Abstract
Bimetallic paddlewheel complexes derived from imides of (S)-t-leucine adopt ‘chiral crown’ configurations in which the four imide groups are projected in a chiral arrangement on one face, and the four t-butyl groups are projected on the opposite face. In this contribution, the generality of the chiral crown conformation is examined through crystallographic studies where the metal and the nature of the chiral ligands are altered. Based upon these observations, a model is proposed to explain the factors which create bias for the chiral crown configuration.
Chiral bimetallic paddlewheel complexes are important structures for asymmetric catalysts. In particular, chiral dirhodium (II) paddlewheel complexes have been used extensively across a broad range of synthetic transformations, including C-H activation, cyclopropenation, cyclopropanation, X-H insertions, and carbonyl ylide forming reactions.1
Chiral paddlewheel complexes are capable of adopting multiple conformations, some of which are depicted in Figure 1 using the graphical convention of Davies.2 In this convention, the bimetallic paddlewheel is depicted as a disk, and blocking groups are projected on either of the arbitrarily defined α– or β–faces of the paddlewheel. The importance of conformers of type A [(α, α, β, β), e.g. dirhodium carboxamidates3] or type B [(α, β, α, β), e.g. Rh2(S-DOSP)44] is well established in enantioselective catalysis.2 In conformations A and B, the chiral environment of the two metal atoms is equivalent. Conformations of type C (α, α, α, α), in which the chiral environment of the two metal atoms differ, were until recently not considered to be important to asymmetric catalysis.
Fig 1.
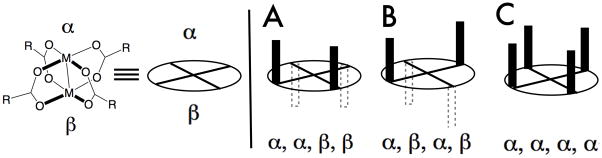
Conformations of chiral paddlewheel complexes
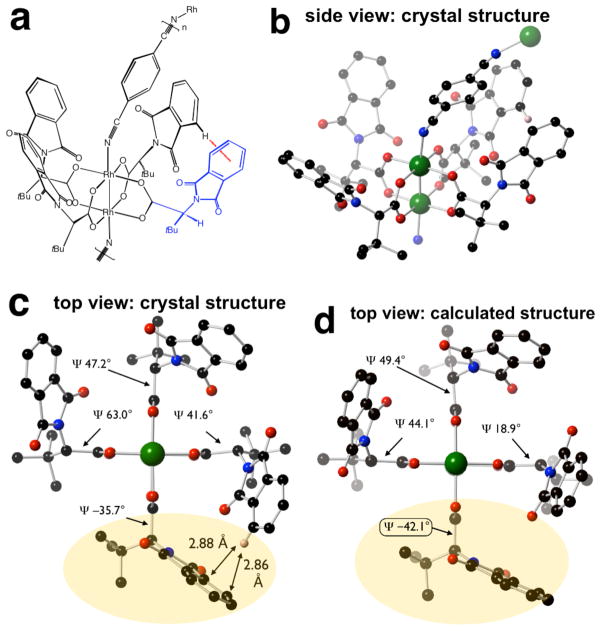
In the context of work on the enantioselective cyclopropanation of alkenes by α-alkyl-α-diazoesters, our group recently reported crystallographic and computational studies5 on dirhodium(II) tetrakis[N-phthaloyl-(S)-t-leucinate [Rh2(S-PTTL)4, 1]— a catalyst that was first described by Hashimoto.6 Previously, the only reported structure of a Rh(II)-carboxylate complex derived from an N-phthaloyl amino acid was dirhodium(II) tetrakis[N-phthaloyl-(S)-phenylalanate]•(4-t-butylpyridine)2, which crystallized in the α, α, β, β-conformation.7 In both the crystal structure and lowest energy calculated structure of 1, the α, α, α, α-conformation was observed (Fig 2a–c). Compound 1 crystallized with a single ethyl acetate as an axial ligand. Ignoring solvent, the crystal structure of 1 is approximately C2 symmetric, and the cavity of the catalyst has a wide (~15 Å) and narrow dimension (~11 Å) (Fig 2b). The calculated structure of 1 is displayed in Fig 2c. In both structures, each of the C-(t-Bu) bonds are roughly parallel to the central Rh-Rh bond, and the four phthalimide groups are projected on the opposite face of the catalyst in a “chiral crown” structure. The dimensions of the cavity of calculated 1 differed somewhat from the crystal structure: 14.1 Å for the wide dimension and 12.8 Å for the narrow dimension.‡ However, the calculated structure displays an overall strong correlation with the crystallized structure of 1.
