Abstract
Axons navigating through the developing nervous system are instructed by external attractive and repulsive cues. Emerging evidence suggests the same cues control dendrite development, but it is not understood how they differentially instruct axons and dendrites. We studied a C. elegans motor neuron whose axon and dendrite adopt different trajectories and lengths. We found that the guidance cue UNC-6 (Netrin) is required for both axon and dendrite development. Its repulsive receptor UNC-5 repelled the axon from the ventral cell body, whereas the attractive receptor UNC-40 (DCC) was dendritically enriched and promotes antero-posterior dendritic growth. Although the endogenous ventrally secreted UNC-6 instructs axon guidance, dorsal or even membrane-tethered UNC-6 could support dendrite development. Unexpectedly, the serine-threonine kinase PAR-4 (LKB1) was selectively required for the activity of the UNC-40 pathway in dendrite outgrowth. These data suggest that axon and dendrite of one neuron interpret common environmental cues with different receptors and downstream signaling pathways.
The precise assembly of neural circuits during development is a prerequisite for intact behavior in the adult organism. Assembly begins with symmetry breaking of each neuron when the neuron decides when and where to form an axon and dendrites, resulting in two molecularly, morphologically and functionally distinct compartments that are each fundamental to its function. Whereas many attractive and repulsive cues determining axonal trajectories have been identified, less is known about cues promoting or limiting dendrite growth.
Recently, several in vivo studies indicated that axon guidance molecules are also involved in dendrite development. Semaphorin (Sema)-3A secreted at the cortical marginal zone attracts the apical dendrites of pyramidal neurons toward superficial layers1. In Drosophila melanogaster olfactory glomeruli, discrete groups of olfactory receptor neuron axons and projection neuron dendrites must connect. A Sema-1a gradient in the antennal lobes cell-autonomously targets projection neuron dendrites to the correct location in the glomeruli2. Netrin and Slit, initially described as axon guidance molecules, have since been shown to regulate dendrite guidance3,4. In zebrafish, midline netrin1 guides octavolateralis efferent neuron dendrites across it without affecting neuronal fate or axon guidance5. Fly motor neuron axons and dendrites respond to Slit and Netrin in both outgrowth and midline guidance events6,7. However, as these dendrites extend from axon segments bilaterally of the midline, it is impossible to distinguish the molecules’ effects on axons and dendrites. As dendrites respond distinctly to these cues, there must be mechanistic differences in the cell’s interpretation of its environment when forming an axon versus a dendrite. Hence the challenge ahead of us appears not only to be identifying new genes, but also teasing apart how a neuron interprets identical molecular cues in different ways.
One mechanism by which cues could be interpreted distinctly is by implementing task-specific downstream molecules. Multiple signaling pathways downstream of UNC-6 (Netrin) in axon guidance have been explored, starting with different receptors. The UNC-5 receptor binds UNC-6 to repel axons. In C. elegans, this response allows ventral motor neurons to dorsally guide their axons away from the ventral UNC-6 source toward their muscle targets3. In mouse, Unc5h3 is required for the formation of the corticospinal tract and its guidance at the pyramidal decussation8. UNC-6’s attractive function is mediated by the receptor UNC-40 (DCC), which guides axons toward ventral UNC-6 in C. elegans, chick and mouse3,9,10. However, a neuron’s response specificity to UNC-6 exceeds receptor choice. In C. elegans, a bifurcation of pathways downstream of UNC-40 has been identified. The UNC-40 cytoplasmic portion consists of three P domains (P1, P2 and P3), which associate with specific downstream molecules that may deliver distinct signals to the actin cytoskeleton. The functions of the actin-binding protein UNC-34 (Enabled) in axon guidance and filopodium outgrowth have been shown in vitro and vivo in C. elegans and Drosophila neurons11,12. In UNC-6 signaling, UNC-34 acts downstream of the P1 domain. MIG-10 (Lamellipodin) and UNC-34 cooperate to promote filopodium outgrowth in the C. elegans neuron HSN13. In parallel, the UNC-40 P2 domain acts through the Rac GTPase CED-10 and the actin-binding protein UNC-115 (abLIM). Both P1 and P2 domain–dependent pathways contribute to filopodium formation, but their activity is not entirely redundant, as within these pathways each protein preferentially contributes to filopodium formation, number or orientation. Hence the molecular diversity orchestrates distinct events in UNC-6 signaling downstream of UNC-40 (refs. 14,15).
Several molecules have been identified as acting downstream of vertebrate DCC. These include kinases such as focal adhesion kinase (FAK), the Src family kinase Fyn and phospholipase C (PLC)-γ and phosphatidylinositol-3-OH kinase (PI3K)pathways16,17. DCC has also been shown to interact with the Rac1-activating guanine nucleotide exchange factor DOCK180 and with unconventional myosin X, which stimulates filopodium formation and modulates neuritic DCC localization18,19. p130CAS has been proposed to link the Src family kinases downstream of DCC with the small GTPases Rac1 and Cdc42 (ref. 20). These findings highlight the commonalities between pathways used for axon guidance and other cell motility events.
Here we show how specificity can be achieved while generating a dendrite using the same extracellular cues used for axonal development. We use the C. elegans motor neuron DA9 to describe how UNC-6 regulates dendrite outgrowth. Its function in dendrite development is executed by short-range signaling through UNC-40, whereas the repulsive receptor UNC-5 is critical for axon guidance. We also show that PAR-4 is required for dendrite, but not axon, outgrowth in DA9 and acts downstream of UNC-6 andUNC-40. Therefore, the axon and dendrite of one neuron interpret common environmental cues with different receptors and downstream signaling pathways.
