Abstract
Background
Heart failure (HF) is an important contributor to both the burden and cost of national healthcare expenditures, with more older Americans hospitalized for HF than for any other medical condition. With the aging of the population, the impact of HF is expected to increase substantially.
Methods and Results
We estimated future costs of HF by adapting a methodology developed by the American Heart Association to project the epidemiology and future costs of HF from 2012 to 2030 without double counting the costs attributed to comorbid conditions. The model assumes that HF prevalence will remain constant by age, sex, and race/ethnicity and that rising costs and technological innovation will continue at the same rate. By 2030, >8 million people in the United States (1 in every 33) will have HF. Between 2012 and 2030, real (2010$) total direct medical costs of HF are projected to increase from $21 billion to $53 billion. Total costs, including indirect costs for HF, are estimated to increase from $31 billion in 2012 to $70 billion in 2030. If one assumes all costs of cardiac care for HF patients are attributable to HF (no cost attribution to comorbid conditions), the 2030 projected cost estimates of treating patients with HF will be 3-fold higher ($160 billion in direct costs).
Conclusions
The estimated prevalence and cost of care for HF will increase markedly because of aging of the population. Strategies to prevent HF and improve the efficiency of care are needed.
Keywords: AHA Scientific Statements, heart failure
Heart failure (HF) is an important healthcare issue because of its high prevalence, mortality, morbidity, and cost of care. As of 2012, 2.4% of the US population has HF, with prevalence increasing with age such that among those ≥80 years of age, almost 12% of both men and women have HF.1 Mortality is high, with 50% of Medicare beneficiaries not surviving 3 years after an HF hospitalization.2 Although hospitalizations for HF have decreased slightly in recent years,3 the cost of HF care is high and will remain a significant concern for the US healthcare system. If one assumes a continuation of present care practices, an increase in costs is expected, in part because patients with HF will survive longer because of the development and implementation of life-prolonging therapies, as well as aging of the population, which will lead to more patients at risk for developing HF.
Previously, the American Heart Association (AHA) evaluated the overall prevalence and medical costs of cardiovascular diseases.4 The AHA used a methodology that assumed continued trends in HF epidemiology and avoided double counting of disease costs across categories5 and estimated that HF would grow faster than other cardiovascular diseases because of its higher prevalence among older Americans. The purpose of the present study is to update and expand on prior work and provide an in-depth look at how the changing demographics in the United States will impact the prevalence and cost of care for HF for different US populations. Projections can be interpreted as the most likely scenario if no further action is taken to reduce the health and economic burden of HF; however, we expect that economic and political forces will require major changes in healthcare delivery and spending before these projections become a reality. These projections can be used to judge the effectiveness of any health policy changes related to HF care.
Data and Methods
Overview
HF prevalence and costs (direct and indirect) were projected using the following steps: First, HF prevalence and average cost per person were estimated by age group (18–44, 45–64, 65–79, ≥80 years), sex (male, female), and race/ethnicity (white non-Hispanic, white Hispanic, black, other). HF prevalence was assumed to remain constant for each of the 32 age, sex, and race/ethnicity groups included in the model. The initial HF cost per person was determined for each demographic group and was assumed to increase in real terms based on the historical rate of growth of overall medical spending (direct) and real wages (indirect), with the assumption that rising prices and technological innovation will continue at the same rate for the next 18 years. Then, total HF population prevalence and costs were projected by multiplying prevalence rates and average costs by the US Census–projected population of each demographic group. Thus, projections reflect expected changes in population demographics but assume no change in prevalence and average relative cost within a demographic group.
Projections of HF Prevalence
Prevalence estimates for HF were determined with data from the 1999–2008 National Health and Nutrition Examination Survey and US Census Bureau projected population counts for years 2012 to 2030. Additional details are provided in Appendix A.
Projected population counts for years 2012 to 2030 were obtained from the 2008 population projections of the US resident population by age, sex, race, and Hispanic origin generated by the US Census Bureau based on Census 2000 data. The US Census Bureau used a cohort-component method6 with assumptions regarding future births, deaths, and migration. We multiplied the prevalence estimates of HF condition in each sex/age/race group by the projected population counts in the corresponding category for years 2012 to 2030 to project the number of people with HF in each category for each year. Then, projected overall HF prevalence and prevalence by overall demographic characteristic were calculated.
Projections of Direct Medical Costs
Medical costs of HF were estimated with the 2004–2008 Medical Expenditure Panel Survey (MEPS).7 Details of the MEPS data and their use in estimating cost of care are provided in Appendix B. Briefly, estimates of future direct medical costs of HF were determined in several steps. First, we estimated per person medical costs for people with HF as a function of health conditions using a 2-part regression model that controlled for cardiovascular disease conditions and other potentially costly or prevalent medical conditions and sociodemographic variables. Second, expenditures attributable to HF were calculated as the difference in predicted expenditures for a person with HF and predicted expenditures for a similar person without the condition. Double counting of expenditures in individuals with multiple conditions was avoided by use of a previously developed procedure (described in Appendix B).5 Third, we adjusted the per person cost estimates to account for nursing home spending. Fourth, we inflated the dollar values from MEPS to 2010. Total medical costs of HF were then estimated by multiplying the per person cost of each HF condition by the projected number of people with HF. Thus, estimates do not assume that all costs of care for a patient with HF are attributable to HF. Instead estimates provide an estimate of the incremental cost of care attributable to HF.
Projections of Indirect Costs
Indirect costs of lost productivity from morbidity and premature mortality were estimated. Morbidity costs represent the value of lost earnings attributable to HF and include loss of work among currently employed individuals and those too sick to work, as well as home productivity loss, which is the value of household services performed by household members who do not receive pay for the services.8,9 Per capita work loss and home productivity loss costs attributable to HF were estimated with 2001–2008 MEPS data and a negative binomial model for annual days of work missed (work loss) and annual days in bed (home productivity loss) attributable to illness or injury as a function of HF, other comorbid conditions, and sociodemographic variables. We generated total work loss and home productivity loss costs by multiplying per capita work days lost attributable to HF by (1) prevalence of HF, (2) the probability of employment given HF (for work loss costs only), (3) mean per capita daily earnings, and (4) US Census population projection counts.
Mortality costs represent the value of lost earnings from premature death attributable to HF. To calculate total mortality costs, we first multiplied death rates estimated from the 2006 National Vital Statistics data by Census population projections to project the number of HF deaths, which were then multiplied by the remaining lifetime earnings. More details of indirect cost calculations are provided in Appendix B.
Results
Prevalence
Table 1 displays the projected number of people in the United States with HF from 2012 to 2030 for different age groups. By 2030, >8 million Americans will be living with HF, with 2 million of these >80 years of age (>26% of all HF patients). Accordingly, the prevalence of HF in the United States is expected to increase by 23%, from 2.42% in 2012 to 2.97% in 2030. With the growth of the US population, the total number of Americans living with HF will increase by 46% from 2012 to 2030.
Table 1.
Projections of the US Population With HF From 2010 to 2030 for Different Age Groups
| Year | All | 18–44 y | 45–64 y | 65–79 y | ≥80 y |
|---|---|---|---|---|---|
| 2012 | 5 813 262 | 396 578 | 1 907 141 | 2 192 233 | 1 317 310 |
| 2015 | 6 190 606 | 402 926 | 1 949 669 | 2 483 853 | 1 354 158 |
| 2020 | 6 859 623 | 417 600 | 1 974 585 | 3 004 002 | 1 463 436 |
| 2025 | 7 644 674 | 434 635 | 1 969 852 | 3 526 347 | 1 713 840 |
| 2030 | 8 489 428 | 450 275 | 2 000 896 | 3 857 729 | 2 180 528 |
HF indicates heart failure.
