Abstract
Objectives
We aimed to identify the frequency, pattern, and prognostic significance of left ventricular (LV) late gadolinium enhancement (LGE) in patients with atrial fibrillation (AF).
Background
There are limited data on the presence, pattern, and prognostic significance of LV myocardial fibrosis in patients with AF. Late gadolinium enhancement during cardiac magnetic resonance (CMR) is a marker for myocardial fibrosis.
Methods
We studied a consecutive group of 664 patients without known prior myocardial infarction being referred for radiofrequency ablation of AF. CMR was requested to assess pulmonary venous anatomy.
Results
Overall, 73% were male, with an average age of 56 years, and an ejection fraction of 55±10%. Left ventricular LGE was found in 88 patients (13%). The endpoint was all-cause mortality, and in this cohort we observed 68 deaths over a median follow-up period of 42 months. On univariable analysis, age (HR 1.05, CI 1.03–1.08, LRχ2 15.2, p=0.0001), diabetes (HR 2.39, CI 1.41–4.09, LRχ210.3, p=0.001), a history of heart failure (HR 1.78, CI 1.09–2.91, LRχ2 5.37, p=0.02), left atrial dimension (HR 1.04, CI 1.01–1.08, LRχ2 6.47, p=0.01), presence of LGE (HR 5.08, CI 3.08–8.36, LRχ2 28.8, p<0.0001), and LGE extent (HR 1.15, CI 1.10–1.21, LRχ2 35.6, p<0.0001) provided the strongest association with mortality. The mortality rate was 8.1% per patient-years in patients with LGE vs. 2.3% patients without LGE. In the best overall multivariable model for mortality, age and the extent of LGE were independent predictors of mortality. Indeed, each 1% increase in LGE associated with a 15% increased risk of death.
Conclusions
In patients with AF, LV LGE is a frequent finding and is a powerful predictor of mortality.
Keywords: Late Gadolinium Enhancement, Atrial Fibrillation, Cardiac Magnetic Resonance
Atrial fibrillation (AF) is the most common clinical cardiac arrhythmia with estimates suggesting that it affects approximately 1 in 25 adults over the age of 60 in the United States (1). The occurrence of AF is associated with an increase in both cardiovascular and all-cause mortality (2,3). Catheter ablation offers a viable alternative in symptomatic patients that are refractory to pharmacological therapy (4,5), and the use of catheter ablation is increasing (6). The pulmonary veins are the key targets for the ablation of AF (4). For this reason, detailed anatomic imaging of the left atrium and pulmonary veins is routinely performed prior to performance of a catheter ablation (6,7). Imaging is performed to allow the use of advanced mapping systems during the procedure, to detect anatomical variants, and to minimize complications (8). Multiple different techniques exist for anatomical imaging including angiography, computerized tomography, ultrasound, and cardiac magnetic resonance (CMR) imaging, and there are currently no guidelines and limited clinical data to support an advantage for one imaging modality over another (9). Cardiac magnetic resonance provides accurate and detailed pulmonary vein anatomy prior to pulmonary vein isolation (10), and CMR imaging may also provide complementary information. Specifically, left ventricular (LV) myocardial fibrosis identified using late gadolinium enhancement (LGE) has been shown to be a predictor of adverse outcomes in broad groups of patients (11–15). However, there are limited data on the presence, pattern, and prognostic significance of LV LGE in patients with AF (16). Therefore, the aim of this study was to determine the incidence, pattern, and prognostic significance of unanticipated LV LGE in patients with AF. We hypothesized that unanticipated LV LGE would be a frequent occurrence and that the presence of LV LGE would be associated with adverse outcomes.
Methods
Study population
We prospectively collected data on all consecutive patients from September 2005 through June 2011 that underwent a CMR study prior to pulmonary vein isolation. The study indication was specifically for identification of pulmonary vein anatomy (7). All patients at our institution, in whom pulmonary vein isolation is being planned, and without a contra-indication to the performance of a magnetic resonance study, undergo a CMR for imaging of pulmonary venous anatomy. Contraindications to a CMR study included the presence of a permanent pacemaker, severe claustrophobia, and severe impairment of renal function (glomerular filtration rate <30 mL/min/1.73 m2). Paroxysmal AF was defined as AF that terminated spontaneously less than 7 days after onset, while persistent AF was defined as that those extending beyond 7 days. Hypertension was defined as a systolic blood pressure of above 139 mm Hg systolic or diastolic above 89 mm Hg diastolic on multiple measurements or use of antihypertensive medication. Heart failure was defined as a clinical history of heart failure or reduced left ventricular (LV) ejection fraction (EF). We defined recurrence of AF was defined as AF occurring >3 months after pulmonary vein isolation and confirmed by either EKG or cardiac monitoring. We subsequently excluded patients who had prior myocardial infarction (MI) by either clinical evidence of an MI per electronic medical records or EKG evidence defined by Minnesota codes 1.1.1–1.2.8(17). We also obtained the LV measurements measured using echocardiography that was performed at the time of the planned ablation. The Human Subjects Research Review Committee of our institution approved the study protocol.
CMR protocol
All images were acquired with EKG gating, breath-holding, and with the patient in a supine position. Subjects were imaged on either a 1.5 or 3.0-T CMR system (SignaHDxt, General Electric Healthcare, Waukesha, Wisconsin; Tim Trio, Siemens, Erlangen, Germany, respectively). The CMR protocol consisted of cine steady-state free precession (SSFP) imaging for cardiac function (typical repetition time, 3.4 ms; echo time, 1.2ms; in-plane spatial resolution, 1.6 × 2 mm), pulmonary vein anatomy imaging, and LGE imaging (repetition time, 4.8 ms; echo time, 1.3 ms; inversion time, 200 to 300 ms). For LGE imaging, a segmented inversion-recovery pulse sequence was used starting 10–15 minutes after a single bolus dose of 0.15-mmol/kg of gadolinium DTPA (Magnevist®, Bayer HealthCare). Cine imaging and LV LGE imaging were obtained in 8 to 14 matching short-axis (8 mm thick with0-mm spacing) and 3 radial long-axis planes. This CMR prescription was to ensure whole-heart coverage was obtained for complete LV and RV assessment. LGE was interpreted as present or absent by the consensus of 2 CMR-trained physicians. LGE was considered present only if confirmed on both short-axis and matching long-axis myocardial locations. LGE extent was quantified by a semi-automatic detection method using a previously validated research tool (Mass Research, Leiden University Medical Center, Belgium), with the extent of LGE defined using the full-width at half maximum (FWHM) criteria (18). The mass of LV LGE was measured in grams and was expressed as a percentage of the total LV mass. The distribution of LGE was characterized as subendocardial, transmural, mid-wall, epicardial, or focal/involving the RV insertion points.