RESULTS
UNC-6 and UNC-40 are required for DA9 dendrite development
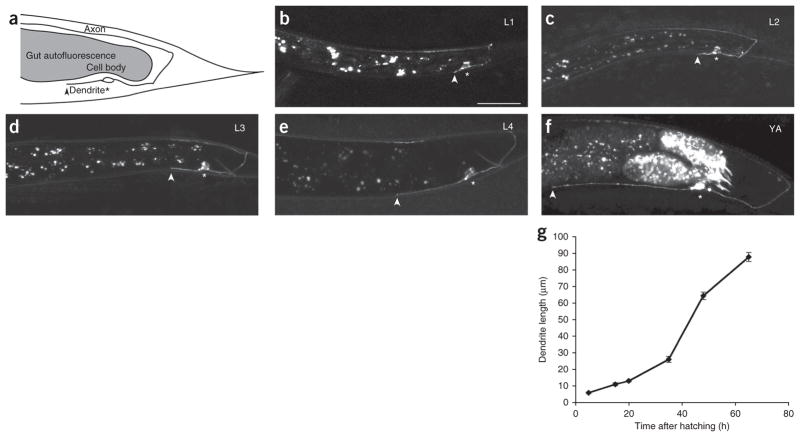
DA9 is an embryonically born, cholinergic, DA-class motor neuron. The somata of DA motor neurons located in the ventral nerve cord extend a single axon posteriorly and form a commissure to reach the dorsal nerve cord. In the dorsal cord, the DA axons grow anteriorly and form en passant synapses with a subset of dorsal body wall muscles and ventral D reciprocal inhibitory neurons21. DA motor neuron axon outgrowth and neuromuscular junction formation occur embryonically. DA9 is the most posterior of DA neurons. It has a single, unbranched dendrite that extends anteriorly in the ventral nerve cord and receives inputs from command interneurons and sensory neurons21. To characterize DA9 dendrite growth, we used a fluorescently labeled transmembrane protein driven under a promoter22 (Pmig-13::mig-13::gfp) that, in the tail, is predominantly expressed in DA9 during larval stages 1 through 4 (L1–L4), and cytoplasmic mCherry expression under a DA9-specific promoter23 that is expressed predominantly after L4 (Pitr-1::mCherry). Using these markers, we measured dendrite length from its anterior tip to the soma at different developmental time points. Unlike axon development, we found that most dendrite outgrowth in DA9 takes place postembryonically (Fig. 1a–g). In L1 larvae, when the axon has already completed its dorsal and anterior growth, the dendrite is very short (Fig. 1b). The dendrite continues to grow throughout larval development (Fig. 1c–f), occupying an increasing proportion of the tail (Supplementary Fig. 1 and Supplementary Table 1). Hence, dendrite growth is not a secondary effect of body size, as it is not directly coupled to the worm’s growth. The rate of dendrite extension falls off in older adults. Dendrite development in DA9 is temporally and spatially distinct from axon outgrowth and guidance, and so it is an appropriate system in which to study mechanisms differentially specifying the developing dendrite.
Figure 1.
Development of the DA9 motor neuron in wild-type C. elegans. (a) Schematic diagram of DA9. The DA9 cell body is located in the tail. Its axon extends posteriorly in the ventral cord, dorsally through a commissure and anteriorly in the dorsal cord. The ventrally located soma elaborates a single, unbranched dendrite anteriorly. Gut autofluorescence, within gray patch, should be disregarded in all images. Asterisk, cell body; arrowhead, tip of dendrite. (b–f) Lateral view of wild-type worms expressing Pmig-13::mig-13::gfp in DA9, at developmental stages L1–L4 and young adult (YA). (b) An L1 worm. Worms hatch with a fully formed axon, while the dendrite is a stump. The dendrite orientation is already determined by L1. (c–f) The dendrite continues to grow anteriorly throughout the life of the worm (images L2–YA). (g) Growth curve of dendrite over time. Early larval dendritic growth takes place at a steady pace (L1–L3), followed by a growth spurt between L4 and YA and slowing in older adults. All pictures are lateral view with ventral down and anterior to the left; all quantification from anterior edge of soma to anterior tip of dendrite. Scale bar, 20μm. n ≥ 20. Error bars, s.e.m.
In asking what processes underlie DA9 dendrite outgrowth, we considered UNC-6 as a candidate. UNC-6 is an evolutionarily conserved axon guidance cue in invertebrates and vertebrates24. In C. elegans, ventral epidermoblasts and then ventral cord pioneer neurons provide UNC-6 as a diffusible cue25. Its expression by these cells presumably creates a ventral to dorsal gradient providing directional information for axons. Neurons with dorsal somata use UNC-6’s attractive receptor UNC-40 to guide their axons up the gradient toward the ventral cord, while ventral neurons send their axons down the gradient through the repulsive activity of the UNC-5 signaling pathway3,26.
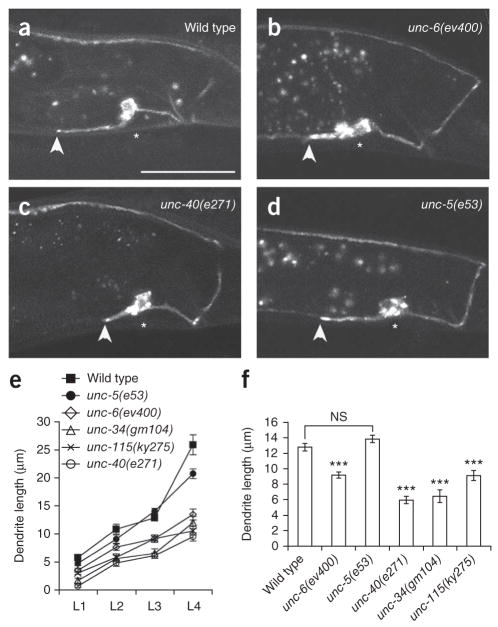
The DA9 axon migrates dorsally and its dendrite grows along the ventral cord, suggesting that its axon uses the UNC-5 repulsive pathway. Consistent with this, we have previously found that DA9 dorsal guidance is partially affected in unc-6 or unc-5 mutants27. To understand how UNC-6 affects dendrite growth, we measured DA9 dendrite length at various developmental stages. We found that the DA9 dendrite in unc-6 mutants was shorter compared with that in wild-type worms (Fig. 2). This defect could be detected in all postembryonic developmental stages (Fig. 2e, f). Similar phenotypes were found in unc-40 mutants and in worms lacking the previously characterized signaling molecules UNC-34 and UNC-115 (Fig. 2e, f). Of note, unc-5 mutants, which exhibit defects in axon dorsal guidance27, showed a weak outgrowth phenotype in L4 stage, but an otherwise normal dendrite length (Fig. 2d, e). The dendrite orientation was normal in all the mutants mentioned above. The average dendrite length in DA9 neurons with misguided versus properly routed axons in unc-6 mutants was not significantly different, suggesting that axon guidance and dendrite growth are independent events (Supplementary Fig. 2). To test whether unc-6 mutant dendrites have lost their molecular identity, we examined the subcellular distribution of two somato-dendritically localized proteins, fibrillin and dystrophin. Their polarized distribution was unaffected in unc-6 mutants, suggesting that the remaining neurite maintains dendritic characteristics27. These data suggest that the high concentration of UNC-6 promotes DA9 dendrite growth along the ventral nerve cord.
Figure 2.
UNC-6 (Netrin) regulates dendrite outgrowth through UNC-40 (DCC). (a–d) Micrographs of wild-type and unc-6, unc-40 and unc-5 mutants expressing Pmig-13::mig-13::gfp, revealing truncated dendrites in unc-6 and unc-40 mutants. (e) Growth curves showing dendrite length in L1–L4 wild-type and unc-6 signaling mutants. A minimal defect in dendrite growth in L1 worms increases over larval growth, with a trend toward unc-40 signaling mutant phenotypes being more severe than unc-5. (f) Dendrite lengths in L3 worms. All pictures are lateral views with ventral down and anterior to the left, all quantification from anterior edge of soma to anterior tip of dendrite. Scale bar, 20 μm. n ≥ 20. Error bars, s.e.m. ***P < 0.001; NS, not significant; Student’s t-test.