Cost of Care
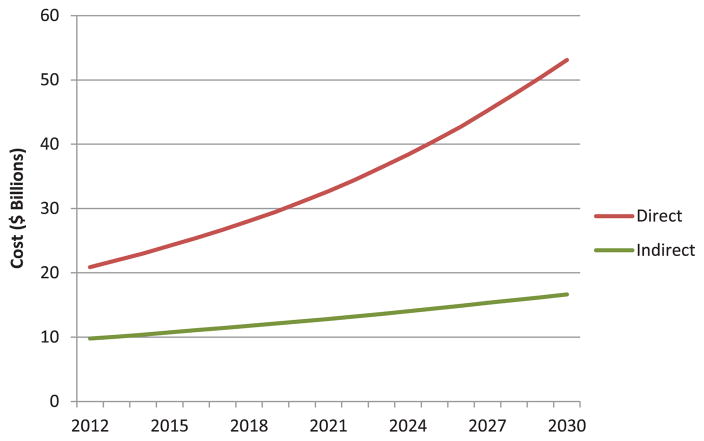
Total medical costs are projected to increase from $20.9 billion in 2012 to $53.1 billion in 2030 (Table 2; Figure 1), a 2.5-fold increase. The majority (80%) of the costs attributed to HF are related to hospitalization, assuming continuation of current hospitalization practices. Indirect costs are expected to rise as well, but at a lower rate, from $9.8 billion to $16.6 billion, an increase of 69%. The total cost of HF (direct and indirect costs) is expected to increase from $30.7 billion to $69.8 billion. This is equivalent to $244 for every US adult in 2030.
Table 2.
Projections of Total Cost of Care ($ Billions) for HF for Different Age Groups of the US Population*
| Year | All | 18–44 y | 45–64 y | 65–79 y | ≥80 y |
|---|---|---|---|---|---|
| 2012 | |||||
| Medical | 20.9 | 0.33 | 3.67 | 8.46 | 8.42 |
| Indirect: Morbidity | 5.42 | 0.52 | 1.92 | 2.05 | 0.93 |
| Indirect: Mortality | 4.35 | 0.66 | 2.53 | 0.98 | 0.18 |
| Total | 30.7 | 1.51 | 8.12 | 11.5 | 9.53 |
| 2020 | |||||
| Medical | 31.1 | 0.43 | 4.58 | 14.2 | 11.8 |
| Indirect: Morbidity | 7.09 | 0.66 | 2.20 | 3.11 | 1.12 |
| Indirect: Mortality | 5.39 | 0.79 | 2.89 | 1.49 | 0.22 |
| Total | 43.6 | 1.88 | 9.67 | 18.8 | 13.2 |
| 2030 | |||||
| Medical | 53.1 | 0.59 | 5.86 | 23.3 | 23.4 |
| Indirect: Morbidity | 9.80 | 0.91 | 2.54 | 4.48 | 1.87 |
| Indirect: Mortality | 6.84 | 0.98 | 3.32 | 2.16 | 0.37 |
| Total | 69.7 | 2.48 | 11.7 | 29.9 | 25.6 |
HF indicates heart failure.
Excludes HF care costs that have been attributed to comorbid conditions.
Figure 1.
The projected increase in direct and indirect costs attributable to HF from 2012 to 2030 is displayed. Direct costs (cost of medical care) are expected to increase at a faster rate than indirect costs because of lost productivity and early mortality. HF indicates heart failure.
The above estimates, although avoiding double counting, do not indicate the increased cost of treating all patients with HF. If one assumes all costs of care for HF patients are attributable to HF (ie, no cost attribution to comorbid conditions), the 2030 projected cost estimates of treating HF are 3-fold higher (ie, $160 billion in direct costs).
Age Subgroups
Because of aging of the US population, the total cost of care for older Americans with HF will increase faster than for younger ages. Cost of HF care will increase almost 3-fold for those >65 years of age, whereas increases will be less for younger age groups (a 1.6-fold increase for those aged 45–64 years and a 2-fold increase for those aged 18–44 years).
The fraction of total HF expenditures consumed to treat those ≥65 years will increase from 69% in 2012 to 80% in 2030. Those >65 years of age have fewer indirect costs because they are less likely to be employed than younger patients.
Sex and Race Subgroups
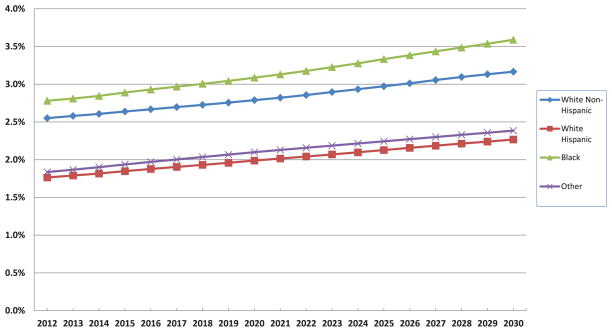
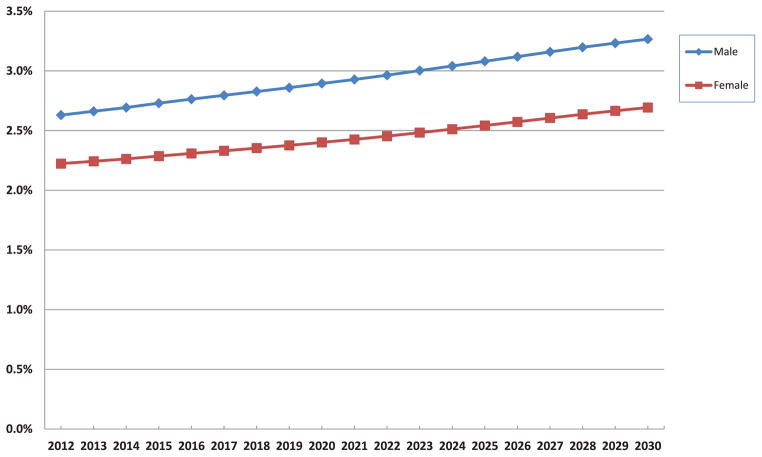
The prevalence of HF among different racial and ethnic groups is expected to increase substantially (Figure 2). The highest prevalence will remain among blacks and will rise by 29% between 2012 and 2030, from 2.8% to 3.6%. In 2030, white Hispanic and other non-Hispanic nonblack patients will have the lowest prevalence, 2.3% and 2.4%, respectively. The fraction of men and women with HF is expected to grow similarly over the next 18 years (Figure 3), with a higher prevalence among men.
Figure 2.
Projected US prevalence of HF from 2012 to 2030 is shown for different races. The prevalence of HF remains lowest among white Hispanics and highest among blacks. HF indicates heart failure.
Figure 3.
Projected prevalence of HF from 2012 to 2030 is shown for men and women in the United States. The prevalence of HF remains highest among men throughout the period, although it increases among both groups over time. HF indicates heart failure.
The aging of the population and the growth in per capital medical spending are the primary drivers of these projected increases in HF costs, which are expected to grow the fastest for ≥65 years of age. Aging of the population has less of an impact on indirect costs than direct costs because of lower rates of employment among older Americans. Annual HF costs for people aged 65 to 79 years are projected to increase by 160%, from $11.50 billion to $29.93 billion.