Methods of clinical follow-up
The endpoint of interest was all-cause mortality. We ascertained patient mortality using the Social Security Death Index (SSDI) and reviewed electronic medical records of all patients. When electronic medical records of a patient provided insufficient follow-up information, the primary provider of the patient was contacted regarding clinical events. Complete follow-up was available for all patients.
Statistical analysis
Continuous data are presented as mean ± SD. Continuous data were compared using an unpaired Student t-test or Mann–Whitney non-parametric test as appropriate. Variables lacking a normal distribution and evaluated with non-parametric tests are summarized with medians and quartiles. Nominal data are presented as number and percentages and were compared with a Fisher exact test or a Chi-squared test, whichever was appropriate. The hazard ratio for the prediction of the event was calculated for mortality using a Cox regression model using three cohorts: all patients, patients without evidence of MI by clinical history or EKG, and patients without evidence of MI by clinical history, EKG, or LGE imaging. We considered all the significant variables in the univariable analysis, and sought the best-overall multivariable models for mortality, by stepwise-forward selection with a probability to enter set at p=0.01 and to remove the effect from the regression at p=0.01. Event curves were determined according to the Kaplan–Meier method and comparisons of mortality rates were performed by the log-rank test. A two-tailed p value of < 0.05 was considered significant for all other analyses. SAS was used for statistical analysis (SAS Institute Inc, Cary, NC).
Results
In total, 720 consecutive patients were referred for a CMR in preparation for pulmonary vein isolation. Of the entire cohort, 56 patients had a prior MI by clinical history or EKG. Cohort characteristics from the entire cohort of 720 patients, the 664 patients without known MI by clinical history or EKG, and this cohort further stratified according to the presence or absence of LGE are presented in Table 1. In brief, among this cohort, there were 484 males (73%) with an average age of 56±11 years (range 24–85 years). Patients presented a median of 49 months after first symptomatic onset of atrial (range 12 months to 12 years); 435 (65%) patients had persistent atrial fibrillation, 229 (35%) had paroxysmal atrial fibrillation, and 430 (65%) of patients were in sinus rhythm at the time of the study. There were 324 (49%) of patients with hypertension, 130 (20%) of patients with sleep apnea, 98 (15%) had diabetes, and 172 (26%) had heart failure. In total, 429 patients (69%) were on a class 1 or class 3 anti-arrhythmic.
Table 1.
Characteristics of all Patients, Patients without a prior MI by clinical History or EKG, and stratified by the presence or absence of LGE
| Variable | Entire Cohort (720) | No Prior MI (664) | LGE Positive (88) | LGE Negative (576) | p Value |
|---|---|---|---|---|---|
| Age (yrs) | 56 (10) | 56 (11) | 59 (10) | 55 (10) | 0.007 |
| Male, n (%) | 531 (74) | 484 (73) | 63 (71) | 421 (73) | 0.69 |
| Duration of AF (median, IQR) | 50 (29–83) | 49 (29–84) | 54 (34–84) | 49 (16–77) | 0.71 |
| Paroxysmal Atrial Fibrillation, n (%) | 250 (35) | 229 (35) | 32 (36) | 197 (34) | 0.72 |
| Persistent Atrial Fibrillation, n (%) | 472 (66) | 435 (65) | 49 (60) | 386 (67) | 0.72 |
| Prior AF Ablation, n (%) | 173 (24) | 160 (24) | 24 (27) | 136 (24) | 0.50 |
| Cardiovascular Risk Factors, n (%): | |||||
| Diabetes Mellitus | 106 (15) | 98 (15) | 18 (20) | 80 (14) | 0.11 |
| Hypertension | 365 ((51) | 324 (49) | 50 (57) | 274 (48) | 0.11 |
| Heart Failure | 186 (26) | 172 (26) | 32 (36) | 140 (24) | 0.02 |
| Obstructive Sleep Apnea | 142 (20) | 130 (20) | 27 (31) | 103 (18) | 0.009 |
| Valvular Heart Disease | 77 (11) | 70 (11) | 12 (14) | 58 (10) | 0.35 |
| Hyperthyroidism | 34 (5) | 32 (5) | 5 (6) | 27 (5) | 0.60 |
| Hypercholesterolemia | 240 (33) | 203 (31) | 31 (35) | 172 (30) | 0.32 |
| Alcohol Excess | 59 (8) | 54 (8) | 5 (6) | 49 (8) | 0.53 |
| Family History AF | 88 (12) | 84 (13) | 10 (11) | 74 (13) | 0.73 |
| Medication, n (%): | |||||
| Aspirin | 325 (45) | 291 (44) | 39 (44) | 252 (44) | 0.95 |
| Beta-blocker | 491 (68) | 446 (67) | 66 (75) | 380 (66) | 0.11 |
| Calcium channel blocker | 164 (23) | 148 (22) | 19 (22) | 129 (22) | 1.00 |
| ACE/ARB | 261 (36) | 234 (35) | 34 (39) | 200 (35) | 0.47 |
| Class 1 Anti-arrhythmic | 157 (22) | 157 (24) | 16 (18) | 141 (24) | 0.22 |
| Class 3 Anti-arrhythmic | 341 (47) | 298 (45) | 45 (51) | 253 (44) | 0.21 |
| Digoxin | 64 (9) | 56 (8) | 8 (9) | 48 (8) | 0.38 |
| Spironolactone | 19 (3) | 17 (3) | 3 (3) | 14 (2) | 0.48 |
| Diuretics | 126 (18) | 117 (18) | 20 (23) | 97 (17) | 0.18 |
| Statin | 237 (33) | 188 (28) | 30 (34) | 158 (27) | 0.21 |
| BMI (kg/m2) | 29 (5) | 29.5 (5) | 30.6 (5) | 29.2 (5) | 0.03 |
| Systolic Blood Pressure (mmHg) | 127 (17) | 127 (17) | 126 (19) | 127 (17) | 0.89 |
| Diastolic Blood Pressure (mmHg) | 75 (12) | 75 (12) | 74 (12) | 75 (12) | 0.56 |
| Heart Rate (beats/min) | 72 (17) | 72 (17) | 73 (19) | 72 (17) | 0.88 |
| EKG Parameters: | |||||
| Sinus Rhythm at presentation, n (%) | 459 (64) | 430 (65) | 56 (64) | 374 (65) | 0.81 |
| AV Delay (ms) | 172 (31) | 172 (32) | 185 (33) | 170 (31) | 0.006 |
| QRS Duration (ms) | 96 (15) | 96 (15) | 100 (18) | 95 (14) | 0.006 |
| QTc Duration (ms) | 442 (33) | 441 (33) | 445 (28) | 440 (33) | 0.15 |
| LVH by EKG (Sokolov Criteria), n (%) | 56 (8) | 52 (8) | 8 (9) | 44 (8) | 0.67 |
| GFR (ml/min/1.73m2) | 83 (17) | 83 (17) | 78 (17) | 84 (17) | 0.001 |
All data are number (percentage) or mean and SD unless otherwise indicated; BMI = body mass index; ACE/ARB = angiotensin converting enzyme inhibitor/angiotensin receptor blocker; GFR = glomerular filtration rate using the Modification of Diet in Renal Disease formula done at the time of the CMR p value = LGE positive vs. LGE negative patients without a prior clinical history of MI.