Fig 2.
The chiral crown conformation of Rh2(S-PTTL)4 (a) side view, (b) top view of crystal structure (axial ligand not shown), (c) top view of minimum energy structure after sequential MCMM and DFT calculations
Charette and coworkers recently described concomitant studies, in which they elegantly demonstrated that Rh(II)-carboxylate catalysts derived from N-tetrahalophthaloyl amino acids are useful in highly enantioselective and diastereoselective cyclopropanations with α-nitro diazoacetophenones.8 The authors crystallized four Rh(II)-carboxylate catalysts: dirhodium(II) tetrakis[N-phthaloyl-(S)-valinate, dirhodium(II) tetrakis[N-tetrachlorophthaloyl-(S)-valinate, dirhodium(II) tetrakis[N-tetrabromophthaloyl-(S)-t-leucinate, and dirhodium(II) tetrakis[N-tetrabromophthaloyl-(S)-t-leucinate. Each of these complexes crystallized with two axial ligands in the α, α, α, α-chiral crown conformation. Based on qualitative NOE studies, the authors proposed that Rh2(S-PTTL)4 adopts conformations other than the crown conformation, whereas the chlorinated analog Rh2(S-TCPTTL)4 was proposed to be more rigid. However, the structures and prevalence of any alternate conformations of Rh2(S-PTTL)4 are currently unclear. A goal of the study presented here was to better understand the generality of the crown conformation, and to provide insight into the factors that enforce the crown conformer across varied catalyst types.
Crystallography is an important tool for probing 3-dimensional structure. To better understand the generality of the crown conformation in chiral paddlewheel complexes, we determined the effects of varying metal (Cu vs Rh) and ligand size (naphthoyl vs phthaloyl) in paddlewheel complexes 2 and 3, respectively (Fig 3). We also studied the complexation of Rh2(S-PTTL)4 with 1,4-dicyanobenzene in a 1-dimensional chiral coordination polymer 5.
Fig 3.
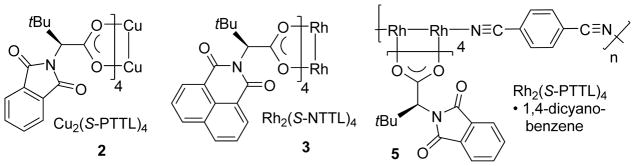
Complexes studied in this work
Crystals of the copper paddlewheel complex Cu2(S-PTTL)4 (2) were prepared by refluxing N-phthaloyl-(S)-t-leucine with Cu(OAc)2 (Fig 4). Crystals were grown from an acetonitrile solution that was subjected to slow vapor diffusion with 1:1 EtOH/H2O. Complex 2 crystallized with two different axial ligands, with ethanol on the α-face of the structure, and acetonitrile on the β-face. Crystalline 2 adopts a chiral crown conformation, with each of the C-(t-Bu) bonds positioned roughly parallel to the central Cu-Cu bond. Like 1, the chiral cavity of crystalline 2 has a wide (13.0 Å) and narrow (11.7 Å) dimension. The proportions of the chiral cavity for crystalline 2 are more similar to those of the calculated structure of 1 than to the crystalline structure of 1 (Fig 2). The C–Cα torsion angles (ψ) of 2 oscillate in magnitude (ψ 49.0°, 36.2°, 47.0°, 42.8°), and are more similar to those of the calculated structure of 1 than to the crystallized structure of 1.
Fig 4.
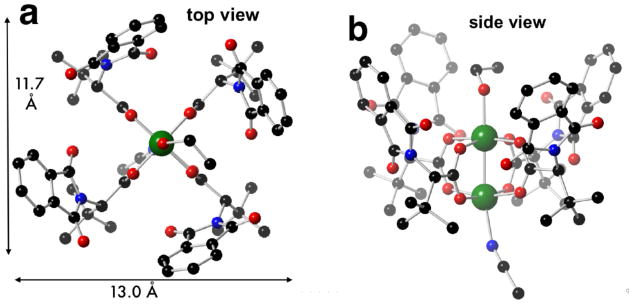
Crystal structure of Cu2(S-PTTL)4 (2). Ethanol and acetonitrile are the axial ligands on the α- and β-faces, respectively.
