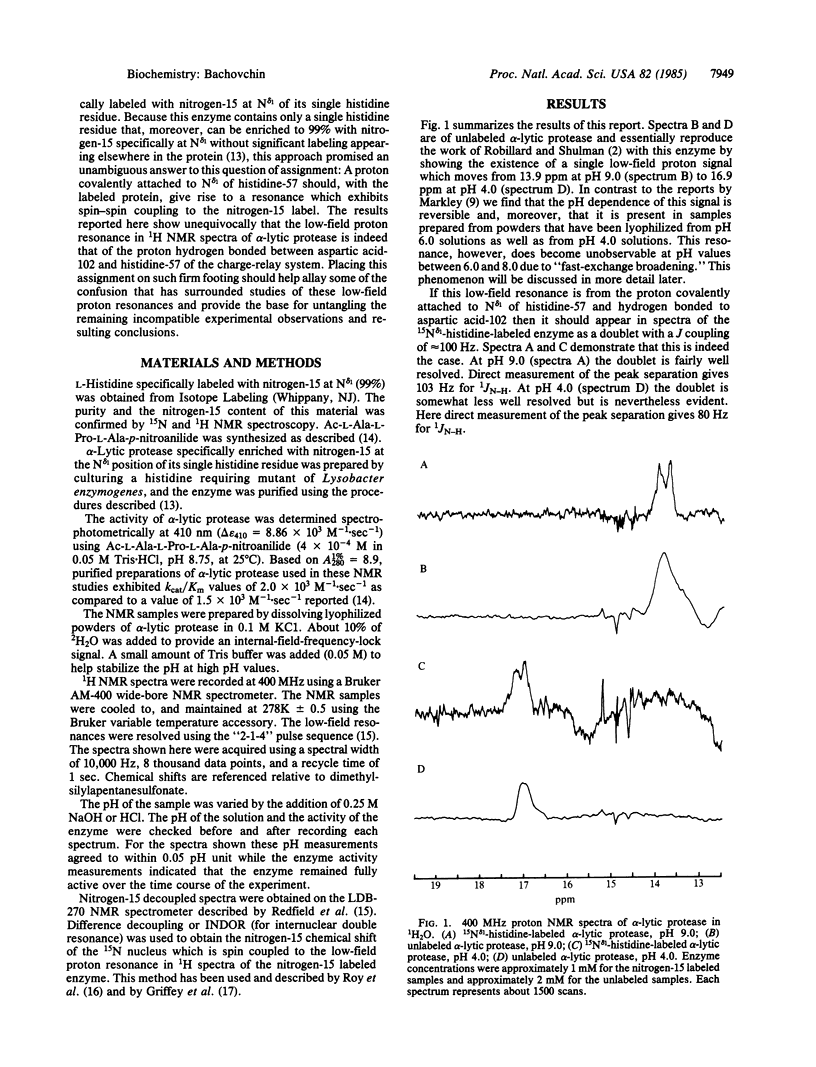
Abstract
Proton NMR spectra of serine proteases in 1H2O solutions typically show a single resonance at very low magnetic field--i.e., 14-18 ppm from dimethylsilylapentanesulfonate. This resonance has been assigned to the proton hydrogen bonded between aspartic acid-102 and histidine-57 (chymotrypsin numbering system) of the "charge-relay system" or catalytic triad of serine proteases [Robillard, G. & Shulman, R. G. (1972) J. Mol. Biol. 71, 507-511]. Since then, there have been a number of reports that have cast doubt on its correctness. In the present work we have tested this assignment using alpha-lytic protease (EC 3.4.21.12, Myxobacter alpha-lytic proteinase), a bacterial serine protease homologous to elastase, which is specifically labeled with nitrogen-15 at N delta 1 of its single histidine residue. The low-field region of the proton spectra of this labeled enzyme shows a single resonance having the properties reported [Robillard, G. & Shulman, R. G. (1974) J. Mol. Biol. 86, 519-540], which, in addition, exhibits spin-spin splitting to the nitrogen-15 label. The observation of this 15N delta 1-H coupling makes the assignment of this resonance to the charge-relay proton unequivocal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Kraut J., Freer S. T. A detailed structural comparison between the charge relay system in chymotrypsinogen and in alpha-chymotrypsin. Biochemistry. 1976 Oct 5;15(20):4481–4485. doi: 10.1021/bi00665a023. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Forgac M. D., Richards J. H. Mechanism of action of serine proteases: tetrahedral intermediate and concerted proton transfer. Biochemistry. 1976 Dec 14;15(25):5581–5588. doi: 10.1021/bi00670a024. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Smallcombe S. H., Whitaker D. R., Richards J. H. Carbon nuclear magnetic resonance studies of the histidine residue in alpha-lytic protease. Implications for the catalytic mechanism of serine proteases. Biochemistry. 1973 Nov 6;12(23):4732–4743. doi: 10.1021/bi00747a028. [DOI] [PubMed] [Google Scholar]
- Jordan F., Polgár L. Proton nuclear magnetic resonance evidence for the absence of a stable hydrogen bond between the active site aspartate and histidine residues of native subtilisins and for its presence in thiolsubtilisins. Biochemistry. 1981 Oct 27;20(22):6366–6370. doi: 10.1021/bi00525a013. [DOI] [PubMed] [Google Scholar]
- Markley J. L. Hydrogen bonds in serine proteinases and their complexes with protein proteinase inhibitors. Proton nuclear magnetic resonance studies. Biochemistry. 1978 Oct 31;17(22):4648–4656. doi: 10.1021/bi00615a010. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Ibañez I. B. Zymogen activation in serine proteinases. Proton magnetic resonance pH titration studies of the two histidines of bovine chymotrypsinogen A and chymotrypsin Aalpha. Biochemistry. 1978 Oct 31;17(22):4627–4640. doi: 10.1021/bi00615a008. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Porubcan M. A. The charge-relay system of serine proteinases: proton magnetic resonance titration studies of the four histidines of porcine trypsin. J Mol Biol. 1976 Apr 15;102(3):487–509. doi: 10.1016/0022-2836(76)90330-2. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Birktoft J. J., Freer T., Kraut J. Re-examination of the charge relay system in subtilisin comparison with other serine proteases. J Biol Chem. 1977 Dec 25;252(24):8875–8883. [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance studies of the active site of chymotrypsin. I. The hydrogen bonded protons of the "charge relay" system. J Mol Biol. 1974 Jul 5;86(3):519–540. doi: 10.1016/0022-2836(74)90178-8. [DOI] [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance studies of the active site of chymotrypsin. II. Polarization of histidine 57 by substrate analogues and competitive inhibitors. J Mol Biol. 1974 Jul 5;86(3):541–558. doi: 10.1016/0022-2836(74)90179-x. [DOI] [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance study of the histidine--aspartate hydrogen bond in chymotrypsin and chymotrypsinogen. J Mol Biol. 1972 Nov 14;71(2):507–511. doi: 10.1016/0022-2836(72)90366-x. [DOI] [PubMed] [Google Scholar]
- Roy S., Papastavros M. Z., Sanchez V., Redfield A. G. Nitrogen-15-labeled yeast tRNAPhe: double and two-dimensional heteronuclear NMR of guanosine and uracil ring NH groups. Biochemistry. 1984 Sep 11;23(19):4395–4400. doi: 10.1021/bi00314a024. [DOI] [PubMed] [Google Scholar]