Imaging characteristics
Imaging characteristics from the entire cohort of 720 patients, the 664 patients without known MI by clinical history or EKG, and this cohort separated according to the presence or absence of LGE are presented in Table 2. By echocardiography, mean LVEF was 55±10%, mean LV end-diastolic dimension was 49±5 mm, mean left atrial dimension was 41±7 mm, and mean estimated pulmonary artery systolic pressure was 29±7 mmHg. By CMR, mean LV end-diastolic volume was 167±42 mls, mean LVEF was 56±10%, mean LV mass indexed to body surface area was 71±12 grams, mean right ventricular end-diastolic volume was 163±42 mls, and the mean right ventricular EF was 52±8% (Table 2).
Table 2.
Imaging Characteristics of all Patients, Patients without a prior MI by clinical History or EKG, and stratified by the presence or absence of LGE
| Variable | Entire Cohort (720) | No Prior MI (664) | LGE Positive (88) | LGE Negative (576) | p Value |
|---|---|---|---|---|---|
| Echocardiographic Parameters: | |||||
| LV Ejection Fraction (%) | 55 (10) | 55 (10) | 54 (13) | 55 (10) | 0.21 |
| LV Diastolic Dimension (mm) | 49 (5) | 49 (5) | 49 (7) | 49 (5) | 0.62 |
| Estimated PASP (mmHg) | 29 (7) | 29 (7) | 28 (7) | 29 (7) | 0.28 |
| Left Atrial Dimension (mm) | 41 (6) | 41 (6) | 43 (6) | 41 (6) | 0.0004 |
| Cardiac Magnetic Resonance: | |||||
| LV EDV (ml) | 168 (43) | 167 (42) | 165 (42) | 167 (42) | 0.68 |
| LV ESV (ml) | 75 (28) | 74 (27) | 76 (30) | 73 (26) | 0.37 |
| LV Ejection Fraction (%) | 56 (10) | 56 (10) | 54 (12) | 57 (9) | 0.006 |
| LV Mass (grams) | 149 (33) | 148 (33) | 154 (37) | 148 (33) | 0.08 |
| LV Mass index (grams/m2) | 72 (12) | 71 (12) | 74 (14) | 71 (12) | 0.01 |
| RV EDV (ml) | 164 (42) | 163 (42) | 154 (43) | 164 (42) | 0.07 |
| RV ESV (ml) | 80 (26) | 80 (26) | 75 (29) | 81 (25) | 0.10 |
| RV Ejection Fraction (%) | 52 (7) | 52 (8) | 53 (8) | 52 (7) | 0.32 |
| Left Atrial Dimension (mm) | 41 (7) | 41 (7) | 44 (8) | 40 (7) | <0.0001 |
| LV LGE, n (%) | 108 (15) | 88 (13) | |||
| LV LGE FWHM (% of LV mass) | 6.4 (3.5) | 5.9 (3) | |||
| LV LGE Location (n, %): | |||||
| Subendocardial | 50 (46) | 38 (43) | |||
| Transmural | 14 (13) | 6 (7) | |||
| Epicardial | 1 (1) | 1 (1) | |||
| Mid-myocardial | 32 (30) | 32 (37) | |||
| Insertion points | 11 (10) | 11 (12) | |||
All data are number (percentage) or mean and SD unless otherwise indicated; LV = left ventricular; PASP = pulmonary artery systolic pressure; LV EDV = left ventricular end diastolic volume; LVESV = left ventricular end systolic volume; RVEDV = right ventricular end diastolic volume; RVESV = right ventricular end systolic volume; RVEF = right ventricular ejection fraction; LGE FWHM = LGE using full width half maximum method. p value = LGE positive vs. LGE negative patients without a prior clinical history of MI.
Late gadolinium enhancement
Among the entire cohort, LGE was detected in 108 patients (15%). Among the entire cohort, the LGE pattern was ischemic in 59% (transmural in 14 (13%) and subendocardial in 50 (46%)) and non-ischemic in 41% (mid-myocardial in 32 (30%), insertion point in 11 (10%), and epicardial in 1 (1%), Table 2). When patients with a clinical history of or EKG evidence for an MI were excluded, LGE detected in 88 (13%, Table 2). The pattern of LGE pattern was ischemic in 50% (transmural in 6 (7%) and subendocardial in 38 (43%)) and non-ischemic in 50% (mid-myocardial in 32 (37%), insertion point in 11 (12%), and epicardial in 1 (1%), representative images are displayed in Figure 1). The average extent of LGE was 5.9±3% (median 5.2%, range from 1.2% to 14.6%). Patients were grouped according to the presence or absence of LGE (Table 1, Table 2). There were baseline differences among the cohorts with and without LGE. Patients with LGE were on average older, and were more likely to have heart failure and sleep apnea. Patients with LGE were also more likely to have a lower GFR, a lower LV EF, increased LV mass, increased left atrial dimensions, a longer PR interval and a wider QRS interval. We performed clinical follow-up on the patients with unanticipated LGE. There were 44 patients with LGE in an ischemic distribution. Of these 44 patients, 42 underwent stress testing with imaging, 26 had evidence of ischemia, 21 had evidence of significant CAD on angiography, and 18 had a revascularization procedure. Of the patients with LGE in a non-ischemic distribution (44 patients), 38 underwent stress testing or angiography. Of these patients, 5 had evidence of significant CAD, and 2 underwent a revascularization procedure. In comparison, 85 of the 576 patients (16%) without LGE underwent subsequent assessment for the presence of obstructive coronary disease (p < 0.001). We conclude that, despite limited by verification bias, that an ischemic pattern of LGE was strongly associated with significant angiographic coronary stenosis and subsequent coronary revascularization.
Figure 1. Representative LGE images comparing a normal patient (A), a patient with mid-myocardial late gadolinium enhancement typically seen in dilated cardiomyopathy (B), a patient with a subendocardial myocardial infarct (C), and a patient with subepicardial late gadolinium enhancement (D).