UNC-40 acts cell-autonomously and localizes dendritically
To establish whether UNC-40 acts cell-autonomously in DA9, we created mosaic unc-40 worms by expressing wild-type unc-40 cDNA under the mig-13 promoter in unc-40 mutants. The mig-13 promoter is expressed in only DA9 and VA12 in the tail of the worm, with expression in VA12 becoming evident after the L3 stage23. Expression of unc-40 in DA9 fully rescued dendrite outgrowth (Fig. 3), suggesting that UNC-6 signaling is acting in DA9 itself to promote dendrite outgrowth.
Figure 3.
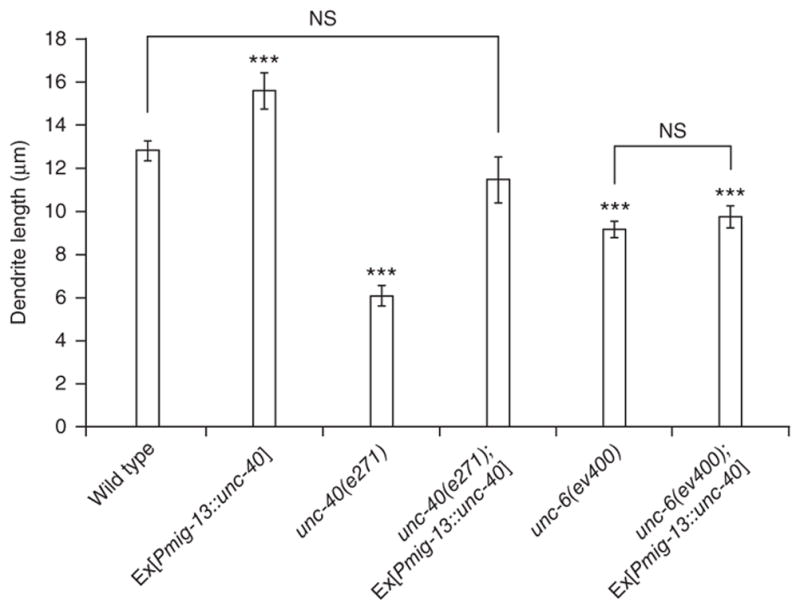
UNC-40 is necessary and sufficient for dendrite outgrowth. Graph of dendrite lengths in wild-type and with UNC-40 transgenes in various mutant background. All quantification is from anterior edge of soma to anterior tip of dendrite. n ≥ 20. Error bars, s.e.m. ***P < 0.001; NS, not significant; Student’s t-test.
To further test whether UNC-6–UNC-40 signaling is the rate-limiting molecular event for DA9 dendrite growth, we examined worms wild-type for unc-40 expressing mig-13::unc-40. We reasoned that the dendrite of these worms might be longer than wild type if the signaling strength of the UNC-6–UNC-40 pathway instructs growth. Indeed, we observed that transgenic worms showed significantly longer DA9 dendrites compared with wild-type worms. If this UNC-40 ‘gain-of-function’ effect represents overactivation of endogenous UNC-6 signaling, the dendrite overextension should be UNC-6 dependent. Indeed, the overextension phenotype was significantly suppressed in unc-6 worms (P = 0.0001; Fig. 3). Hence, UNC-6–UNC-40 signaling is necessary and sufficient for dendrite outgrowth in DA9, and signaling through UNC-40 represents the ‘rate-limiting’ step.
As UNC-40 acts cell-autonomously, we investigated whether it acts in a specific subcellular compartment. It has previously been observed in some signaling events during neuronal development that the receptor localizes specifically to its site of activity28,29. We tested this in DA9 by expressing a functional unc-40::gfp fusion. We found that UNC-40 was markedly enriched in the dendrite and soma compared to the axon (Supplementary Fig. 3a). To address the mechanism responsible for spatially restricting UNC-40, we asked whether the receptor localization may be subject to its own ligand, as has been shown to be the case for UNC-40 localization during the polarization of the soma of neuron HSN and in neuron AIY13,28. We examined UNC-40::GFP localization in unc-6 mutants and observed that the dendritic restriction of UNC-40 was no longer fully maintained. Instead, we saw UNC-40 localizing in the dendrite and axon in many individuals (Supplementary Fig. 3b). This suggests that downstream UNC-40 signaling might be restricted to the dendrite through UNC-40 subcellular localization in vivo. However, dorsal muscle expression of unc-6 revealed that unc-6 was not sufficient to relocalize dendritic UNC-40 to the axon and that even low levels of dendritic UNC-40 were sufficient to support dendrite outgrowth (Fig. 4 and Supplementary Fig. 3c).
Figure 4.
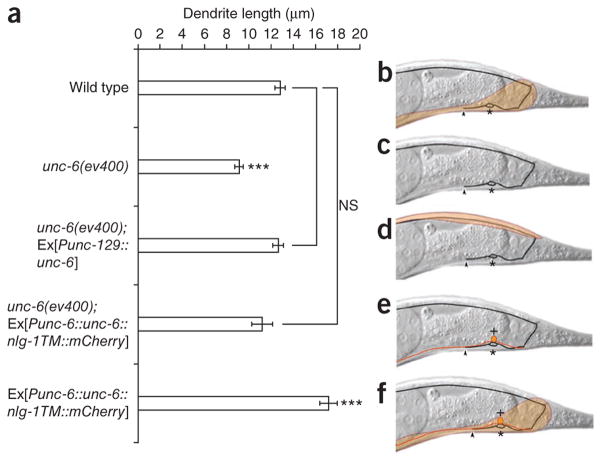
Local, non-graded UNC-6 signaling is sufficient for dendrite outgrowth. (a) Graph of dendrite length in different genetic backgrounds. (b–f) Schematics of the corresponding UNC-6 expression pattern (orange shading) in each background. Dendrite lengths in wild-type (b) and unc-6 worms (c), as well as rescue of outgrowth by dorsal muscle unc-6 expression (unc-6(ev400); Ex[Punc-129::unc-6]) (d) and membrane-tethered unc-6 expression (unc-6(ev400); Ex[Punc-6::unc-6::nlg-1TM:: mCherry]). The soma of the neighboring VA12 motor neuron (red) is marked by + (e). Overexpression of membrane-tethered unc-6 is sufficient for dendrite overshooting (f). All quantification from anterior edge of soma to anterior tip of dendrite. n ≥ 25. Error bars, s.e.m. ***P < 0.001; NS, not significant; Student’s t-test.