Discussion
This study projects that the burden of HF for the US healthcare system will grow substantially during the next 18 years if current trends continue. It is estimated that by 2030, the prevalence of HF in the United States will increase by 25%, to 3.0%. Because of the increase in the size of the US population, the number of patients with HF will increase by 46%, to >8 million patients by 2030. If one assumes that the rate of medical care inflation from the past decade continues over the next 2 decades, the cost of HF care will increase >2-fold. Although our primarily analysis avoided double counting, the cost estimates underestimate the cost of treating all patients with HF. The direct cost of treating patients with HF could be as much as 3-fold greater ($160 billion by 2030).
Because of aging of the population, the increase in HF will be greatest for older Americans. Among those >80 years of age, the number of patients with HF is expected to grow by 66% by 2030. Large increases are expected for all sex and racial/ethnic subgroups.
Causes and Stages of HF
If the projections for accelerating HF costs are to be avoided, an understanding of the different causes of HF and their risk factors is helpful. HF is a clinical syndrome that results from a variety of disorders of the myocardium (eg, idiopathic dilated cardiomyopathy), cardiac valves, pericardium, or vasculature (eg, ischemic heart disease). HF is generally a symptomatic disease marked by shortness of breath, fatigue, and swelling. Coronary artery disease, valvular disease, hypertension, and dilated cardiomyopathy are the causes of HF in the majority of patients in the Western world.10
In 2001, the American College of Cardiology and AHA practice guidelines for chronic HF introduced a classification system that encompasses 4 sequential stages of HF.11 Stages A and B are considered precursors to the clinical syndrome of HF and are meant to alert healthcare providers to known risk factors for HF and the available therapies aimed at mitigating disease progression. Stage A patients are at risk for HF related to conditions such as hypertension, atherosclerotic heart disease, and diabetes mellitus. Patients with stage B have developed structural heart disease from a variety of potential insults to the heart muscle, ranging from previous myocardial infarction to valvular heart disease, but remain asymptomatic. Stages C and D represent the symptomatic phases of HF. Most HF therapeutic interventions, including dietary salt restriction, medications known to prolong survival, and implantable devices such as pacemakers and defibrillators, are targeted at patients with symptomatic HF (stage C). In the end stages of HF (stage D), patients develop marked symptoms at rest or with minimal activity despite optimal medical therapy.
Risk Factors
An understanding of risk factors for HF is important for the development of interventions aimed at prevention. Classic demographic risk factors for the development of HF include older age, male sex, ethnicity, and low socioeconomic status.12 Specific comorbid and disease states also contribute significantly to the development of HF. Ischemic heart disease is thought to be the most important risk factor for HF. Hypertension is associated with a smaller relative risk of development of HF than that associated with ischemic heart disease but contributes more to the overall population burden of HF because of its greater prevalence.13 Diabetes mellitus, insulin resistance, and obesity are also linked to HF development, with diabetes mellitus increasing the risk of HF by ≈2-fold in men and up to 5-fold in women.14,15 Smoking remains the single largest preventable cause of disease and premature death in the United States, and current smokers have a significantly higher risk for the development of HF than ex-smokers and nonsmokers.16,17 Although ischemic heart disease and smoking have declined, any associated reduction in future HF may be offset by the growing rates of diabetes mellitus and obesity.
Potential Strategies to Reduce Future Costs
Prevention and treatment of HF can be improved through enhanced community-based cardiovascular health strategies, new therapies for prevention and treatment of HF, and improved implementation of existing preventative measures and therapies.12 In an effort to increase the use of evidence-based prevention and treatment approaches, the AHA, alone or in partnership with the American College of Cardiology and other professional societies, has produced guidelines for the prevention and treatment of HF.12,18 Other prevention-oriented guidelines for hypertension, cholesterol, smoking, obesity, and physical activity, if successfully implemented, would also be expected to reduce the incidence of HF.19–23 Primordial prevention strategies have substantial potential to reduce the population burden of HF by preventing the development of adverse risk factors for HF.24
In multiple studies, disparities and variations in use of evidence-based therapies in eligible patients with or at risk for HF have been demonstrated.25,26 As a result, patients may develop incident HF, be hospitalized, and experience fatal events that could have been prevented with more effective implementation of guideline-recommended therapy.26 Improved implementation of guideline-based therapies can prevent the onset of HF in those at risk and substantially improve survival in patients with established HF.27,28 Thus, there remain substantial opportunities to improve implementation of existing therapies to both prevent and treat HF.
Performance measures help focus quality measurement and improvement efforts on guideline-based strategies or processes that have the greatest clinical impact.29 The AHA, the American College of Cardiology, The Joint Commission, the Centers for Medicare and Medicaid Services, and other organizations have developed performance measures for patients with, and at risk for, cardiovascular diseases.29–33 By facilitating measurements of cardiovascular healthcare quality, performance measures may serve as vehicles to accelerate appropriate translation of scientific evidence into clinical practice.29 Performance measure sets for HF treatment, as well as primary and secondary cardiovascular prevention in the ambulatory setting, have been developed.30–32
Performance improvement programs have facilitated the implementation of evidence-based therapies in both hospital and ambulatory care settings.34–37 Not only have hospitals improved HF, coronary artery disease, and stroke care substantially over time, those providing the highest levels of care based on the performance measures have better patient survival rates than hospitals not performing at the highest level.35 Substantial quality improvement has also been demonstrated in the outpatient practice setting.37 Thus, guidelines, performance measures, and performance improvement programs can have a substantial impact on cardiovascular prevention and treatment and will be important tools for limiting the burden of HF. The AHA strongly recommends the use of programs such as the AHA’s Get With The Guidelines, the AHA/American Cancer Society/American Diabetes Association’s The Guideline Advantage, the American Heart Association’s Heart 360, and the American College of Cardiology’s Practice Innovation and Clinical Excellence (PINNACLE) to identify appropriate patients for therapy, provide practitioners with useful reminders based on the guidelines, and continuously assess the success achieved in providing guideline-based therapies to patients who can benefit from them.
Care Transitions and Coordination
Hospitalizations (including readmissions) account for a substantial portion of the cost of HF care. To achieve the best clinical outcomes and reduce preventable hospitalizations, care coordination is necessary. Care coordination may be challenging because of patient, family, or caregiver factors; disparities in care; and complex and sometimes confusing medical regimens. With aging, patients are likely to have comorbid conditions, including atrial fibrillation, sleep-disordered breathing, and anemia, all of which cause dyspnea and fatigue, which makes it difficult for patients to determine the specific causative condition that requires attention.38 Furthermore, social support may be important if all patients with HF are to obtain recommended care.39
Care transition programs by hospitals have become more widespread in an effort to reduce avoidable readmissions. The interventions used by these programs include initiating discharge planning early in the course of hospital care, actively involving patients and families or caregivers in the plan of care, providing new processes and systems that ensure patient understanding of education about the plan of care before discharge from the hospital, and improving quality of care by continually monitoring adherence to national evidence-based guidelines. In multiple studies, self-care adherence to the HF plan of care was associated with reduced all-cause mortality or HF hospitalization.40,41
Although many care coordination and transitions programs were found to benefit patients by decreasing readmissions,42–46 decreasing medication discrepancies,47 and reducing cost of care,45,48,49 not all programs were effective.48,50–52 It is possible that care transition programs may increase appropriate admissions while decreasing inappropriate admissions, which would have an uncertain impact on the 30-day all-cause readmission rate that has become a focus of public reporting and pay for performance.
Provider Workforce for Managing Patients With HF
The needs of the growing HF population cannot be met by physician and nursing subspecialists alone. Rather, a marked expansion of competency across the broad provider work-force is needed. Key factors driving this growing and shifting demand include (1) the burgeoning patient numbers, largely a result of population aging; (2) a shift of care from inpatient to outpatient settings; and (3) consolidation of provider services away from small group practices and toward integrated systems. The net result will likely be a significant increase in the need for specialized HF physicians, general cardiologists, primary care providers with expanded competency in HF, advanced practice nurses, and other practitioners, including pharmacists.