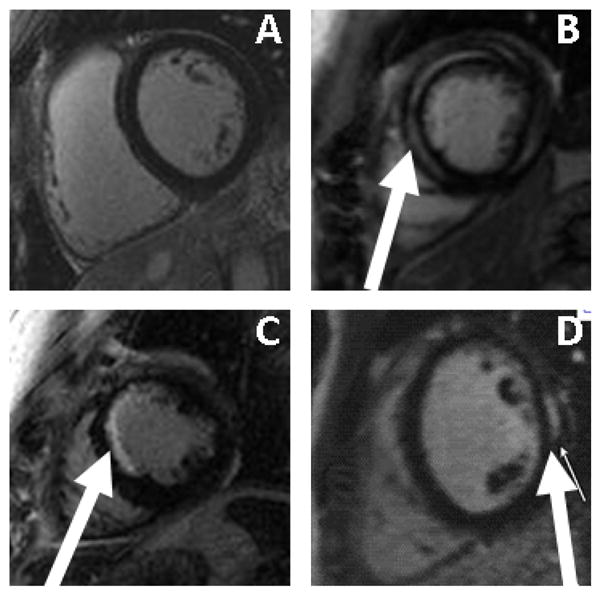
Regions of LGE are highlighted using white arrows.
Mortality
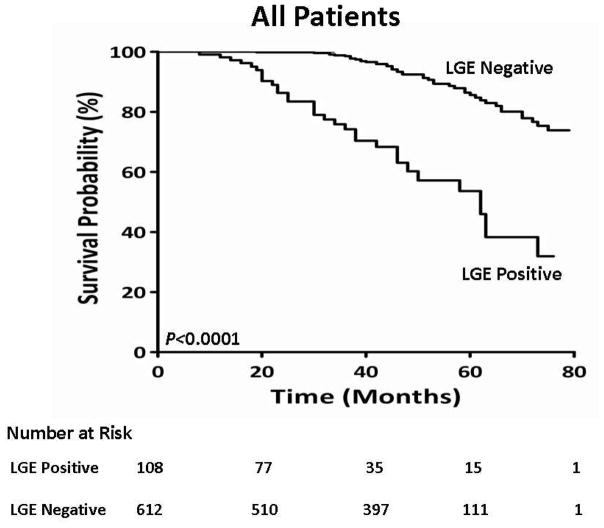
There were 68 deaths over a median of 42 months of follow-up. The mortality rate of the whole cohort was 2.9% per patient-years. There were 46 deaths among 582 patients without LGE (2.3% mortality rate per patient-years) as compared to 22 deaths among 88 patients with LGE (8.1% mortality rate per patient-years).
Univariable and multivariable associations with mortality
We tested the associations with mortality among three cohorts; all patients, patients without evidence of MI by clinical history or EKG, and patients without evidence of MI by clinical history, EKG, or LGE imaging. Among the entire cohort of all patients, there were 78 deaths. On univariate analysis among all patients (Table 3), age (HR 1.05, CI 1.02–1.07, LRχ2 14.9, p = 0.0001), diabetes (HR 2.07, CI 1.23–3.50, LRχ2 7.56, p = 0.006), hypertension (HR 1.72, CI 1.10–2.71, LRχ2 5.55, p = 0.02), heart failure (HR 1.76, CI 1.17–2.80, LRχ2 5.92, p = 0.01), left atrial dimension (HR 1.04, CI 1.01–1.08, LRχ2 7.36, p = 0.007), the presence of LGE (HR 6.09, CI 3.88–9.55, LRχ2 25.5, p <0.0001), and the extent of LGE (HR 1.17, CI 1.10–1.24, LRχ2 25.8, p <0.0001) provided the strongest unadjusted association with mortality among the entire cohort. In a multivariable model among all patients, age (HR, 1.04, 95% CI 1.01–1.06, LRχ2 8.81, p = 0.003) and the extent of LGE provided the strongest adjusted association with mortality (HR, 1.16, 95% CI 1.10–1.22, LRχ2 24.5, p <0.0001). A Kaplan-Meier curve showing the difference in mortality between all patients according to the presence or absence of LGE is shown (Figure 2). In a second cohort, we excluded patients with a clinical history of MI or evidence of an MI by EKG. In that cohort, on univariable analysis, age (HR 1.05, CI 1.03–1.08, LRχ2 15.2, p=0.0001), diabetes (HR 2.39, CI 1.41–4.09, LRχ2 10.3, p=0.001), heart failure (HR 1.78, CI 1.09–2.91, LRχ2 5.37, p=0.02), left atrial dimension (HR 1.04, CI 1.01–1.08, LRχ2 6.47, p=0.01), the presence of LGE (HR 5.08, CI 3.08–8.36, LRχ2 28.8, p<0.0001), and the extent of LGE (HR 1.15, CI 1.10–1.21, LRχ2 35.6, p<0.0001) provided the strongest association with mortality (Table 4). In a multivariable model, age (HR, 1.05, 95% CI 1.02–1.08, LRχ2 11.1, p=0.009) and the extent of LGE, again provided the strongest adjusted association with mortality (HR, 1.15, 95% CI 1.10–1.21, LRχ2 32.5, p<0.0001). A Kaplan-Meier curve showing a significant difference in survival among this cohort, according to the presence or absence of LGE, is presented in Figure 3. In the third cohort, we excluded patients with a prior history of MI by clinical history, EKG, or an ischemic LGE pattern on CMR (Table 5). In this third cohort, the extent of LGE had the strongest unadjusted association with mortality (HR 1.24, CI 1.13–1.35, LRχ2 22.4, p<0.0001)).
Table 3.