UNC-6 action is local and gradient-independent
UNC-6 produced by ventral neurons is secreted and diffuses to produce a putative ventro-dorsal gradient. This gradient is required for ventral axon guidance in, for example, AVM, PDE and HSN neurons3. However, it has also been reported that UNC-6 (Netrin) can act locally on the cell surface, without diffusing. This has been shown in the crossing of commissural axons at the Drosophila midline, and immobilized Netrin-1 has recently been shown to produce traction with axonal growth cones that is sufficient to reorient them30,31. We attempted to test whether the UNC-6 gradient is required for anterior dendrite growth. We disrupted the endogenous gradient by expressing unc-6 in dorsal muscles of unc-6 mutants using a fragment of the unc-129 (tgf-β) promoter (Fig. 4a–d). This manipulation should result in a reverse gradient (dorsal high, ventral low). Unexpectedly, the presence of UNC-6 in a changed distribution was sufficient to completely rescue the dendrite truncation, suggesting that UNC-6 distribution is not important for its function in promoting DA9 dendrite growth (Fig. 4a). Notably, this artificial dorsal-high UNC-6 gradient worsened the axon phenotype. In unc-6 worms, 24% of axons failed to reach the dorsal cord, indicating that UNC-6 is important, but not essential, for DA9 guidance. In unc-6 mutants carrying the unc-129::unc-6 transgene, 49% of axons failed to reach the dorsal cord, suggesting that the artificial gradient further prevents the axons’ dorsal migration, possibly through UNC-5 (Supplementary Fig. 4).
Given that a ventro-dorsal UNC-6 gradient is not required, we asked whether UNC-6 from neurons adjacent to DA9 might be sufficient. To test this, we expressed the membrane-tethered UNC-6 extracellular domain under its own promoter. This manipulation fully rescued the unc-6 mutant dendrite defect, and expression of membrane-tethered UNC-6 resulted in dendrite overshooting in wild-type worms, suggesting that UNC-6 is acting as a short-range signal (Fig. 4a, e, f). Consistent with this, UNC-6 is expressed by neurons in the ventral nerve cord that fasciculate with the DA9 dendrite. Among these are the interneuron AVA and the motor neuron VA12 (refs. 21,25). Notably, unc-6 mutants expressing membrane-tethered UNC-6 showed a penetrance of axon guidance phenotypes similar to that of the unc-6 mutants alone, suggesting that axon guidance requires secreted UNC-6 (Supplementary Fig. 4). Hence, UNC-6 from neighboring neurons is probably promoting dendrite outgrowth.
These results highlight how differently UNC-6 affects DA9’s axon and dendrite development. Axon guidance requires a ventro-dorsal UNC-6 gradient and is mediated by the UNC-5 receptor, whereas short-range UNC-6 is sufficient to promote adjacent
PAR-4 acts downstream of UNC-6 and UNC-40
Genes identified acting downstream of UNC-6 and UNC-40 in C. elegans include the actin-binding proteins UNC-34, UNC-115 and MIG-10 (ref. 14). We tested whether dendrite outgrowth was mediated by any of these genes. We found that mutants for unc-34 and unc-115, but not mig-10, showed a shortened dendrite (Fig. 1 and data not shown). Similar to the unc-40 phenotype, the unc-34 phenotype is slightly more severe than the unc-6 phenotype.
These data suggest that molecules originally described in axon guidance also function during dendrite outgrowth1,6. Given that dendrite and axon growth seem to be mediated by common pathways, we tested a gene originally identified in early embryonic polarity and more recently in axon outgrowth, the serine-threonine kinase gene par-4 (lkb1)32,33. Unexpectedly, par-4 mutants exhibited normal axon outgrowth and guidance (Fig. 5a, b). However, they showed shortened dendrites, a phenotype similar to but less severe than that of unc-6, unc-40 and unc-34 mutants (Fig. 5a, b). DA9-specific par-4 cDNA expression in par-4 mutants was able to rescue the defect (Fig. 5c). Furthermore, par-4 overexpression in wild-type worms was sufficient to cause marked dendritic overshooting (Fig. 5c). Hence, like unc-40, par-4 is acting cell-autonomously in DA9 and is necessary and sufficient for dendrite outgrowth.
Figure 5.
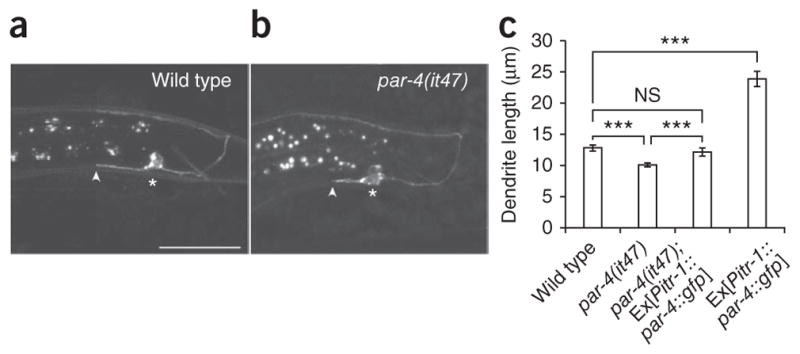
PAR-4 (LKB1) is necessary and sufficient for dendrite outgrowth. (a, b) Lateral view of wild-type and par-4 mutants expressing Pmig-13:: mig-13::gfp at L3 stage. (c) Graph of dendrite length in L3 wild-type, par-4, par-4 rescue (par-4(it47); Ex[Pitr-1::par-4::gfp]) and par-4 overexpression (Ex[Pitr-1::par-4::gfp]). All worms are L3 stage, all pictures are lateral view with ventral down and anterior to the left, all quantification from anterior edge of soma to anterior tip of dendrite. Scale bar, 20 μm. n ≥ 30. Error bars, s.e.m. ***P < 0.001; NS, not significant; Student’s t-test.
As the par-4 phenotype resembles that of unc-6 signaling mutants, we asked whether these genes were acting in parallel or in a hierarchical pathway. With few exceptions, double mutants for genes within one genetic pathway show a phenotype not significantly different from that of the more severe of the two mutants. If par-4 and unc-6 are acting independently, par-4; unc-6 or unc-40; par-4 double mutants should show a significantly shorter dendrite than single mutants. The unc-40; par-4 mutants showed a dendrite length not significantly different from that of unc-40 mutants alone, and the par-4; unc-6 mutant dendrite length was not significantly different from that of unc-6 mutants (Fig. 6a). We found this also to be the case at L4 and adult stages (Supplementary Fig. 5) and conclude that these genes are likely acting in the same pathway.
Figure 6.