When considering professional staffing in HF management, administrators should recognize the dichotomous nature of the population and practice: (1) Acute and chronic “standard” HF management, including palliative care, for most patients and (2) “advanced HF” management, including heart transplantations and ventricular assist devices for select patient subsets. Both types of HF care require a multidisciplinary approach, with standard HF care requiring management of the multiple comorbidities of the older population and advanced HF care driven by the complex and technical nature of medical and transplant cardiology practice.
Much of advanced HF care is currently provided by large practices with at least 10 full-time equivalent staff.53 These larger programs often include multiple disciplines such as financial coordinators, social workers, exercise physiologists, nutritionists, psychologists, and pharmacologists. Small practices (<4 full-time equivalent staff; 43% of all HF practices) provide less advanced care, whereas the majority of standard HF care is provided outside of HF practices by providers in primary care and general cardiology. It is likely that an increase in staffing needs will be proportionately weighted more heavily toward ambulatory medical management than advanced therapies, potentially with a proportionately greater involvement of nonphysician staff.
It is likely that the number of providers pursuing advanced HF training will need to increase to meet future demands for advanced HF care. In 2005, the Heart Failure Society of America identified only 48 active US advanced HF training programs, although 17 additional institutions were considering initiating programs. However, the American Board of Internal Medicine’s recent designation of Advanced Heart Failure and Transplant Cardiology as a certifiable secondary subspecialty54 has sparked expanded interest in the field by cardiologists and cardiology trainees. The Accreditation Council for Graduate Medical Education is in the final stages of preparation for training program accreditation. For nurses, HF certification examination is now offered by the American Association of Heart Failure Nurses, and >200 nurses have taken the examination. Economic factors may also increase provider supply. New reimbursement models, such as bundled payments for an HF population, will increasingly link rewards to improved efficiency, quality, and clinical outcomes, again driving organizational resources into HF management.
Racial Disparities in HF Care
Diversity in race, ethnicity, and socioeconomic culture should not lead to disparities for HF prevention or care, yet disparities of care have been observed across ethnic and racial minority groups. Although guidelines can be applied across all groups, it is important to remember that certain racial/ethnic groups have a higher prevalence of risk factors, such as hypertension among black women or diabetes mellitus in women of Mexican-American descent.55
By the year 2050, 1 of 3 individuals in the United States will be of Hispanic origin. Costs will be amplified because Hispanics are younger at onset of HF, as recently confirmed in the Get With The Guidelines–Heart Failure registry.56 Relative to non-Hispanic whites, blacks and Hispanics with HF and preserved or reduced ejection fraction were more likely to have a greater proportion of significant risk factors such as diabetes mellitus, hypertension, and obesity.55 Furthermore, Hispanics may be more likely to have less insurance and access to care. Some disparity is caused in part by limited acculturation and a lower socioeconomic level. Patients with HF who are foreign-born and do not speak English as their primary language have a greater risk of rehospitalization, independent of clinical factors and race/ethnicity.57 If improvement programs, such as Get With The Guidelines, are implemented in hospitals, quality measures can increase across all groups, thus benefitting the Hispanic population as well.56
Although the number of black patients with HF is not expected to increase as quickly as the number of Hispanic patients, there is concern that disparities in access to high-quality chronic care may perpetuate a greater burden of HF for this group. In a study of Medicare beneficiaries, hospitalizations for HF declined less for black patients than for other patient groups.3 Although black patients had higher rates of readmission for HF than whites within the first year of discharge,58 mortality rates at 30 days and 1 year were lower for blacks than whites. Lower mortality by black race was ascribed to the success of Medicare in allowing access to the healthcare system. Thus, the importance of a social net may be significant in preventing disparities by race. Ongoing research should address the underrepresentation of some racial groups in HF trials and thus the potential lack of understanding of group differences regarding effective therapy.59
End-of-Life Care
For the foreseeable future, the majority of patients with HF will experience worsening symptoms, decreased quality of life, accelerating episodes of decompensation, and a refractory terminal phase of disease60,61 (AHA/American College of Cardiology stage D).18 In 2008, HF was listed on 1 in 9 US death certificates, and for 56 565 individuals, it was given as the underlying cause of death.1 Additionally, a substantial and increasing proportion of patients have comorbidities that further worsen quality of life and, in some cases, will be the cause of death.62,63
Although the median survival for patients with symptomatic HF is ≈5 years, the clinical course for an individual patient is typically nonlinear and relatively unpredictable, with acute episodes of decompensation often separated by relative periods of stability.64,65 The relative uncertainty in prognosis, compared with the more predictable linear decline of patients with advanced cancer, for example, complicates the already difficult process of planning for the terminal phase of disease.
Advanced therapies for HF are frequently discussed in the setting of stage D disease, but such advanced options are unlikely to be appropriate for the majority of patients. Use of cardiac transplantation is constrained by a limited supply of donor hearts, a situation that will not likely change in the foreseeable future.66 The use of mechanical circulatory support may increase as the technology improves but is likely to remain inappropriate for the majority of patients with HF because of the predominance of HF with normal ejection fraction, multiple comorbidities, or very advanced age.25,67
Prolongation of the final stages of the disease will impose an even heavier burden of limitation and suffering onto not only patients but also families and the medical system. More than a quarter of Medicare spending occurs in the last year of life,68 and the costs of care during the last 6 months for a patient with HF have been increasing (by 11% from 2000 to 2007).69 Increasing prevalence and length of end-of-life care for patients with stage D HF will require ongoing integration of multiple aspects of care, patient priorities, and shared decisions that have not been adequately emphasized under prior systems of care.
Palliative care, including formal hospice care, is increasingly advocated for patients with advanced HF.65,70,71 Offering palliative care to patients with HF may lead to more conservative (and less expensive) treatment that is consistent with many patients’ goals for care. Although cancer remains the most prevalent hospice diagnosis, the use of hospice services is growing among the HF population, with HF now the second most common reason for entering hospice. As mentioned above, a challenge to timely hospice referral for patients with HF is the difficulty of predicting life expectancy, even for patients with advanced disease. A recent study of patients in hospice care found that patients with HF were more likely than patients with cancer to use hospice services longer than 6 months or to be discharged from hospice care alive.72
Study Limitations
The present analysis has several limitations. First, we estimated costs of HF care using survey data, which are subject to sampling error. Thus, there is uncertainty in our point estimates that is difficult to quantify. We used the human capital approach to estimate indirect costs and did not include the time value of informal caregivers of those with HF.73 The human capital approach also undervalues the morbidity costs of those not in the labor force (psychological costs), which is often the case for patients with HF. Our analysis did not examine types of HF (eg, type of cardiomyopathy, valve disease, arrhythmia), and it is likely that the relative prevalence of the causes of HF will change over time.
Our study did not assume any change in mortality or admission rates once HF occurred. Recent studies have found that hospitalizations for HF and mortality have both declined.3 If such trends continue, the impact on our estimates will be mixed. Considerable effort is under way to develop more sophisticated home management strategies and to disincentivize hospital utilization through financial incentives and altered reimbursement models. Some have projected these efforts to markedly reduce the trajectory of HF-related hospitalization rates. Although lower hospitalization rates would lead to less cost, longer life expectancy with HF could increase resource use and result in higher costs. If better adherence to guidelines occurs or new treatments are developed, patients may live longer but also healthier. Our study also assumed that the rate of growth of healthcare spending would continue based on historical trends. Costs may be reduced if the rate of development of new HF technologies slows or major changes in the structure of financing of healthcare change resource use patterns.