Univariable Analyses for Association with Mortality Among All Patients
| Variable | HR | CI | LRχ2 | p Value |
|---|---|---|---|---|
| Age | 1.05 | 1.02–1.07 | 14.9 | 0.0001 |
| Male | 0.78 | 0.47–1.31 | 0.87 | 0.35 |
| Duration of AF | 1.00 | 0.99–1.00 | 0.43 | 0.51 |
| History of Hypertension | 1.72 | 1.10–2.71 | 5.55 | 0.02 |
| History of Prior AF Ablation | 0.80 | 0.45–1.41 | 0.59 | 0.44 |
| History of MI | 1.59 | 0.69–3.67 | 1.19 | 0.27 |
| EKG MI | 2.48 | 1.00–6.17 | 3.84 | 0.05 |
| History of Diabetes Mellitus | 2.07 | 1.23–3.50 | 7.56 | 0.006 |
| History of Obstructive Sleep Apnea | 0.98 | 0.56–1.71 | 0.03 | 0.95 |
| History of Valvular Heart Disease | 1.16 | 0.74–2.80 | 1.68 | 0.28 |
| History of Heart Failure | 1.76 | 1.17–2.80 | 5.92 | 0.01 |
| Beta-blockers | 1.49 | 0.90–2.49 | 2.39 | 0.12 |
| ACE/ARB Inhibitor | 1.58 | 1.02–2.46 | 4.10 | 0.05 |
| Class I Anti-arrhythmic | 0.53 | 0.29–1.02 | 3.78 | 0.08 |
| Class III Anti-arrhythmic | 1.51 | 0.87–2.13 | 1.91 | 0.17 |
| Diuretic Therapy | 1.17 | 0.67–2.06 | 1.17 | 0.57 |
| Statin Use | 1.73 | 0.63–2.57 | 0.51 | 0.47 |
| Aspirin Use | 0.75 | 0.48–1.17 | 1.59 | 0.20 |
| Systolic Blood Pressure | 0.99 | 0.98–1.01 | 0.86 | 0.35 |
| Diastolic Blood Pressure | 0.99 | 0.97–1.01 | 1.44 | 0.23 |
| Heart Rate | 1.01 | 0.10–1.02 | 2.56 | 0.11 |
| BMI | 1.04 | 0.99–1.09 | 3.12 | 0.08 |
| Sinus Rhythm (at presentation) | 0.90 | 0.56–1.45 | 0.19 | 0.66 |
| AV Delay | 1.02 | 0.99–1.01 | 0.28 | 0.60 |
| QRS Duration | 1.01 | 1.00–1.02 | 4.02 | 0.05 |
| QTc Duration | 1.00 | 1.00–1.01 | 0.74 | 0.39 |
| Echocardiographic Parameters: | ||||
| LV Ejection Fraction | 0.99 | 0.97–1.01 | 1.51 | 0.22 |
| Estimated PASP | 1.00 | 0.97–1.04 | 0.05 | 0.83 |
| LV Diastolic Dimension | 1.01 | 0.97–1.06 | 0.23 | 0.63 |
| Left Atrial Dimension | 1.04 | 1.02–1.07 | 4.38 | 0.04 |
| Cardiac Magnetic Resonance | ||||
| LV EDV | 1.00 | 0.99–1.00 | 0.02 | 0.90 |
| LV ESV | 1.01 | 1.00–1.01 | 1.85 | 0.17 |
| LV EF | 0.98 | 0.96–1.02 | 2.99 | 0.08 |
| LV Mass Index | 1.01 | 0.99–1.03 | 0.96 | 0.39 |
| RV EDV | 1.00 | 0.99–1.00 | 0.10 | 0.92 |
| RV ESV | 0.99 | 0.98–1.00 | 1.27 | 0.26 |
| RV EF | 1.00 | 0.97–1.03 | 0.43 | 0.51 |
| Left Atrial Dimension | 1.04 | 1.01–1.08 | 7.36 | 0.007 |
| Late Gadolinium Enhancement: | ||||
| Presence of LGE | 6.09 | 3.88–9.55 | 25.5 | <0.0001 |
| Mid-myocardial LGE | 5.41 | 3.28–8.15 | 18.7 | 0.0001 |
| Sub-endocardial LGE | 5.92 | 3.18–8.60 | 23.2 | <0.0001 |
| Extent of LGE | 1.17 | 1.10–1.24 | 25.8 | <0.0001 |
Figure 2. Kaplan Meier curves displaying survival probability in cohorts according to the presence of absence of LGE.
Results were compared using a Log-Rank test with a p value of < 0.0001.
Table 4.
Univariable Analyses for Association with Mortality in Patients Without a Prior MI by History or EKG
| Variable | HR | CI | LRχ2 | p Value |
|---|---|---|---|---|
| Age | 1.05 | 1.03–1.08 | 15.2 | 0.0001 |
| Male | 0.72 | 0.42–1.24 | 1.37 | 0.24 |
| Duration of AF | 1.00 | 0.99–1.00 | 0.12 | 0.73 |
| History of Hypertension | 1.58 | 0.98–2.56 | 3.51 | 0.06 |
| History of Prior AF Ablation | 0.89 | 0.49–1.62 | 0.15 | 0.70 |
| History of Diabetes Mellitus | 2.39 | 1.41–4.09 | 10.3 | 0.001 |
| History of Obstructive Sleep Apnea | 1.52 | 0.97–2.02 | 2.08 | 0.18 |
| History of Valvular Heart Disease | 1.51 | 0.75–3.06 | 1.36 | 0.24 |
| History of Heart Failure | 1.78 | 1.09–2.91 | 5.37 | 0.02 |
| History of Paroxysmal AF | 1.00 | 0.69–1.46 | 0.01 | 0.95 |
| History of Persistent AF | 1.01 | 0.69–1.46 | 0.01 | 0.98 |
| AF Recurrence post-PVI | 1.39 | 0.99–1.96 | 3.67 | 0.06 |
| Beta-blockers | 1.36 | 0.79–2.32 | 1.23 | 0.27 |
| Calcium Channel Blockers | 1.25 | 0.71–2.19 | 0.62 | 0.43 |
| ACE/ARB Inhibitor | 1.22 | 0.74–2.03 | 0.60 | 0.44 |
| Class I Anti-arrhythmic | 0.59 | 0.32–1.08 | 2.91 | 0.08 |
| Class III Anti-arrhythmic | 1.27 | 0.77–2.10 | 0.85 | 0.36 |
| Diuretic Therapy | 0.14 | 0.61–2.05 | 0.13 | 0.71 |
| Statin Use | 0.83 | 0.19–1.44 | 1.57 | 0.23 |
| Systolic Blood Pressure | 0.99 | 0.98–1.01 | 0.73 | 0.39 |
| Diastolic Blood Pressure | 0.99 | 0.97–1.01 | 1.39 | 0.24 |
| Heart Rate | 1.01 | 0.99–1.02 | 2.32 | 0.12 |
| Body Mass Index | 1.04 | 0.99–1.09 | 2.65 | 0.10 |
| Sinus Rhythm (at presentation) | 0.86 | 0.51–1.44 | 0.33 | 0.57 |
| AV Delay | 1.00 | 0.99–1.01 | 0.57 | 0.45 |
| QRS Duration | 1.01 | 1.00–1.03 | 4.03 | 0.05 |
| QTc Duration | 1.01 | 1.00–1.01 | 2.17 | 0.14 |
| Echocardiographic Parameters: | ||||
| LV Ejection Fraction | 0.99 | 0.97–1.02 | 0.03 | 0.86 |
| Estimated PASP | 1.02 | 0.98–1.05 | 0.99 | 0.32 |
| LV Diastolic Dimension | 1.00 | 0.96–1.06 | 0.03 | 0.85 |
| Left Atrial Dimension | 1.03 | 1.00–1.07 | 3.15 | 0.08 |
| Cardiac Magnetic Resonance Parameters: | ||||
| LV EDV | 1.00 | 0.99–1.00 | 0.09 | 0.76 |
| LV ESV | 1.00 | 0.99–1.01 | 0.55 | 0.46 |
| LV EF | 0.99 | 0.97–1.01 | 0.66 | 0.41 |
| LV Mass | 1.00 | 0.99–1.01 | 0.03 | 0.85 |
| LV Mass Index | 1.00 | 0.98–1.03 | 0.23 | 0.63 |
| RV EDV | 0.99 | 0.99–1.00 | 0.05 | 0.83 |
| RV ESV | 0.99 | 0.99–1.00 | 0.08 | 0.76 |
| RV EF | 0.99 | 0.96–1.02 | 0.44 | 0.50 |
| Left Atrial Dimension | 1.04 | 1.01–1.08 | 6.47 | 0.01 |
| Late Gadolinium Enhancement: | ||||
| Presence of LGE | 5.08 | 3.08–8.36 | 28.8 | <0.0001 |
| Mid-myocardial LGE | 5.91 | 3.58–11.6 | 26.7 | <0.0001 |
| Sub-endocardial LGE | 3.71 | 1.95–7.10 | 15.9 | 0.0001 |
| Extent of LGE | 1.15 | 1.10–1.21 | 35.6 | <0.0001 |
Figure 3. Kaplan Meier curves displaying survival probability in a sub-cohort without a clinical or EKG history of MI.