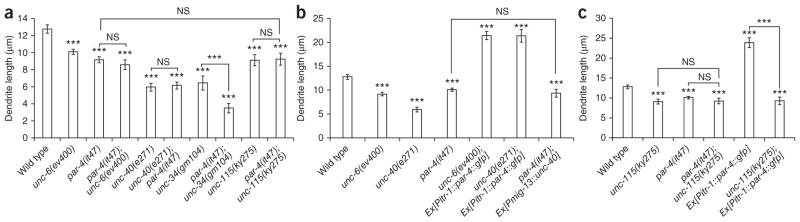
PAR-4 acts downstream of UNC-6 and UNC-40. (a) Graph of dendrite length in L3 worms in par-4, unc-6, unc-40, unc-34 and unc-115 mutants and their double mutants with par-4. The par-4; unc-6, the unc-40; par-4 and the par-4; unc-115 mutants do not enhance, whereas unc-34; par-4 mutants show a marked enhancement in dendrite truncation. (b) Dendrite length in L3 worms expressing a par-4 cDNA construct in unc-6 and unc-40 mutants (unc-6(ev400); Ex[Pitr-1::par-4::gfp] and unc-40(e271); Ex[Pitr-1::par-4::gfp] and worms expressing unc-40 cDNA in a par-4 mutant (par-4(it47); Ex[Pmig-13::unc-40]). Overexpression of par-4 can compensate for unc-6 or unc-40 loss, whereas unc-40 overexpression does not rescue par-4. (c) Graph of dendrite length in L3 worms. par-4; unc-115 double mutants do not enhance compared to single mutants, and the overextension caused by Ex[Pitr-1::par-4::gfp] expression is suppressed in unc-115 mutants, suggesting that unc-115 acts downstream of par-4. All quantification is from anterior edge of soma to anterior tip of dendrite. n ≥ 20. Error bars, s.e.m. ***P < 0.001; NS, not significant; Student’s t-test.
One could imagine several ways in which UNC-6, UNC-40 and PAR-4 could be hierarchically organized, given their functional and molecular characteristics. PAR-4 is a serine-threonine kinase that was cloned in a screen for genes mediating the asymmetric localization of P-granules in early embryonic worm development34. Furthermore, PAR-4 has been shown to modulate the localization of the zinc finger protein MEX-5 (ref. 35). Hence, PAR-4 could act upstream of UNC-6 and UNC-40 and be responsible for the asymmetric distribution of UNC-40 or other proteins required for dendrite outgrowth downstream of UNC-6 and UNC-40. The second possibility would be that PAR-4 acts downstream of UNC-6 and UNC-40 to regulate cytoskeletal modulators. This second scenario would resemble PAR-4’s function in mouse axon formation, in which PAR-4 is activated by BDNF and phosphorylates the SAD-A and SAD-B kinases33,36.
To establish a hierarchy between PAR-4 and UNC-6, we carried out several experiments. If PAR-4 were acting upstream of UNC-40 in regulating its localization, we would expect UNC-40 localization to change in par-4 mutants, as in unc-6 mutants. However, UNC-40 localization was unaltered in par-4 mutants (data not shown). If PAR-4 were acting upstream of UNC-40 without affecting its localization, we would expect unc-40 expression in par-4 mutants to rescue dendrite outgrowth. However, the same unc-40 cDNA that rescued in unc-40 mutants did not in par-4 mutants (Fig. 6b). If PAR-4 acts downstream of UNC-6 and UNC-40, its overexpression should rescue. Overexpression of par-4 in both unc-6 and unc-40 mutants rescued dendrite outgrowth and resulted in marked overshooting (Fig. 6b). These data taken together indicate that PAR-4 is a new type of effector of UNC-6.
Two pathways downstream of UNC-40 in attractive axon guidance have already been described, one of which is UNC-34 dependent, and one of which is UNC-115 dependent14. To determine whether PAR-4 acts in either of these or represents a third pathway, we constructed double mutants of par-4 with either unc-34 or unc-115. The phenotype of unc-34; par-4 double mutants was strongly enhanced (Fig. 6a). The severe dendrite truncation indicates that in mutating these genes we have tapped into the two main pathways responsible for dendrite outgrowth in DA9. In contrast, the par-4; unc-115 double mutants show a dendrite length similar to that of par-4 and unc-115 single mutants (Fig. 6a), suggesting they function in one genetic pathway. Furthermore, the dendrite overshooting produced when expressing par-4 in DA9 is blocked by a mutation in unc-115, suggesting that par-4 acts upstream of unc-115 (Fig. 6c).
We wondered whether par-4 acts in the same pathway in other neurons that have been characterized as having unc-40- and unc-34-dependent growth. One such neuron is the touch receptor AVM, whose process makes a ventral turn in part owing to the attractive guidance of unc-6 (ref. 12) (Fig. 7a). In unc-34 mutants, 3% of worms had guidance defects and failed to turn ventrally (Fig. 7b, c). Whereas we observed no ventral guidance defects in par-4 mutants, the incidence of ventral guidance defects increased to 10% in unc-34; par-4 double mutants. Furthermore, the double mutants showed a 10% incidence of processes that terminate before the pharynx terminal bulb, which was not observed in single mutants or wild-type worms (Fig. 7d). These data suggest that par-4 acts downstream of unc-40 and in parallel with unc-34.
Figure 7.
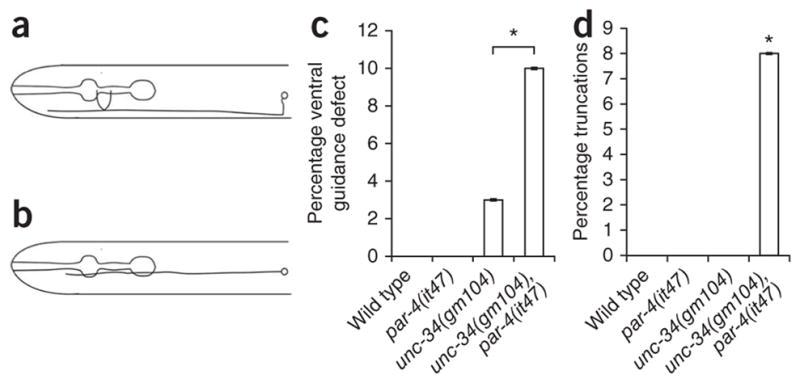
unc-34 and par-4 act in parallel in AVM development. (a) The AVM soma is located laterally in the worm and extends a process which first migrates ventrally, then anteriorly into the head (ventral down, anterior to the left). (b) unc-34 and unc-40 mutants show a guidance defect in which the AVM process fails to make a ventral turn before extending12. (c) Graph of percentage of guidance defects in AVM. Wild-type and par-4 single mutants do not show ventral guidance defects, whereas unc-34 mutants show a low-penetrance defect. The frequency is enhanced in unc-34; par-4 double mutants. (d) Graph of percentage of outgrowth defects in the AVM process. Wild-type, par-4 and unc-34 single mutants consistently have AVM processes that grow beyond the pharynx terminal bulb, whereas par-4, unc-34 double mutants show a low-penetrance outgrowth defect. n ≥ 100. Error bars, standard error of proportion; **P < 0.01, *P < 0.05, χ2 test.