We recognize that differences exist between our estimates of HF cost and those previously published in the Heart Disease and Stroke Statistics—2010 Update.74 The present study used more recent data and different methods that avoid double counting of disease costs. Our cost estimates are 3-fold higher if we assume all medical costs for a patient with HF are attributable to HF.
Conclusions
Assuming continuation in present practice patterns, the cost of HF is projected to increase markedly over the next 18 years based on demographic changes in the population. The cost would be substantial, with each US adult, on average, paying $244 annually by 2030 to care for the 10 million patients with HF. The best solution is prevention, which is possible through treatment of predisposing conditions such as coronary artery disease, hypertension, and diabetes mellitus. Prevention strategies need to be applied broadly across diversely ethnic and racial groups as well. Further research on HF prevention by sex is also needed. In addition, a shift in the care model directed toward reducing inpatient hospitalization use could have a significant impact on the trajectory of overall HF-related costs. Health policy should continue to expand its focus on prevention of HF to continue to improve the health of the US population and to reduce use of limited healthcare resources.
Appendix A: Data Definitions
Questions/Measures and ICD-9-CM Codes Used to Define HF Conditions in NHANES and MEPS
| Condition | Qualifying Questions/Measures From NHANES | ICD-9 Codes From MEPS |
|---|---|---|
| Hypertension | Were you told on 2 or more different visits that you had hypertension, also called high blood pressure? Are you now taking prescribed medicine for your high blood pressure? Average SBP ≥140 mm Hg or average DBP ≥90 mm Hg |
401, 403 |
| Coronary heart disease | Has a doctor or other health professional ever told you that you had coronary heart disease? Has a doctor or other health professional ever told you that you had angina, also called angina pectoris? Has a doctor or other health professional ever told you that you had a heart attack (also called myocardial infarction)? Rose Questionnaire |
410, 411, 412, 413, 414 |
| Heart failure | Has a doctor or other health professional ever told you that you had congestive heart failure? | 428 |
| Stroke | Has a doctor or other health professional ever told you that you had a stroke? | 430, 431, 433, 434, 436, 438 |
| Other heart failure, including cerebrovascular | NA | 390, 391, 393–400, 402, 404, 405, 415–427, 429, 432, 435, 437, 440–448, 450–459, 745–747 |
DBP indicates diastolic blood pressure; HF, heart failure; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; MEPS, Medical Expenditure Panel Survey; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; and SBP, systolic blood pressure.
Appendix B: Detailed Data and Methods
Projections of HF Prevalence
Prevalence of HF was estimated with data from the 1999–2008 National Health and Nutrition Examination Survey. The National Health and Nutrition Examination Survey is a survey of a nationally representative sample administered by the National Center for Health Statistics, which is part of the Centers for Disease Control and Prevention. The survey includes an interview and a physical examination component; the interview includes demographic, socioeconomic, dietary, and health-related questions, and the examination component consists of medical, dental, and physiological measurements, as well as laboratory tests administered by highly trained medical personnel. Prevalence of HF was based on patient self-report. A list of qualifying measures and questions used to define HF is presented in Table A1.
We estimated the prevalence of HF using logit regression models controlling for survey year and demographics (age, sex, and race/ethnicity). Stepwise regressions were used to determine significant interactions of demographics to be included in the models. We predicted prevalence of HF in each sex/age/race cell for 2007 to 2008 using coefficients from the logit regressions. Prevalence estimates were adjusted to account for the nursing home care population using data from the 2004 National Nursing Home Survey.
Prevalence estimates were then combined with Census projections of population counts for years 2012 to 2030 to generate the projected number of people with HF and projected HF prevalence for years 2012 to 2030. Projected population counts for years 2012 to 2030 were obtained from the 2008 population projections of the US resident population by age, sex, race, and Hispanic origin generated by the US Census Bureau. The 2008 projections are based on Census 2000 and were produced by use of a cohort-component method. The projections are based on assumptions about future births, deaths, and net international migration. We multiplied predicted prevalence of HF in each sex/age/race cell by the projected population counts in the corresponding cells for years 2012 to 2030 to project the number of people with HF in each cell in each of the years. We then aggregated the number of people with HF by sex, by age, and by race and calculated projected HF prevalence overall and by each demographic characteristic.
Projections of HF Direct (Medical) Costs
The main data source for the generation of projections of medical costs of HF was the 2004–2008 MEPS.7 MEPS is a nationally representative survey of the civilian noninstitutionalized population administered by the Agency for Healthcare Research and Quality. MEPS provides data on participants’ use of medical services and the corresponding medical costs. Medical conditions are identified in MEPS Medical Condition files based on self-reports of conditions that led to medical visits or treatment within the interview year. Medical conditions are classified with International Classification of Diseases, Ninth Revision, Clinical Modification codes based on self-reported conditions that were transcribed by professional coders. HF was defined with International Classification of Diseases, Ninth Revision, Clinical Modification codes, with a full list of the codes presented in Appendix A. The MEPS data measure total annual medical spending, including payments by insurers and out-of-pocket spending (copayments, deductibles, and payments for noncovered services). The costs captured by MEPS represent payments (not charges) from the payer to the provider. MEPS spending data are obtained through a combination of self-report and validation from payers (eg, private insurers).
Projections of the direct medical costs of HF were estimated by point of service. The following point-of-service categories were used (MEPS expenditure files listed in parentheses): Hospital (inpatient, outpatient, emergency department), physician (office-based visits), prescription (prescription), home health (home health), and other (vision, medical supplies, dental). Nursing home costs were estimated with the 2004 National Nursing Home Survey (see below).
For each point of service, projections of the direct medical costs of HF were estimated by use of the following steps. First, we estimated per person medical costs as a function of health conditions using a 2-part regression model. In the first part of the 2-part model, we used a logistic regression model to predict the probability of any expenditures. For the second part of the model, we used a generalized linear model with a gamma distribution and a log link to estimate total annual medical expenditures for people having any expenditures. We used an algorithm for choosing among alternative nonlinear estimators recommended by Manning and Mullahy75 and found that this type of model was the most appropriate for the data. Our model controlled for cardiovascular disease conditions and other potentially costly or prevalent medical conditions and sociodemographic variables.
Second, expenditures attributable to HF were calculated as the difference in predicted expenditures for a person with HF and predicted expenditures for a similar person without HF. We estimated the per person cost attributable to HF for each age/sex/race cell based on coefficients from the national pooled model.
Disease-attributable expenditures are typically calculated by predicting expenditures using observed diseases and subtracting from that predicted expenditures, setting the disease of interest (eg, HF) to zero and leaving all other covariates and diseases as they are in the data. However, in previous work, we have shown that in nonlinear models, such as the model used here, this approach will lead to double counting of expenditures for concurrent diseases, regardless of whether one disease causes the other.5 Double counting of expenditures is a particular problem in cases in which >1 condition is treated during a single office visit or hospitalization. We used a technique, termed complete classification and described in a previous study, to ensure that no double counting occurred.5 Using the parameters of the econometric model, we specifically treated each disease and combination of diseases observed in the data as its own separate entity when calculating the attributable costs. For example, HF alone and HF with hypertension would be treated as 2 different diseases in the attributable expenditure calculation described above. We then divided the total expenditures attributable to the combinations of diseases back to the constituent diseases using the parameters from the model to construct shares for each constituent disease within a combination (ie, a share of all HF with hypertension disease costs that are attributable to HF). The shares attribute a greater share of the joint expenditures to the disease with the larger coefficient in the main effect. The formula to construct the shares is given in Trogdon, Finkelstein, and Hoerger.5
Our third step in calculating projections of direct medical costs was to adjust the per person cost estimates to account for nursing home spending by use of data from the 2004 National Nursing Home Survey and National Health Accounts. We assumed that per person, non–nursing home expenditures attributable to cardiovascular disease were the same for the nursing home population as for the noninstitutionalized population.
