. 1974;249:5544–5550. [PubMed] [Google Scholar]
- Liu WK, Young JD, Ward DN. Deglycosylated ovine lutropin: preparation and characterization by in vitro binding and steroidogenesis. Molecular and Cellular Endocrinology. 1984;37:29–39. doi: 10.1016/0303-7207(84)90125-4. [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz JE, Thomas RM, Nachamen CA, Muda M, Palmer S, Dias JA. FSH receptor is synthesized as a dimer and proteolytically processed at the cell surface. Faseb Journal. 2006;20:A115–A115. [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley ARJ. Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology. 1997;138:424–432. doi: 10.1210/endo.138.1.4892. [DOI] [PubMed] [Google Scholar]
- Parsons TF, Bloomfield GA, Pierce JG. Purification of an alternate form of the α subunit of the glycoprotein hormones from bovine pituitaries and identification of its O-linked oligosaccharide. J Biol Chem. 1983;258:240–244. [PubMed] [Google Scholar]
- Plant TM, Nakai Y, Berchetz P, Keogh E, Knobil E. The sites of action of estradiol and phentolamine in the inhibition of the pulsatile, circhoral discharges of LH in the rhesus monkey (Macaca mulatta) Endocrinology. 1978;102:1015–1018. doi: 10.1210/endo-102-4-1015. [DOI] [PubMed] [Google Scholar]
- Reame N, Sauder SE, Kelch RP, Marshall JC. Pulstatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotopin-releasing hormon secretion. J Clin Endocrinol Metab. 1984;59:328–337. doi: 10.1210/jcem-59-2-328. [DOI] [PubMed] [Google Scholar]
- Renwick AGC, Mizuochi T, Kochibe N, Kobata A. The asparagine-linked sugar chains of human follicle-stimulating hormone. J Biochem. 1987;101:1209–1221. doi: 10.1093/oxfordjournals.jbchem.a121985. [DOI] [PubMed] [Google Scholar]
- Stanton PG, Hearn MT. The iodination sites of bovine thyrotropin. J Biol Chem. 1987;262:1623–1632. [PubMed] [Google Scholar]
- Timossi C, Barrios-de-Tomasi J, Gonzalez-Suarez R, Arranz MC, Padmanabhan V, Conn PM, Ulloa-Aquirre A. Differential effects of the charge variants of human follicle-stimulating hormone. J Endocrinol. 2000;165:193–205. doi: 10.1677/joe.0.1650193. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Chappel SC. Multiple species of follicle-stimulating hormone exist within the anterior pituitary gland of male golden hamsters. J Endocrinol. 1982;95:257–266. doi: 10.1677/joe.0.0950257. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Cravioto A, Damian-Matsumura P, Jimenez M, Zambrano E, Diaz-Sanchez V. Biological characterization of the naturally occurring analogues of intrapituitary human follicle-stimulating hormone, Hum. Reprod. 1992;7:23–30. doi: 10.1093/oxfordjournals.humrep.a137550. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Damian-Matsumura P, Jimenez M, Zambrano E, Diaz-Sanchez V. Biological characterization of the isoforms of urinary human follicle-stimulating hormone contained in a purified commercial preparation, Hum. Reprod. 1992;7:1371–1378. doi: 10.1093/oxfordjournals.humrep.a137576. [DOI] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton WJ, Nguyen VT, Butnev VY, Singh V, Moore WT, Bousfield GR. Characterization of human follicle-stimulating hormone isoforms reveals a non-glycosylated β-subunit In addition to the conventional glycosylated β-subunit. J Clin Endocrinol Metab. 2001;86:3675–3685. doi: 10.1210/jcem.86.8.7712. [DOI] [PubMed] [Google Scholar]
- Ward DN, Glenn SD, Nahm HS, Wen T. Characterization of cleavage products in selected human lutropin preparations, Int. J Peptide Prot Res. 1986;27:70–78. doi: 10.1111/j.1399-3011.1986.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Weisshaar G, Hiyama J, Renwick AGC. Site-specific N-glycosylation of human chorionic gonadotropin—structural analysis of glycopeptides by one- and two-dimensional 1H NMR spectroscopy. Glycobiology. 1991;1:393–404. doi: 10.1093/glycob/1.4.393. [DOI] [PubMed] [Google Scholar]
- West CR, Carlson NE, Lee JS, McNeilly AS, Sharma TP, Ye W, Padmanabhan V. Acidic mix of FSH isoforms are better facilitators of ovarian follicular maturation and E2 production than the less acidic. Endocrinology. 2002;143:107–116. doi: 10.1210/endo.143.1.8601. [DOI] [PubMed] [Google Scholar]
- Wide L. Male and female forms of human follicle-stimulating hormone in serum. J Clin Endocrinol Metab. 1982;55:682–688. doi: 10.1210/jcem-55-4-682. [DOI] [PubMed] [Google Scholar]
- Wide L. Median charge and charge heterogeneity of human pituitary FSH, LH and TSH. II Relationship to sex and age. Acta Endocrinol (Copenh) 1985;109:190–197. doi: 10.1530/acta.0.1090190. [DOI] [PubMed] [Google Scholar]
- Wide L. Evidence for diverse structural variations of the forms of human FSH within and between pituitaries. Acta Endocrinol. 1987;115:7–15. doi: 10.1530/acta.0.1150007. [DOI] [PubMed] [Google Scholar]
- Wide L, Bakos O. More basic forms of both human follicle-stimulating hormone and luteinizing hormone in serum at midcycle compared with the follicular or luteal phase. J Clin Endocrinol Metab. 1993;76:885–889. doi: 10.1210/jcem.76.4.8473400. [DOI] [PubMed] [Google Scholar]
- Wide L, Ericksson K. Dynamic changes in glycosylation and glycan composition of serum FSH and LH during natural ovarian stimulation, Upsala j. Med Sci. 2013;118:153–164. doi: 10.3109/03009734.2013.782081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Olivares A, Mendez JP, Guerrero L, Diaz-Cueto L, Veldhuis JD, Ulloa-Aguirre A. Dynamics of basal and gonadotropin-releasing hormone-releasable serum follicle-stimulating hormone charge isoform distribution throughout the human menstrual cycle. J Clin Endocrinol Metab. 1995;80:1647–1656. doi: 10.1210/jcem.80.5.7745013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.