The naphthalene based complex 3 was prepared as described previously.9 Crystals of 3 were grown by slow evaporation of a solution of 3 in 18:1:1 toluene/EtOAc/THF. Like 1 and 2, complex 3 crystallizes in the crown conformation with ethyl acetate on the α-face of the complex, and THF on the β-face. The ψ angles are more similar (46.5°, 50.0°, 47.6°, 49.6°) in 3 than was observed for 1 and 2. Still, the chiral cavity of 3 has wide and narrow dimensions that are significantly different (16.8 Å and 15.0 Å, Fig 5).
Fig 5.
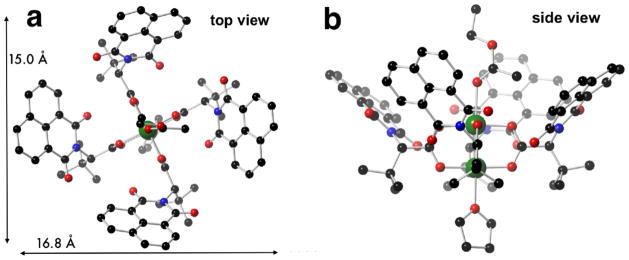
Crystal structure of Rh2(S-NTTL)4 (3). Ethyl acetate and THF are axial ligands on the α- and β-faces, respectively.
The steric effect of the bulky t-butyl groups are one factor that biases for the crown structure. Analogous complexes derived from phenylalanine adopt the α, α, β, β-conformation. Hashimoto had shown that dirhodium(II) tetrakis[N-phthaloyl-(S)-phenylalanate] (4) crystallizes with in the α, α, β, β-conformation with two axial 4-t-butylpyridine ligands.7 To demonstrate that this observation was not anomolous, we crystallized 4 from acetonitrile (Fig 6). Crystalline 4•(MeCN)2 also adopts the α, α, β, β-conformation,‡‡ in which two of the phthalimide moieties orient on opposing faces separated by only 3.48 Å (Fig 6b). This arrangement of the phthalimide functions is possible because the relatively small benzyl side chains are permissive of large N–Cα torsion angles (φ 75.6° and 62.6°).
Fig 6.
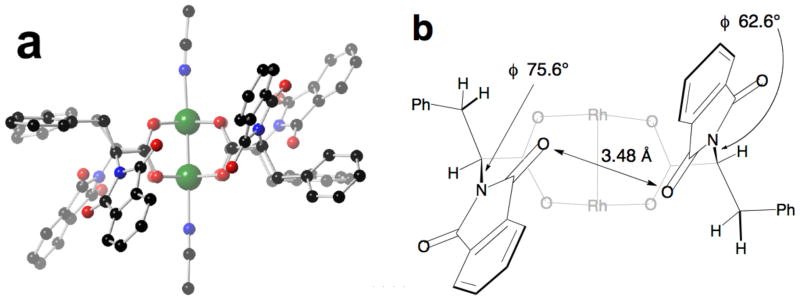
(a) X-ray crystal structure of Rh2(S-PTPA)4 (4) with axial acetonitrile ligands in the α, α, β, β-conformation. (b) representation of the close contacts between two of the phthalimide moieties oriented on opposing faces
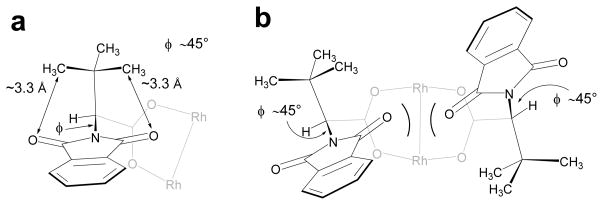
The t-butyl side chains of t-leucine derived catalysts restrain their structures. Analysis of the crystal structures of 1–3 reveals a very narrow range of φ angles (45° +/− 6°). This is a consequence of close contact between the t-butyl group and both of the imide carbonyl oxygens, with average interatomic distances of 3.3 Å (+/− 0.3 Å) in the structures of 1 and 2 (Fig 7a), and 3.1 Å (+/− 0.2 Å) in 3. For M2(S-PTTL)4 or M2(S-NTTL)4 complexes, there would be an unfavorable steric interaction in the α, α, β, β-conformation unless the φ-angles were increased (Fig 7b).