Fourth, to estimate projected costs, we first followed recommendations from the Agency for Healthcare Research and Quality to inflate dollar values in the MEPS data to 2010.76 We then multiplied the per person cost of HF in each sex/age/race cell by the projected number of people treated for HF in the corresponding cells for years 2012 to 2030. The projected number of people treated for HF was calculated by use of a similar methodology as outlined in the “Prevalence” section. However, instead of the National Health and Nutrition Examination Survey data, we used 1996–2008 MEPS data to predict the treated prevalence of HF, because only those patients who receive treatment incur medical costs within a certain year.
Finally, we used Congressional Budget Office assumptions for future healthcare cost growth above and beyond growth attributable to population growth and aging.77 We assumed that the costs of HF would increase at the same rate as overall medical expenditures between 2012 and 2030, an average annual rate of 2.85%.
Projections of Indirect Costs of HF
Two types of indirect costs were calculated: Lost productivity from (1) morbidity and (2) premature mortality.
Morbidity Costs of HF
Morbidity costs represent the value of foregone earnings from lost productivity attributable to HF. Morbidity costs include 3 components: Work loss among currently employed individuals, home productivity loss, and work loss among individuals too sick to work.9 Per capita work loss days attributable to HF by age, sex, and race/ethnicity were estimated with 2001–2008 MEPS data. We estimated a negative binomial model for annual days of work missed because of illness or injury as a function of HF, other comorbid conditions, and sociodemographic variables. Per capita work days lost attributable to HF for each age/sex/race cell were based on coefficients from the national pooled model. As for medical expenditures, we avoided double counting of costs that resulted from individuals with multiple conditions by using the previously cited procedure.5 We generated total work loss costs by multiplying per capita work days lost because of HF by (1) prevalence of HF (by age, sex, and race/ethnicity) from MEPS, (2) the probability of employment given HF (by age, sex, and race/ethnicity) from MEPS, (3) mean per capita daily earnings (by age and sex) from the 2010 Current Population Survey, and (4) Census population projection counts (by age, sex, and race/ethnicity).
Home productivity loss was estimated by valuing days spent in bed because of HF at the replacement cost of housekeeping services.9 Per capita days in bed because of HF by age, sex, and race/ethnicity were estimated with 2001–2008 MEPS data and the same strategy as outlined above for work days lost. We generated total home productivity loss costs by multiplying per capita bed days attributable to HF by (1) prevalence of HF (by age, sex, and race/ethnicity) from MEPS, (2) dollar value of a day of house work (by age and sex),78 and (3) Census population projection counts (by age, sex, and race/ethnicity).
To estimate work loss among individuals too sick to work because of HF, we first estimated the number of people too sick to work who would have been employed except for their HF. For the noninstitutionalized population, we multiplied the number of people not in the labor force because of illness/disability by age from the Current Population Survey79 by the percentage of all work loss attributable to HF based on the MEPS regression analysis for work loss days described above. The assumption was that the percentage of work days missed because of HF was the same for days missed by being out of the labor force and for days missed conditional on working. For the institutionalized population, we multiplied the number of people with a primary diagnosis of HF from the 2004 National Nursing Home Survey (as a percentage of total population) by Census population counts and the probability of employment given HF (by age, sex, and race/ethnicity) from MEPS. The last component accounts for individuals with HF who might not have worked even if they had not been institutionalized. Finally, the sum of the number of noninstitutionalized and institutionalized people too sick to work because of HF was multiplied by 250 work days per year and mean annual earnings from the 2010 Current Population Survey.
Mortality Costs of HF
Mortality costs represent the value of foregone earnings from premature mortality attributable to HF. We began with estimates of lifetime earnings by sex and age provided by the National Heart, Lung, and Blood Institute to the AHA (unpublished data). We then expressed these 2003 values in real 2010 dollars using the Census’s price deflator and adjusted the values based on observed changes in real earnings between 2003 and 2010.80
We estimated death rates for each HF category by age, sex, and race/ethnicity using 2006 National Vital Statistics data.81 Assuming the death rates remained constant within age, sex, and race/ethnicity cell, we multiplied the death rates by Census population projections to project the number of HF deaths by age, sex, race/ethnicity, and year through 2030. Finally, we multiplied age- and sex-specific remaining lifetime earnings by the projected number of deaths in the corresponding age/sex cells to obtain projections of total mortality costs. The real value of indirect costs (morbidity and mortality) was assumed to grow at the Congressional Budget Office’s average annual growth rate of real earnings (1.54%) through 2030.82
Disclosures
Writing Group Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Paul A. Heidenreich | Stanford University | VA Health Care System†; American Society of Echocardiography† | None | None | None | None | Stanford University VA Health Care System*; AHA* (unpaid); American College of Cardiology* (unpaid); Institute for Health Care Improvement (IHI)* (unpaid); Heart Failure Society of America* (unpaid); NHLBI* (unpaid) | VA Palo Alto Health Care System†; Stanford University†; Heart Failure Society of America* (unpaid); Institute for Health Care Improvement* (unpaid); VA Health Care System* (unpaid) |
| Nancy M. Albert | Cleveland Clinic Foundation | None | None | None | None | None | None | None |
| Larry A. Allen | University of Colorado | None | None | None | None | None | None | None |
| David A. Bluemke | NIH | None | None | None | None | None | None | None |
| Javed Butler | Emory University | None | None | None | None | None | None | None |
| Gregg C. Fonarow | UCLA | None | None | Medtronic* | None | None | Novartis†; SC Member, ASTRONAUT trial† | None |
| John S. Ikonomidis | Medical University of South Carolina | NIH R01 grant recipient† | None | None | None | None | None | None |
| Olga Khavjou | RTI | None | None | None | None | None | CDC† | None |
| Marvin A. Konstam | Tufts Medical Center | Otsuka*; Amgen* | None | None | None | None | Merck*; Cardiokine*; Johnson & Johnson* | None |
| Thomas M. Maddox | Department of Veterans Affairs | None | None | None | None | None | None | None |
| Graham Nichol | University of Washington–Harborview Medical Center | Resuscitation Outcomes Consortium (NIH U01 HL077863-05) 2010–2015, Co-PI†; Evaluation of Video Self-Instruction in Compressions-Only CPR (Asmund S. Laerdal Foundation for Acute Medicine) 2007–2010, PI†; Randomized Trial of Hemofiltration After Resuscitation from Cardiac Arrest (NIH NHLBI R21 HL093641-01A1) 2009– 2011, PI†; Randomized Field Trial of Cold Saline IV After Resuscitation From Cardiac Arrest (NIH NHLBI R01 HL089554-03) 2007–2012, Co-I†; Novel Methods of Measuring Health Disparities (NIH 1RC2HL101759-01) 2009–2011, Co-I†; Cascade HeartRescue Program (Medtronic Foundation) 2010–2015, PI† | None | None | None | None | Gambro Renal Inc, Lakewood, CO*; Sotera Wireless, San Diego, CA*; Lifebridge Medizintechnik AG, Ampfing, Germany* | Chair, AHA Executive Database Steering Committee* (unpaid); Chair, Mission: Lifeline EMS Task Force* (unpaid); Cochair, AHA Resuscitation Science Symposium Planning Committee* (unpaid); Member, AHA Advanced Cardiac Life Support Subcommittee* (unpaid); Member, AHA Epidemiology and Statistics Committee* (unpaid); Member, Pacific Mountain Affiliate Board of Directors, American Heart Association* (unpaid); received travel reimbursement, AHA*; University of Washington† |
| Michael Pham | Department of Veterans Affairs | St. Jude Medical (PI; LAPTOP-HF)*; NIH (PI; ACE Inhibition and Cardiac Allograft Vasculopathy)*; NIAID (PI; Prevention of Cardiac Allograft Vasculopathy Using Rituximab [Rituxan] Therapy in Cardiac Transplantation)* | None | None | Sedgwick, LLP† | None | None | None |
| Ileana L. Piña | Montefiore-Einstein Medical Center | None | None | None | None | None | None | None |
| Justin G. Trogdon | RTI | None | None | None | None | None | CDC† | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (1) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (2) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Eldrin F. Lewis | Brigham & Women’s Hospital | Amgen, Inc†; Novartis†; Sanofi-Aventis* | None | None | None | None | None | None |
| Veronique L. Roger | Mayo Clinic | NHLBI† | None | None | None | None | None | None |
| Jeff Teuteberg | University of Pittsburgh | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (1) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (2) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Advocacy Coordinating Committee on January 25, 2013. A copy of the document is available at http://my.americanheart.org/statements by selecting either the “By Topic” link or the “By Publication Date” link. To purchase additional reprints, call 843-216-2533 or e-mail kelle.ramsay@wolterskluwer.com.