Next we sought to identify how par-4 is regulated by unc-40. While no enzymatic activity has been associated with unc-40, three evolutionarily conserved cytoplasmic motifs, P1, P2 and P3, have been identified37,38. P1 and P2 have previously been identified as mediating netrin attraction through unc-34, unc-115, ced-10 and mig-10 (refs. 14,15). We asked whether par-4 also promotes outgrowth through one of the P motifs. To do this, we characterized the gain-of-function phenotypes in AVM when expressing myristoylated unc-40 (myr::unc-40) in touch cells. Overexpression of myr::unc-40 caused defects in soma morphology, axon guidance and branching in 90% of worms (Supplementary Fig. 6). A partial suppression and trend toward suppression of these gain-of-function phenotypes was achieved by deleting the P1 and P2 motifs, respectively (myr::unc-40ΔP1 and myr::unc-40ΔP2), but less so upon deleting the P3 motif. Losing unc-34 did not further suppress excess outgrowth in the myr::unc-40ΔP1 background, confirming that unc-34 acts through the P1 motif. Similarly, we confirmed that loss of unc-115 did not further suppress excess outgrowth in myr::unc-40ΔP2 worms, suggesting that unc-115 acts through the P2 motif. When we inspected these strains in par-4 mutants, we found that loss of par-4 suppressed myr::unc-40ΔP1 gain-of-function phenotypes but, like unc-115, did not suppress excess outgrowth in myr::unc-40ΔP2 worms (Supplementary Fig. 6). This suggests that unc-40 regulates par-4 through its intracellular P2 motif and is consistent with par-4 acting upstream of unc-115.
We conclude that short-range UNC-6 signaling regulates dendrite outgrowth in DA9 by means of a previously undescribed pathway involving UNC-40 acting upstream of PAR-4. This extends the role of UNC-6, originally described as an axon outgrowth and guidance cue, and more recently as a regulator of synaptic trafficking, to another domain of neural development. This trifunctional capacity within one cell is presumably achieved by means of specificity in downstream signaling molecules, such as PAR-4.
DISCUSSION
Dendrite growth and guidance is a key event in neural circuit formation. We have identified UNC-6 as regulating dendrite outgrowth by a pathway in which local UNC-6 signals through UNC-40 and PAR-4.
UNC-6 functions in multiple events during DA9 development
The contribution of UNC-6 to axon guidance of DA class motor neurons has previously been described3,39. In C. elegans, lateral and dorsal neurons in the nerve ring use UNC-40 to grow toward UNC-6. Ventral motor neurons, including the DA class, use ventral UNC-6 as a repulsive cue for dorsal guidance through UNC-5. Hence UNC-6 governs the dorsal guidance in DA motor neurons, which is the first event in their morphogenesis.
The trafficking of axon-specific molecules, such as presynaptic vesicles and active zone proteins, appears to take place simultaneously with axon growth and is also mediated by UNC-6 in an UNC-5-dependent fashion. In unc-6 and unc-5 mutants, vesicle-associated and active zone proteins are detected in the dendrite, whereas in wild-type worms, their localization is restricted to a zone within the dorsal axon27. The discovery that a diffusible molecule might instruct the molecular polarity of a neuron was unexpected. In particular, the fact that UNC-6 acts as a local inhibitor is noteworthy, as the contribution of other diffusible molecules to synapse formation has been primarily positive. It is also of note that the exclusion or ‘repulsion’ of synaptic components is UNC-5 dependent, as is the axonal growth-cone repulsion away from ventral UNC-6 (ref. 27).
We have found that dendrite outgrowth is another event orchestrated by UNC-6 during DA9 development. Postembryonically, UNC-6 acts as a growth-promoting signal on the dendrite, much like the outgrowth-promoting function by which Netrin was originally identified as the UNC-6 homolog in the chick floor plate9. UNC-6 mediates these three functions in DA9 by using distinct downstream signaling pathways.
UNC-6 differentially regulates axon and dendrite growth
The fact that axons and dendrites use identical cues to instruct distinct growth trajectories creates the conundrum of how a neuron can interpret the same external cues to generate different subcellular responses. Conceptually, the answer must lie in the spatial confinement and temporal regulation of signaling components. Although the temporal aspect remains poorly understood, we have uncovered several mechanisms by which a motor neuron differentiates its response to UNC-6 during dendrite and axon outgrowth.
First, unc-40 mutants, but not unc-5 mutants, show dendrite truncations. Hence the primary receptor mediating dendrite outgrowth is UNC-40, whereas both axon guidance and dendritic exclusion of presynaptic components are regulated by UNC-5. Therefore, axon and dendrite growth are regulated by distinct UNC-6 receptors (Supplementary Fig. 7a). The next question is how the distinct receptors are confined to subcellular compartments. It turns out that UNC-40 is targeted to the dendrite in part by UNC-6 during dendrite outgrowth; that is, UNC-6 compartmentalizes DA9 to allow a receptor-specific response. It is possible that UNC-6 recruits or retains dendritic UNC-40 by direct binding. Such ligand-dependent receptor localization has been observed in C. elegans for UNC-6 and LIN-44 (Wnt)23,28. As unc-6 cannot relocalize UNC-40 away from the dendrite, it is likely that other molecules, such as integrins or the clathrin adaptor protein AP1, contribute to UNC-40’s dendritic localization40,41.
Second, we found that membrane-tethered UNC-6 is sufficient for dendrite outgrowth, suggesting that UNC-6 is acting at short range. This is in contrast to the long-range, UNC-6 gradient–dependent growth and guidance events during DA9 axon development. Rather than acting as an orienting cue, UNC-6 acts at the surface of UNC-6-positive neighboring neurons, allowing the DA9 dendrite to grow along them. This is reminiscent of Drosophila Netrin at the ventral midline, where Netrin is not required for attracting axons toward the midline, but rather for promoting their growth across it30. Several pieces of our data suggest that a second pathway also promotes dendrite outgrowth. Among them, the dendrite was significantly shortened, but not lost, in all single mutants. Furthermore, both unc-34 and unc-40 phenotypes were more severe than that of unc-6, suggesting they respond to another ligand as well. The unc-40 cDNA expression was able to partially rescue the unc-6 phenotype, also speaking to its having an unc-6-independent function. Ventral UNC-6 seems to have a permissive function in dendrite outgrowth, but does not convey directional (antero-posterior) information. This would suggest that there is an unidentified directional signal that is acting in conjunction with UNC-6. The ability of the Pmig-13::unc-40 overexpression to have effects in wild-type but not unc-6 worms suggests that unc-40 also has a nonautonomous role in DA9 dendritic extension.