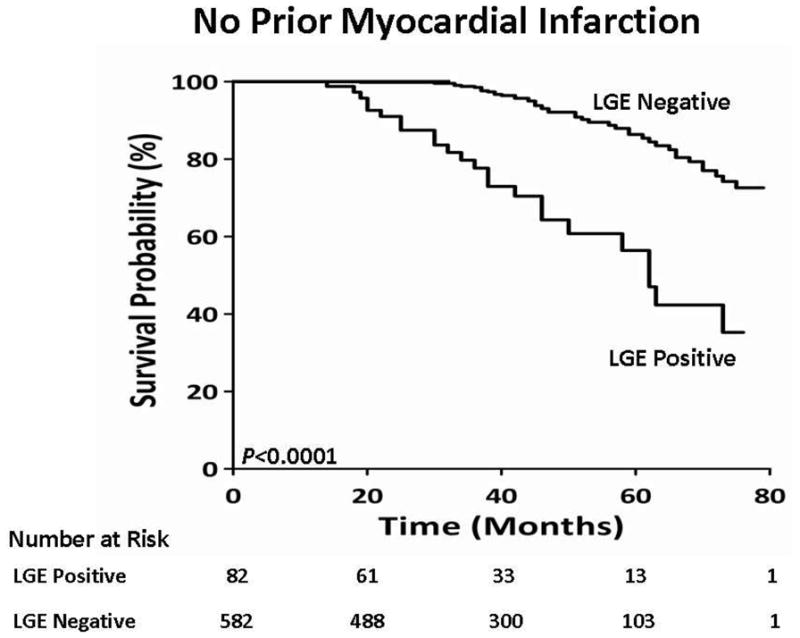
Results were compared using a Log-Rank test with a p value of < 0.0001.
Table 5.
Univariable Analyses for Association with Mortality in Patients Without Evidence of Myocardial Infarction by clinical history, EKG, or LGE imaging
| Variable | HR | CI | LRχ2 | p Value |
|---|---|---|---|---|
| Age | 1.06 | 1.03–1.09 | 17.4 | <0.0001 |
| Male | 0.69 | 0.40–1.22 | 1.6 | 0.21 |
| Duration of AF | 0.99 | 0.99–1.00 | 0.93 | 0.33 |
| History of Hypertension | 1.58 | 0.94–2.66 | 2.91 | 0.09 |
| History of Prior AF Ablation | 0.76 | 0.39–1.48 | 0.64 | 0.42 |
| History of Diabetes Mellitus | 2.65 | 1.49–4.69 | 11.2 | 0.0008 |
| History of Obstructive Sleep Apnea | 1.56 | 0.94–2.02 | 2.28 | 0.16 |
| History of Valvular Heart Disease | 1.60 | 0.76–3.39 | 1.53 | 0.22 |
| History of Heart Failure | 2.02 | 1.19–3.41 | 6.84 | 0.009 |
| Beta-blockers | 1.43 | 0.81–2.50 | 1.56 | 0.21 |
| Calcium Channel Blockers | 0.60 | 0.30–1.23 | 1.92 | 0.17 |
| ACE/ARB Inhibitor | 1.42 | 0.85–2.40 | 1.77 | 0.18 |
| Class I Anti-arrhythmic | 0.57 | 0.29–1.10 | 2.80 | 0.09 |
| Class III Anti-arrhythmic | 1.03 | 0.61–1.75 | 0.01 | 0.89 |
| Diuretic Therapy | 1.23 | 0.65–2.32 | 0.39 | 0.53 |
| Statin Use | 1.75 | 1.02–3.03 | 4.05 | 0.04 |
| Systolic Blood Pressure | 0.99 | 0.98–1.01 | 0.10 | 0.74 |
| Diastolic Blood Pressure | 1.01 | 0.98–1.03 | 0.81 | 0.37 |
| Heart Rate | 1.01 | 0.99–1.02 | 2.17 | 0.14 |
| Body Mass Index | 1.04 | 0.99–1.10 | 2.60 | 0.11 |
| Sinus Rhythm (at presentation) | 0.95 | 0.55–1.63 | 0.04 | 0.85 |
| AV Delay | 1.00 | 0.99–1.00 | 0.11 | 0.74 |
| QRS Duration | 1.01 | 1.00–1.03 | 4.81 | 0.03 |
| QTc Duration | 1.01 | 0.99–1.02 | 3.32 | 0.07 |
| Echocardiographic Parameters: | ||||
| LV Ejection Fraction | 0.99 | 0.97–1.02 | 0.34 | 0.56 |
| Estimated PASP | 1.03 | 0.99–1.06 | 2.51 | 0.11 |
| LV Diastolic Dimension | 0.99 | 0.94–1.04 | 0.24 | 0.62 |
| Left Atrial Dimension | 1.02 | 0.98–1.06 | 0.84 | 0.36 |
| Cardiac Magnetic Resonance Parameters: | ||||
| LV EDV | 1.00 | 0.99–1.01 | 0.01 | 0.95 |
| LV ESV | 1.00 | 0.99–1.01 | 0.25 | 0.62 |
| LV EF | 1.00 | 0.98–1.03 | 0.01 | 0.96 |
| LV Mass | 1.00 | 0.99–1.01 | 0.13 | 0.72 |
| LV Mass Index | 1.00 | 0.98–1.02 | 0.04 | 0.85 |
| RV EDV | 1.00 | 0.99–1.01 | 0.26 | 0.61 |
| RV ESV | 1.00 | 0.99–1.01 | 0.26 | 0.61 |
| RV EF | 0.98 | 0.95–1.01 | 1.78 | 0.18 |
| Left Atrial Dimension | 1.02 | 0.98–1.06 | 0.65 | 0.42 |
| Late Gadolinium Enhancement: | ||||
| Presence of LGE | 4.21 | 2.18–8.14 | 18.3 | <0.0001 |
| Extent of LGE | 1.24 | 1.13–1.35 | 22.4 | <0.0001 |
Discussion
We aimed to determine the incidence, pattern, and prognostic significance of myocardial scar in patients with AF undergoing pulmonary vein isolation. We performed a full CMR study including LV LGE imaging in a large series of consecutive patients with AF. The principal findings of this study were:
The incidence of unanticipated LV LGE was 13%;
There were two relatively even patterns of LV LGE noted in this study, an ischemic pattern and a non-ischemic pattern;
The presence of LV LGE had a significant relationship with mortality, even after adjusting for key variables such as gender, diabetes, and heart failure. Similar results were found when we included all patients with and without a prior MI.