Expert peer review of AHA Scientific Statements is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://my.americanheart.org/statements and select the “Policies and Development” link.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. . Heart disease and stroke statistics--2012 update: a report from the American Heart Association [published correction appears in Circulation. 2012;125:e1002] Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ on behalf of the American Heart Association Advocacy Coordinating Committee, Stroke Council, Council on Cardiovascular Radiology and Intervention Council on Clinical Cardiology, Council on Epidemiology and Prevention Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Trogdon JG, Finkelstein EA, Hoerger TJ. Use of econometric models to estimate expenditure shares. Health Serv Res. 2008;43:1442–1452. doi: 10.1111/j.1475-6773.2007.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollmann FW, Mulder TJ, Kallan JE. Methodology and Assumptions for the Population Projections of the United States: 1999 to 2100. Washington, DC: US Census Bureau, Population Projections Branch; 2000. Population Division working paper No. 38. [Google Scholar]
- 7.Cohen JW, Monheit AC, Beauregard KM, Cohen SB, Lefkowitz DC, Potter DE, Sommers JP, Taylor AK, Arnett RH., 3rd The Medical Expenditure Panel Survey: a national health information resource. Inquiry. 1996–1997;33:373–389. [PubMed] [Google Scholar]
- 8.Haddix AC, Teutsch SM, Corso PS. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. 2. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 9.Rice DP, Hodgson TA, Kopstein AN. The economic costs of illness: a replication and update. Health Care Financ Rev. 1985;7:61–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Jacobs AK, Hiratzka LF, Russell RO, Smith SC., Jr ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 12.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 13.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RM, Psaty BM, Kronmal RA. Modifiable risk factors for incident heart failure in the coronary artery surgery study. Arch Intern Med. 1994;154:417–423. [PubMed] [Google Scholar]
- 17.Suskin N, Sheth T, Negassa A, Yusuf S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;37:1677–1682. doi: 10.1016/s0735-1097(01)01195-0. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2010;121:e258] Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report [published correction appears in JAMA. 2003;290:197] JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaén CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers M. Treating Tobacco Use and Dependence: Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2000. [Google Scholar]
- 21.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in Circulation. 2004;110:763] Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 22.Gidding SS, Lichtenstein AH, Faith MS, Karpyn A, Mennella JA, Popkin B, Rowe J, Van Horn L, Whitsel L. Implementing American Heart Association pediatric and adult nutrition guidelines: a scientific statement from the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular Disease in the Young, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council for High Blood Pressure Research. Circulation. 2009;119:1161–1175. doi: 10.1161/CIRCULATIONAHA.109.191856. [DOI] [PubMed] [Google Scholar]
- 23.Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, Gansler T, Glynn T, Smith RA, Taubert K, Thun MJ American Cancer Society; American Diabetes Association; American Heart Association. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109:3244–3255. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 25.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. . Heart disease and stroke statistics–2011 update: a report from the American Heart Association [published corrections appear in Circulation. 2011;124:e426 and Circulation. 2011;123:e240] Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030. e3. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Heywood JT, Liu Y, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Associations between outpatient heart failure process-of-care measures and mortality. Circulation. 2011;123:1601–1610. doi: 10.1161/CIRCULATIONAHA.110.989632. [DOI] [PubMed] [Google Scholar]
- 28.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM GRACE Investigators. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 29.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL for the American College of Cardiology; American Heart Association Task Force on Performance Measures. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111:1703–1712. doi: 10.1161/01.CIR.0000157096.95223.D7. [DOI] [PubMed] [Google Scholar]
- 30.Bonow RO, Bennett S, Casey DE, Jr, Ganiats TG, Hlatky MA, Konstam MA, Lambrew CT, Normand SL, Pina IL, Radford MJ, Smith AL, Stevenson LW, Burke G, Eagle KA, Krumholz HM, Linderbaum J, Masoudi FA, Ritchie JL, Rumsfeld JS, Spertus JA. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) Circulation. 2005;112:1853–1887. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 31.Redberg RF, Benjamin EJ, Bittner V, Braun LT, Goff DC, Jr, Havas S, Labarthe DR, Limacher MC, Lloyd-Jones DM, Mora S, Pearson TA, Radford MJ, Smetana GW, Spertus JA, Swegler EW. AHA/ACCF 2009 performance measures for primary prevention of cardiovascular disease in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on performance measures (Writing Committee to Develop Performance Measures for Primary Prevention of Cardiovascular Disease). [published correction appears in Circulation. 2010;121:e445–e446] Circulation. 2009;120:1296–1336. doi: 10.1161/CIRCULATIONAHA.109.192617. [DOI] [PubMed] [Google Scholar]
- 32.Drozda J, Jr, Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, Bonow RO, Burkiewicz JS, Crouch M, Goff DC, Jr, Hellman R, James T, 3rd, King ML, Machado EA, Jr, Ortiz E, O’Toole M, Persell SD, Pines JM, Rybicki FJ, Sadwin LB, Sikkema JD, Smith PK, Torcson PJ, Wong JB. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement [published correction appears in Circulation. 2011;124:e39] Circulation. 2011;124:248–270. doi: 10.1161/CIR.0b013e31821d9ef2. [DOI] [PubMed] [Google Scholar]
- 33.Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE, Jr, Goodlin SJ, Grady KL, Hundley RF, Jessup M, Lynn TE, Masoudi FA, Nilasena D, Pina IL, Rockswold PD, Sadwin LB, Sikkema JD, Sincak CA, Spertus J, Torcson PJ, Torres E, Williams MV, Wong JB. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2012;125:2382–2401. doi: 10.1161/CIR.0b013e3182507bec. [DOI] [PubMed] [Google Scholar]
- 34.LaBresh KA, Fonarow GC, Smith SC, Jr, Bonow RO, Smaha LC, Tyler PA, Hong Y, Albright D, Ellrodt AG Get With The Guidelines Steering Committee. Improved treatment of hospitalized coronary artery disease patients with the Get With The Guidelines program. Crit Pathw Cardiol. 2007;6:98–105. doi: 10.1097/HPC.0b013e31812da7ed. [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich PA, Lewis WR, LaBresh KA, Schwamm LH, Fonarow GC. Hospital performance recognition with the Get With The Guidelines Program and mortality for acute myocardial infarction and heart failure. Am Heart J. 2009;158:546–553. doi: 10.1016/j.ahj.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, Spertus JA. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010;122:585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 38.Riegel B, Dickson VV, Cameron J, Johnson JC, Bunker S, Page K, Worrall-Carter L. Symptom recognition in elders with heart failure. J Nurs Scholarsh. 2010;42:92–100. doi: 10.1111/j.1547-5069.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 39.Gallagher R, Luttik ML, Jaarsma T. Social support and self-care in heart failure. J Cardiovasc Nurs. 2011;26:439–445. doi: 10.1097/JCN.0b013e31820984e1. [DOI] [PubMed] [Google Scholar]