PAR-4 acts downstream of UNC-40
We found that PAR-4 is a new type of downstream component of UNC-6–UNC-40 signaling in promoting dendrite outgrowth in DA9. PAR-4 is among a group of genes having asymmetrical effects on intra-cellular events, such as cell-division, apical-basal epithelial cell polarization and neurite extension42. These genes were first described in a C. elegans screen for ‘partitioning-defective’ worms showing defects in early cell divisions, P granule distribution and intestinal development34,43. Its fly and mouse homologs lkb1 and LKB1 have since been implicated in cleavage spindle placement, centrosome localization and downstream events such as asymmetric cell division, neuronal migration and axon outgrowth44,45. LKB1 conditional mutants were used to demonstrate that LKB1 is required cell-autonomously for axon outgrowth and phosphorylates SAD-A and SAD-B kinases in cortical neurons in vivo33. As mutants of sad-1, the worm homolog of mammalian SAD kinases, do not show a truncated dendrite, PAR-4 is likely acting through other genes (data not shown). Both the fact that UNC-115 and PAR-4 are regulated by means of the UNC-40 P2 motif and the absence of enhancement observed in the genetic interaction of the par-4; unc-115 mutant suggest that par-4 is acting in one genetic pathway with the actin-binding protein unc-115. As unc-115 loss of function suppresses the excess outgrowth resulting from par-4 overexpression, this may be one avenue by which UNC-6 signals to the cytoskeleton by way of PAR-4 (Supplementary Fig. 7b).
The use of distinct signaling mechanisms within one neuron in response to one extracellular signal explains how a cell can generate local responses independently of each other. In DA9, a dendrite growth response is promoted in the neurite in which UNC-40 is localized preferentially and is present at the tip of the growing neurite. UNC-40 responds to local UNC-6 and acts through genes that do not contribute to axon growth or guidance in DA9. To achieve this response specificity, a simultaneous suppression of the dorsal guidance response is probably achieved by excluding the UNC-5 receptor or its downstream players from the leading edge of the dendrite27. Whereas UNC-5 mediates axon guidance and outgrowth in DA9, other neurons, such as VD inhibitory motor neurons, extend axons in the ventral cord and send dendrites to the dorsal cord using UNC-6–UNC-5 signaling, suggesting that different cells can use similar mechanisms in different ways to specify axon and dendrite outgrowth.
Another example of one guidance cue differentially regulating dendrite and axon development within one neuron was reported in cortical pyramidal neurons. The growth of apical dendrites toward the pial surface is mediated by the diffusible chemoattractant Sema-3A, which also acts as a chemorepellent for cortical axons1,46. In this case, whereas it seems that one receptor is mediating both axon and dendrite responses, a soluble guanylate cyclase is asymmetrically localized to the developing dendrite and is required for the chemo-attractive effect of Sema-3A. Thus, asymmetric localization of intra-cellular signaling components and receptors are mechanisms by which axons and dendrites can be patterned in response to one extracellular cue. As the study of axon and dendrite growth and guidance has not continued to uncover more extrinsic cues in recent years, it is likely that neurons use a plethora downstream signaling mechanisms in a neurite-specific fashion, such as the one described here, which we have yet to discover.
ONLINE METHODS
Strains and genetics
Worms were raised on OP50 Escherichia coli–seeded NGM plates at 22 °C, excepting par-4(it47ts) and par-4(it47ts)–derived strains, which were raised at the permissive temperature 16 °C and analyzed at 25 °C. The following mutant strains were obtained through the Caenorhabditis Genetics Center: CF896 dpy-20(e1282) IV; muIs42 (Pmig-13::mig-13::GFP; dpy-20 +), CB271 unc-40(e271) I, MT464 unc-5(e53) IV, NW434 unc-6(ev400) X, TV2918 unc-34(gm104) V, KK184 par-4(it47ts) V and SK4005 zdIs5 I. CX3079 unc-115(ky275) X was obtained from E. Lundquist’s laboratory. CX4834 zdIs4 IV; kyEx456 (Pmec-7:: myr::unc-40), CX5999 zdIs4 IV; kyEx639 (Pmec-7::myr::unc-40ΔP1) and CX5997 bad-1(ky592) V; zdIs4 IV; kyEx637 (Pmec-7::myr::unc-40ΔP2) were obtained from the Bargmann laboratory, and CX5997 was outcrossed to eliminate bad-1(ky592). N2 Bristol was used as the wild-type reference strain.
par-4 husbandry and temperature shift experiments
par-4(it47ts) worms were raised at the permissive temperature of 16 °C. The PAR-4 requirement is surpassed at the four-cell stage43, and par-4(it47) worms show delayed growth at the permissive temperature. L4 worms were picked onto a plate and let grow to gravid adults and lay eggs, which were shifted to 25 °C at 36 h after picking the L4 stage. These eggs hatched and were raised at 25 °C until the L3 stage, at which point they were imaged for dendrite length.
Cloning and constructs
Expression clones were made in a derivative of pPD49.26 (ref. 47) with additional cloning sites from C.I. Bargmann (Rockefeller University). The following plasmids and strains were generated using standard techniques: wyEx1902 (Pitr-1::mCherry27, wyEx2501 (Pmig-13::unc-40), wyEx2462 (Pmig-13::unc-40::gfp), wyEx2885(Punc-6::unc-6::nlg-1TM::mCherry), wyEx3249 (Punc-129::unc-6) and wyEx3250 (Pitr-1::par-4::gfp). We used the co-injection markers Podr-1::gfp or Podr-1::dsRED at 20 ng μl−1. Transgenes were introduced into C. elegans as previously described48. Pmec-7::myr::unc-40ΔP3 plasmid was obtained from the Bargmann lab and injected at 50 ng μl−1 into zdIs4 IV worms (wyEx4206). For Pmig-13::unc-40 (wyEx2501), a SphI–AscI fragmentcontaining Pmig-13 (ref. 23) was subcloned into unc-40 pSM from Pttx-3::unc-40. Pmig-13::unc-40 was injected at 20 ng μl−1 into unc-40(e271) worms. This array was subsequently outcrossed from unc-40(e271) worms into the N2 background. For Pmig-13::unc-40::gfp (wyEx3208), an SphI–AscI fragment containing Pmig-13 was subcloned into unc-40::gfp pSM from Pttx-3::unc-40::gfp. Pmig-13::unc-40:: gfp was injected at 5 ng μl−1 into N2 worms. Punc-6unc-6::nlg-1TM::mCherry (wyEx2885) was injected at 15 ng μl−1 with the Punc-122::rfp co-injection marker at 20 ng μl−1. For Punc-129::unc-6 (wyEx3249), an SphI–AscI PCR fragment containing a Punc-129 promoter fragment driving expression in dorsal muscle23 cloned into Punc-6::unc-6 was injected at 10 ng μl−1 into unc-6(ev400) worms. For Pitr-1::par-4::gfp (wyEx3250), an SphI–AscI PCR fragment containing Pitr-1pB23 was subcloned into par-4::gfp pSM and was injected at 20 ng μl−1 into N2 worms. par-4 cDNA (K. Kemphues, Cornell University) was amplified using the following primers: 5′-ACATGGCGCGCCATGGATGCTCCGTCGACATCCTCGGGAGC ACA-3′; 5′-CCGGTACCGGAGCACTATCGGTACGAGAACG-3′.