The presence of LV LGE provides strong and complementary prognostic information in patients with congenital heart disease (19), myocardial infarction (14), coronary disease (11), myocarditis (20), aortic stenosis (12), endurance exercise (21), dilated cardiomyopathy (22), and hypertrophic cardiomyopathy (13). However, there are limited data detailing the presence and prognostic significance of LV LGE in patients with AF. In patients with hypertrophic cardiomyopathy, an increased volume of LGE was associated with an increased risk of atrial fibrillation (16,23), however there are no other data supporting myocardial LGE as a predictor of adverse outcomes in patients with AF. In patients with AF, there are robust data showing the association between age, heart failure, diabetes, prior smoking, a murmur, and LVH on death in patients with AF (24). While our results are in a cohort referred for pulmonary vein isolation, there are consistencies between our work and prior data in other AF cohorts. Similar to community data (24), we found in patients referred for ablation that age, diabetes, and heart failure had an unadjusted association with mortality. We also provide additive imaging data and found that imaging provided prognostic information in selected patients with AF. Data are conflicting regarding the role of conventional imaging indices and outcomes in patients with AF (25,26). In the AFFIRM study, heart failure with reduced EF was a stronger predictor of adverse outcomes as compared to heart failure with a preserved EF (25). While in unselected patients presenting to an emergency room with AF, there was no difference in outcomes when separated according to EF (26). We also found that the presence or absence of heart failure was a predictor of mortality, while EF was not.
The data on the prognostic value of LGE in a cohort of patients with AF are complementary and additive to prior data among patients with both a non-ischemic pattern and an ischemic pattern of LGE. In this study, these two broad evenly distributed patterns of myocardial scarring were noted, an ischemic pattern LGE and a non-ischemic pattern. A non-ischemic pattern of LGE has been shown to be an independent predictor of mortality in patients with valvular heart disease (12), in patients with a hypertrophic cardiomyopathy (27), and in patients with a non-ischemic cardiomyopathy (28). Similarly, LGE in an ischemic pattern has been shown to be an independent predictor of mortality in asymptomatic patients (15), in symptomatic patients with known prior MI (11), and in symptomatic patients with a prior MI (29). Finally, among all patients referred for a CMR scan, combined ischemic patterns and non-ischemic patterns of LGE have been shown to predict mortality (30). The mechanisms involved in the development of LV LGE are not clear but are likely different based on LGE pattern. The ischemic pattern of LGE is likely related to silent myocardial infarction and is similar to a data from a large population-based study of volunteers (15). Specifically, Schelbert and colleagues noted a 17% incidence of unrecognized MI (15). We believe that the lower percentage of unrecognized MI in our population is due to a combination of the 20 year age-difference, the percentage of patients with diabetes, and baseline use rate of beneficial medications. However, similar to our study, Schelbert et al., noted that the presence of an unrecognized MI in that study was also strongly associated with subsequent mortality. We believe that the non-ischemic pattern is likely related to the high percentage of patients in our study with heart failure or a reduced EF (22), as over 25% of our study group had a history of heart failure or reduced EF.
There is significant variability in pulmonary vein anatomy and imaging is routinely performed prior to pulmonary vein isolation is randomized studies of patients undergoing AF ablation (31,32), in large clinical registries (6), and is supported by guidelines (7,9). However, there are limited data as to whether imaging is required (33), and multiple modalities exist each with advantages and disadvantages (34). The choice of imaging modality usually depends on local expertise and available equipment, and includes magnetic resonance (10), computerized tomography, angiography (35), and ultrasound (36). There are comparative data between modalities (37,38), but no study integrates all imaging modalities so a complete comparison is lacking. However, cardiac CT and CMR provide superior spatial resolution over ultrasound (39,40), and can also be co-registered with electroanatomical mapping systems (9). Each has advantages and disadvantages. Cardiac CT is widely available, and may also provide additive information beyond pulmonary vein anatomy (34); However, CT is associated with radiation exposure (41), and the presence of incidental findings is considerable (42). Magnetic resonance imaging has less availability, a lower spatial resolution, and has standard contra-indications to its use (7). Allowing for these, both CT and CMR provide equivalent anatomical information (43). In this study, we did not test the ability of one modality over another to provide anatomical information but rather wanted to test whether the accessory information provided by a CMR study would be clinically useful. We found that the additive information provided by a CMR study, the presence of LGE, was an independent predictor of mortality. The CMR study detected infarct and non-infarct pattern LGE, both of which have been shown provide additive information in other cohorts (11,22).
This study should be interpreted within the context of the design format. There are data detailing the association between atrial LGE and AF recurrence in patients with AF (44), however the high-resolution sequence required is not part of our standard CMR imaging protocol. We also did not image all patients with AF; we imaged only patients undergoing pulmonary vein isolation. This likely represents a different phenotype to all patients with AF. We wanted to try and compare this cohort as it relates to all patients with AF. The AFFIRM study enrolled patients with a similar LV EF and percentage of patients with heart failure, and noted an all-cause mortality rate of 4.7%/year; however patients were on average 10 years older than in this study (45). The RACE trial also enrolled patients with similar cardiac function, a similar proportion of patients with diabetes, and a higher proportion of patients with heart failure. In that study they noted a cardiovascular mortality rate of 3.0%/year (46). These data suggest that our cohort has significant similarities with other population of patients with AF and the observed mortality rate is appropriate. Also, we have no data on whether the presence or absence of LV LGE influenced treatment. While imaging of pulmonary veins is part of standard clinical and research practice, there are no randomized data supporting pre-ablation imaging on outcomes after ablation of AF. We recorded the medical therapy at the time of discharge after PVI, and the change in patient-specific anti-arrhythmic therapy over time was not included in this analysis. Finally, we did not perform a comparison of available imaging modalities to test their differential effect on outcomes.
Amongst a large cohort of patients with AF being referred for pulmonary vein isolation, we found a 13% incidence of unanticipated LV LGE. The presence of LV LGE provided strong prognostic information with each adjusted 1% increase in LV LGE was associated with a 15% increased risk of death. Many imaging modalities are available for visualization of the pulmonary vein anatomy prior to ablation of atrial fibrillation, these data support the robust and additive prognostic information provided by CMR imaging, and may support further investigation in this high-risk cohort.