Fig 7.
(a) X-ray structures of 1–3 reveal a narrow range of φ (45° +/− 6°), enforced by close contacts between the t-butyl and the imide oxygens. (b) An unfavorable steric interaction in an α, α, β, β-conformation will result if the idealized 45° angle were maintained for complexes 1–3.
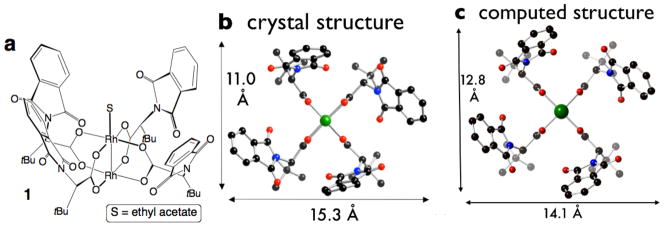
Crystals of coordination polymer 5 were grown from a solution of 2 : 1 Rh2(S-PTTL)4/1,4-dicyanobenzene. The Rh2(S-PTTL)4 subunits in 5 deviate somewhat from the crown structure that was observed in 1–3 (Fig 7) In 5, all four phthaloyl groups are still projected on the same face of the complex. However, for one of the S-PTTL ligands, ψ was −35.7°: a clockwise twist of ~80° relative to what would be expected in a true crown conformation. This ‘twisted crown’ conformation of 5 bears similarity to the second lowest energy conformation of Rh2(S-PTTL)4 from our previous calculations (Fig 7d). This conformer was computed to be 3.86 kcal/mol higher in energy than the crown conformation (Fig 2c). However, our calculations did not take into account the effect of solvent or axial ligation, and the observation of the twisted crown conformation suggests that the calculations may have overestimated the energy difference.
A difference between the calculated conformation in Fig 7d and crystal structure of 5 (Fig 7c) was the observation of a edge-to-face interaction between neighboring phthalimides in the crystal structure. The contact involves the π-system of the ‘twisted ligand’ and an aromatic proton of a neighboring phthalimide (Fig 7c). The close interatomic CH/aromatic distances of 2.86 and 2.88 Å are consistent with a CH-π interaction,10 which may stabilize this conformation. For the twisted ligand, φ is 69.4°. We hypothesize that this deviation of φ from the idealized 45° angle is compensated by the favorable edge-to-face interaction. Such CH-π interactions are not possible in complexes with halogenated phthalimide groups, and are considered unlikely with sterically demanding 1,8-naphthalimide groups. Thus, the twisted crown conformation may not be significant in such complexes.
In conclusion, we have demonstrated the generality of the chiral crown structure for t-leucine-derived paddlewheel complexes by varying the nature of the metal, carboxylate ligands, and the axial ligands. We propose that sterically demanding t-Bu groups enforce the chiral crown structure by restraining the ψ-angles, and thereby destabilize the competing α,α,β,β configuration. In the crystal structure of Rh2(S-PTTL)4•(1,4-dicyanobenzene) (5), an alternate twisted crown conformation is observed, in ψ is twisted by ~80° relative to a true crown conformation. It is hypothesized that this “twisted crown” conformation is stabilized by a CH-π interaction between adjacent phthalimide moieties.
Supplementary Material
Fig 8.
(a) Coordination polymer of Rh2(S-PTTL)4 with 1,4-dicyanobenzene (5). (b) Side view and (c) top view of crystal structure. The PTTL ligand highlighted in yellow is twisted clockwise by ~80° relative to a true crown confromation. (d) second lowest energy conformation from computation.
Acknowledgments
For financial support of this work, we thank the NSF (CHE-0547865) and NIH training grant 1-T32-GM08550 (J. B. L). Spectra were obtained with instrumentation supported by NSF CRIF:MU grants: CHE 0840401 and CHE-0541775.