Microscopy methods, equipment and settings
For all imaging of DA9, worms were immobilized using 10 mM levamisole (Sigma), and for imaging and measuring body length 0.3 M 2,3-butanedione monoxime (BDM) was used. All images were taken at 25 °C. Images of fluorescently tagged fusion proteins were captured in live C. elegans using a Plan-Apochromat ×63, numerical aperture 1.4, oil immersion, differential interference contrast objective on a Zeiss LSM510 confocal microscope. Images were acquired using LSM510 software release 4.0 SP1 in Expert mode using the following settings: pixel depth, 8 bits; stack size (pixels), 1024 × 512 × number of slices (1.8 μm slice depth or scan interval); objective, Plan-Apochromat ×63, 1.4 numerical aperture, oil immersion, differential interference contrast; pixel time, 3.2 μs; main beam splitter 405/488/543/633; dichroic beam splitter 1 635 visible; dichroic beam splitter 2 545; Wavelength, 488 nm; 100% transmission; filter, channel 2, band pass 505–530 nm; pinhole, channel 2, 248 μm. Detector gain, 658; amplifier offset, 0; amplifier gain, 1. Images were processed in Adobe Photoshop CS2 version 9.0 for brightness and contrast. Quantification of dendrite length and body length was carried out by imaging with a Zeiss Axiophot with AxioCam/Axiovision digital imaging. Dendrite length was measured on these images from the anterior point of process extension from the cell body to the process tip. Body length was measured from tip of head to tail.
Statistical analysis
Statistical analysis was performed using two-tailed Student’s t-tests, Pearson’s χ2-test or Mann-Whitney U-test.
Supplementary Material
Acknowledgments
We thank the International Caenorhabditis Genetic Center and the C. Kenyon (University of California, San Francisco), E. Lundquist (University of Kansas) and C. Bargmann (Rockefeller University) laboratories for strains and K. Kemphues laboratory (Cornell University) for par-4 cDNA, as well as C. Gao and Y. Fu for technical assistance. This work was supported by the Human Frontier Science Foundation, the W. M. Keck Foundation and the Howard Hughes Medical Institute (K.S.) and a Boehringer Ingelheim graduate fellowship (H.M.T.).
Footnotes
AUTHOR CONTRIBUTIONS
H.M.T. performed all experiments. H.M.T. and K.S. designed and analyzed the experiments and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 2.Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 4.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 5.Suli A, Mortimer N, Shepherd I, Chien CB. Netrin/DCC signaling controls contralateral dendrites of octavolateralis efferent neurons. J Neurosci. 2006;26:13328–13337. doi: 10.1523/JNEUROSCI.2858-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furrer MP, Kim S, Wolf B, Chiba A. Robo and Frazzled/DCC mediate dendritic guidance at the CNS midline. Nat Neurosci. 2003;6:223–230. doi: 10.1038/nn1017. [DOI] [PubMed] [Google Scholar]
- 7.Furrer MP, Vasenkova I, Kamiyama D, Rosado Y, Chiba A. Slit and Robo control the development of dendrites in Drosophila CNS. Development. 2007;134:3795–3804. doi: 10.1242/dev.02882. [DOI] [PubMed] [Google Scholar]
- 8.Finger JH, et al. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J Neurosci. 2002;22:10346–10356. doi: 10.1523/JNEUROSCI.22-23-10346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serafini T, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 10.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 11.Gertler FB, et al. enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 1995;9:521–533. doi: 10.1101/gad.9.5.521. [DOI] [PubMed] [Google Scholar]
- 12.Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CI. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci. 2002;5:1147–1154. doi: 10.1038/nn956. [DOI] [PubMed] [Google Scholar]
- 13.Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- 15.Chang C, et al. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. doi: 10.1016/j.cub.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 16.Ming G, et al. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu XJ, et al. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol. 2007;9:184–192. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]
- 19.Li X, et al. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G, et al. p130CAS is required for netrin signaling and commissural axon guidance. J Neurosci. 2007;27:957–968. doi: 10.1523/JNEUROSCI.4616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond, B, Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 22.Sym M, Robinson N, Kenyon C. MIG-13 positions migrating cells along the anteroposterior body axis of C. elegans. Cell. 1999;98:25–36. doi: 10.1016/S0092-8674(00)80603-0. [DOI] [PubMed] [Google Scholar]
- 23.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 24.Wadsworth WG. Moving around in a worm: netrin UNC-6 and circumferential axon guidance in C. elegans. Trends Neurosci. 2002;25:423–429. doi: 10.1016/s0166-2236(02)02206-3. [DOI] [PubMed] [Google Scholar]
- 25.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 26.Hamelin M, Zhou Y, Su MW, Scott IM, Culotti JG. Expression of the UNC-5 guidance receptor in the touch neurons of C. elegans steers their axons dorsally. Nature. 1993;364:327–330. doi: 10.1038/364327a0. [DOI] [PubMed] [Google Scholar]
- 27.Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou P, et al. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55:53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 31.Moore SW, Biais N, Sheetz MP. Traction on immobilized netrin-1 is sufficient to reorient axons. Science. 2009;325:166. doi: 10.1126/science.1173851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development. 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- 33.Barnes AP, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 35.Tenlen JR, Molk JN, London N, Page BD, Priess JR. MEX-5 asymmetry in one-cell C. elegans embryos requires PAR-4- and PAR-1-dependent phosphorylation. Development. 2008;135:3665–3675. doi: 10.1242/dev.027060. [DOI] [PubMed] [Google Scholar]
- 36.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Kolodziej PA, et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 38.Chan SS, et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 39.Lim YS, Mallapur S, Kao G, Ren XC, Wadsworth WG. Netrin UNC-6 and the regulation of branching and extension of motoneuron axons from the ventral nerve cord of Caenorhabditis elegans. J Neurosci. 1999;19:7048–7056. doi: 10.1523/JNEUROSCI.19-16-07048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagedorn EJ, et al. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev Cell. 2009;17:187–198. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margeta MA, Wang GJ, Shen K. Clathrin adaptor AP-1 complex excludes multiple postsynaptic receptors from axons in C. elegans. Proc Natl Acad Sci USA. 2009;106:1632–1637. doi: 10.1073/pnas.0812078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 43.Morton DG, Roos JM, Kemphues KJ. par-4, a gene required for cytoplasmic localization and determination of specific cell types in Caenorhabditis elegans embryogenesis. Genetics. 1992;130:771–790. doi: 10.1093/genetics/130.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 45.Asada N, Sanada K, Fukada Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J Neurosci. 2007;27:11769–11775. doi: 10.1523/JNEUROSCI.1938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science. 1998;282:1904–1906. doi: 10.1126/science.282.5395.1904. [DOI] [PubMed] [Google Scholar]
- 47.Fire A, Kondo K, Waterston R. Vectors for low copy transformation of C. elegans. Nucleic Acids Res. 1990;18:4269–4270. doi: 10.1093/nar/18.14.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.