Acknowledgments
Grants: Dr. Neilan is supported by an American Heart Association Fellow to Faculty Grant (12FTF12060588). Dr. Jerosch-Herold and Dr. Kwong are supported by research grants from the National Institutes of Health (RO1HL090634, RO1HL091157, respectively).
Funding: This work was supported by an American Heart Association Fellow to Faculty Grant (12FTF12060588, TGN) and National Institute of Health project grants (R01HL090634-01A1, MJH; R01HL091157, RYK).
We thank the CMR technologists for continued excellence.
Footnotes
Conflict of Interest: The Authors report that they have no relationships relevant to the contents of this manuscript to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Jama. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. Jama. 2011;305:2080–7. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 6.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;3:32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 7.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Ghaye B, Szapiro D, Dacher JN, et al. Percutaneous ablation for atrial fibrillation: the role of cross-sectional imaging. Radiographics. 2003;23(Spec No):S19–33. doi: 10.1148/rg.23si035513. discussion S48–50. [DOI] [PubMed] [Google Scholar]
- 9.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS), Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696. e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Kato R, Lickfett L, Meininger G, et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003;107:2004–10. doi: 10.1161/01.CIR.0000061951.81767.4E. [DOI] [PubMed] [Google Scholar]
- 11.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 12.Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–9. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 13.O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–74. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 15.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. Jama. 2012;308:890–6. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papavassiliu T, Germans T, Fluchter S, et al. CMR findings in patients with hypertrophic cardiomyopathy and atrial fibrillation. J Cardiovasc Magn Reson. 2009;11:34. doi: 10.1186/1532-429X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackburn H, Keys A, Simonson E, Rautaharju P, Punsar S. The electrocardiogram in population studies. A classification system. Circulation. 1960;21:1160–75. doi: 10.1161/01.cir.21.6.1160. [DOI] [PubMed] [Google Scholar]
- 18.Flett AS, Hasleton J, Cook C, et al. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150–6. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Babu-Narayan SV, Kilner PJ, Li W, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–13. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 20.Grun S, Schumm J, Greulich S, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–15. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Breuckmann F, Mohlenkamp S, Nassenstein K, et al. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–7. doi: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 22.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Pujadas S, Vidal-Perez R, Hidalgo A, et al. Correlation between myocardial fibrosis and the occurrence of atrial fibrillation in hypertrophic cardiomyopathy: a cardiac magnetic resonance imaging study. Eur J Radiol. 2010;75:e88–91. doi: 10.1016/j.ejrad.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. Jama. 2003;290:1049–56. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 25.Badheka AO, Rathod A, Kizilbash MA, et al. Comparison of mortality and morbidity in patients with atrial fibrillation and heart failure with preserved versus decreased left ventricular ejection fraction. Am J Cardiol. 2011;108:1283–8. doi: 10.1016/j.amjcard.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 26.Parkash R, Maisel WH, Toca FM, Stevenson WG. Atrial fibrillation in heart failure: high mortality risk even if ventricular function is preserved. Am Heart J. 2005;150:701–6. doi: 10.1016/j.ahj.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–87. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Leyva F, Taylor RJ, Foley PW, et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:1659–67. doi: 10.1016/j.jacc.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 29.Klem I, Shah DJ, White RD, et al. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011;4:610–9. doi: 10.1161/CIRCIMAGING.111.964965. [DOI] [PubMed] [Google Scholar]
- 30.Cheong BY, Muthupillai R, Wilson JM, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–76. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]
- 31.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. Jama. 2010;303:333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 32.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 33.Yokokawa M, Olgun H, Sundaram B, et al. Impact of preprocedural imaging on outcomes of catheter ablation in patients with atrial fibrillation. J Interv Card Electrophysiol. 2012;34:255–62. doi: 10.1007/s10840-011-9660-3. [DOI] [PubMed] [Google Scholar]
- 34.Sohns C, Kruse S, Vollmann D, et al. Accuracy of 64-multidetector computed tomography coronary angiography in patients with symptomatic atrial fibrillation prior to pulmonary vein isolation. Eur Heart J Cardiovasc Imaging. 2012;13:263–70. doi: 10.1093/ejechocard/jer277. [DOI] [PubMed] [Google Scholar]
- 35.Lin WS, Prakash VS, Tai CT, et al. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation. 2000;101:1274–81. doi: 10.1161/01.cir.101.11.1274. [DOI] [PubMed] [Google Scholar]
- 36.Marrouche NF, Martin DO, Wazni O, et al. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation. 2003;107:2710–6. doi: 10.1161/01.CIR.0000070541.83326.15. [DOI] [PubMed] [Google Scholar]
- 37.Wood MA, Wittkamp M, Henry D, et al. A comparison of pulmonary vein ostial anatomy by computerized tomography, echocardiography, and venography in patients with atrial fibrillation having radiofrequency catheter ablation. Am J Cardiol. 2004;93:49–53. doi: 10.1016/j.amjcard.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Jongbloed MR, Bax JJ, Lamb HJ, et al. Multislice computed tomography versus intracardiac echocardiography to evaluate the pulmonary veins before radiofrequency catheter ablation of atrial fibrillation: a head-to-head comparison. J Am Coll Cardiol. 2005;45:343–50. doi: 10.1016/j.jacc.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 39.Toffanin G, Scarabeo V, Verlato R, De Conti F, Zampiero AA, Piovesana P. Transoesophageal echocardiographic evaluation of pulmonary vein anatomy in patients undergoing ostial radiofrequency catheter ablation for atrial fibrillation: a comparison with magnetic resonance angiography. J Cardiovasc Med (Hagerstown) 2006;7:748–52. doi: 10.2459/01.JCM.0000247322.57536.04. [DOI] [PubMed] [Google Scholar]
- 40.To AC, Gabriel RS, Park M, et al. Role of Transesophageal Echocardiography Compared to Computed Tomography in Evaluation of Pulmonary Vein Ablation for Atrial Fibrillation (ROTEA study) J Am Soc Echocardiogr. 2011;24:1046–55. doi: 10.1016/j.echo.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. Jama. 2007;298:317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 42.Schietinger BJ, Bozlar U, Hagspiel KD, et al. The prevalence of extracardiac findings by multidetector computed tomography before atrial fibrillation ablation. Am Heart J. 2008;155:254–9. doi: 10.1016/j.ahj.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamdan A, Charalampos K, Roettgen R, et al. Magnetic resonance imaging versus computed tomography for characterization of pulmonary vein morphology before radiofrequency catheter ablation of atrial fibrillation. Am J Cardiol. 2009;104:1540–6. doi: 10.1016/j.amjcard.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 44.McGann C, Kholmovski E, Blauer J, et al. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J Am Coll Cardiol. 2011;58:177–85. doi: 10.1016/j.jacc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 46.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]