Footnotes
The differing dimensions of the cavities in the crystallized and calculated structures are largely a manifestation of their different ψ angles. In both the crystallized and calculated structures, ψ oscillates in magnitude, but less severely in the calculated structure (ψ 48.1°, 42.1°, 46.3°, 43.2°). There were two similar but non-identical structures in the unit cell of 1: the oscillation of ψ was 53.6°, 37.1°, 56.4°, 41.9° in one structure; 57.7°, 45.8°, 51.6°, 39.9° in the second structure.
Although both 4•(4-t-butylpyridine)2 and 4•(MeCN)2 adopt the α, α, β, β-conformation, there are differences in their conformations. In the crystal structure of 4•(4-t-butylpyridine)2, the ψ angles are smaller than in 4•(MeCN)2. In 4•(4-t-butylpyridine)2, there are close interactions between two of the benzyl groups that are oriented on opposing faces. It is clear that a simple replacement of the benzyl substituents with t-butyl substituents would be untenable for this conformation.
Electronic Supplementary Information (ESI) available: full experimental, characterization and crystallographic details are provided. CIF files for 2–5 have been deposited in the CCDC.
Notes and references
- 1.Hodgson DM, Glen R, Redgrave AJ. Tetrahedron: Asymmetry. 2009;20:754. [Google Scholar]; Davies HML, Manning JR. Nature. 2008:451, 417. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; Denton JR, Cheng K, Davies HML. Chem Commun. 2008:1238. doi: 10.1039/b719175h. [DOI] [PubMed] [Google Scholar]; Shimada N, Anada M, Nakamura S, Nambu H, Tsutsui H, Hashimoto S. Org Lett. 2008;10:3603. doi: 10.1021/ol8013733. [DOI] [PubMed] [Google Scholar]; Tsutsui H, Shimada N, Abe T, Anada M, Nakajima M, Nakamura S, Nambu H, Hashimoto S. Adv Synth Catal. 2007;349:521. [Google Scholar]; Reddy RP, Lee GH, Davies HML. Org Lett. 2006;8:3437. doi: 10.1021/ol060893l. [DOI] [PubMed] [Google Scholar]; Lou Y, Horikawa M, Kloster RA, Hawryluk NA, Corey EJ. J Am Chem Soc. 2004;126:8916. doi: 10.1021/ja047064k. [DOI] [PubMed] [Google Scholar]; Lebel H, Marcoux J, Molinaro C, Charette AB. Chem Rev. 2003;103:977. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]; Buck RT, Coe DM, Drysdale MJ, Ferris L, Haigh D, Moody CJ, Pearson ND, Sanghera JB. Tetrahedron: Asymmetry. 2003;14:791. [Google Scholar]; Saito H, Oishi H, Kitagaki S, Nakamura S, Anada M, Hashimoto S. Org Lett. 2002;4:3887. doi: 10.1021/ol0267127. [DOI] [PubMed] [Google Scholar]
- 2.Hansen J, Davies HML. Coord Chem Rev. 2008;252:545. doi: 10.1016/j.ccr.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle MP. J Org Chem. 2006;71:9253. doi: 10.1021/jo061411m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies HML, Bruzinski PR, Lake DH, Kong N, Fall MJ. J Am Chem Soc. 1996;118:6897. [Google Scholar]
- 5.DeAngelis A, Dmitrenko O, Yap GPA, Fox JM. J Am Chem Soc. 2009;131:7230. doi: 10.1021/ja9026852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe N, Ogawa T, Ohtake Y, Ikegami S, Hashimoto S. Synlett. 1996;1996:85. [Google Scholar]
- 7.Hashimoto S-i, Watanabe N, Sato T, Shiro M, Ikegami S. Tetrahedron Lett. 1993;34:5109. [Google Scholar]
- 8.Lindsay VNG, Lin W, Charette AB. J Am Chem Soc. 2009;131:16383. doi: 10.1021/ja9044955. [DOI] [PubMed] [Google Scholar]
- 9.Müller P, Allenbach Y, Robert E. Tetrahedron: Asymmetry. 2003;14:779.After this manuscript was submitted, a crystal structure of Rh2(S-NTTL)4 in the crown conformation with two axial ethyl acetate ligands was described: Ghanem A, Gardiner MG, Williamson RM, Müller P. Chem Eur J. 2010;16:3291. doi: 10.1002/chem.200903231.
- 10.Suezawa H, Yoshida T, Umezawa Y, Tsuboyama S, Nishio M. Eur J Inorg Chem. 2002;2002:3148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.