- 40.Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–185. doi: 10.1161/01.CIR.0000151811.53450.B8. [DOI] [PubMed] [Google Scholar]
- 41.van der Wal MH, van Veldhuisen DJ, Veeger NJ, Rutten FH, Jaarsma T. Compliance with non-pharmacological recommendations and outcome in heart failure patients. Eur Heart J. 2010;31:1486–1493. doi: 10.1093/eurheartj/ehq091. [DOI] [PubMed] [Google Scholar]
- 42.Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171:1232–1237. doi: 10.1001/archinternmed.2011.278. [DOI] [PubMed] [Google Scholar]
- 43.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 44.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial [published correction appears in J Am Geriatr Soc. 2004;52:1228] J Am Geriatr Soc. 2004;52:675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 45.Stauffer BD, Fullerton C, Fleming N, Ogola G, Herrin J, Stafford PM, Ballard DJ. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med. 2011;171:1238–1243. doi: 10.1001/archinternmed.2011.274. [DOI] [PubMed] [Google Scholar]
- 46.Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, Forsythe SR, O’Donnell JK, Paasche-Orlow MK, Manasseh C, Martin S, Culpepper L. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 48.Leff B, Reider L, Frick KD, Scharfstein DO, Boyd CM, Frey K, Karm L, Boult C. Guided care and the cost of complex healthcare: a preliminary report. Am J Manag Care. 2009;15:555–559. [PubMed] [Google Scholar]
- 49.Wennberg DE, Marr A, Lang L, O’Malley S, Bennett G. A randomized trial of a telephone care-management strategy. N Engl J Med. 2010;363:1245–1255. doi: 10.1056/NEJMsa0902321. [DOI] [PubMed] [Google Scholar]
- 50.Gustafsson F, Arnold JM. Heart failure clinics and outpatient management: review of the evidence and call for quality assurance. Eur Heart J. 2004;25:1596–1604. doi: 10.1016/j.ehj.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 51.Williams G, Akroyd K, Burke L. Evaluation of the transitional care model in chronic heart failure. Br J Nurs. 2010;19:1402–1407. doi: 10.12968/bjon.2010.19.22.1402. [DOI] [PubMed] [Google Scholar]
- 52.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 53.Jessup M, Albert NM, Lanfear DE, Lindenfeld J, Massie BM, Walsh MN, Zucker MJ. ACCF/AHA/HFSA 2011 survey results: current staffing profile of heart failure programs, including programs that perform heart transplant and mechanical circulatory support device implantation: a report of the ACCF Heart Failure and Transplant Committee, AHA Heart Failure and Transplantation Committee, and Heart Failure Society of America. Circ Heart Fail. 2011;4:378–387. doi: 10.1161/HHF.0b013e3182186210. [DOI] [PubMed] [Google Scholar]
- 54.Konstam MA, Jessup M, Francis GS, Mann DL, Greenberg B. Advanced heart failure and transplant cardiology: a subspecialty is born. J Am Coll Cardiol. 2009;53:834–836. doi: 10.1016/j.jacc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Thomas KL, Hernandez AF, Dai D, Heidenreich P, Fonarow GC, Peterson ED, Yancy CW. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161:746–754. doi: 10.1016/j.ahj.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Vivo RP, Krim SR, Krim NR, Zhao X, Hernandez AF, Peterson ED, Piña IL, Bhatt DL, Schwamm LH, Fonarow GC. Care and outcomes of Hispanic patients admitted with heart failure with preserved or reduced ejection fraction: findings from get With The Guidelines-Heart Failure. Circ Heart Fail. 2012;5:167–175. doi: 10.1161/CIRCHEARTFAILURE.111.963546. [DOI] [PubMed] [Google Scholar]
- 57.Peterson PN, Campagna EJ, Maravi M, Allen LA, Bull S, Steiner JF, Havranek EP, Dickinson LM, Masoudi FA. Acculturation and outcomes among patients with heart failure. Circ Heart Fail. 2012;5:160–166. doi: 10.1161/CIRCHEARTFAILURE.111.963561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rathore SS, Foody JM, Wang Y, Smith GL, Herrin J, Masoudi FA, Wolfe P, Havranek EP, Ordin DL, Krumholz HM. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289:2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 59.Ferdinand KC, Serrano CC, Ferdinand DP. Contemporary treatment of heart failure: is there adequate evidence to support a unique strategy for African-Americans? Con position. Curr Hypertens Rep. 2002;4:311–318. doi: 10.1007/s11906-996-0010-2. [DOI] [PubMed] [Google Scholar]
- 60.Adams KF, Jr, Zannad F. Clinical definition and epidemiology of advanced heart failure. Am Heart J. 1998;135(pt 2):S204–S215. doi: 10.1016/s0002-8703(98)70251-0. [DOI] [PubMed] [Google Scholar]
- 61.Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9:684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Carson P, Anand I, O’Connor C, Jaski B, Steinberg J, Lwin A, Lindenfeld J, Ghali J, Barnet JH, Feldman AM, Bristow MR. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial [published correction appears in J Am Coll Cardiol. 2008;51:2197] J Am Coll Cardiol. 2005;46:2329–2334. doi: 10.1016/j.jacc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE I-Preserve Investigators. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 64.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 65.Goodlin SJ, Hauptman PJ, Arnold R, Grady K, Hershberger RE, Kutner J, Masoudi F, Spertus J, Dracup K, Cleary JF, Medak R, Crispell K, Piña I, Stuart B, Whitney C, Rector T, Teno J, Renlund DG. Consensus statement: palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–209. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, Dobbels F, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report–2010. J Heart Lung Transplant. 2010;29:1089–1103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, Fox KA, Rajwani A, Baig W, Groves D, Barlow P, Fisher AC, Batin PD, Kahn MB, Zaman AG, Shah AM, Byrne JA, Lindsay SJ, Sapsford RJ, Wheatcroft SB, Witte KK, Kearney MT. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 68.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45:565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, Peterson ED, Curtis LH. Resource use in the last 6 months of life among Medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 70.Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE. Palliative care in the treatment of advanced heart failure. Circulation. 2009;120:2597–2606. doi: 10.1161/CIRCULATIONAHA.109.869123. [DOI] [PubMed] [Google Scholar]
- 71.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 72.Bain KT, Maxwell TL, Strassels SA, Whellan DJ. Hospice use among patients with heart failure. Am Heart J. 2009;158:118–125. doi: 10.1016/j.ahj.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Hickenbottom SL, Fendrick AM, Kutcher JS, Kabeto MU, Katz SJ, Langa KM. A national study of the quantity and cost of informal caregiving for the elderly with stroke. Neurology. 2002;58:1754–1759. doi: 10.1212/wnl.58.12.1754. [DOI] [PubMed] [Google Scholar]
- 74.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association [published corrections appear in Circulation 2010;121:e260 and Circulation 2011, 124 e425 ] Circulation. 2009 [Google Scholar]
- 75.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 76.Agency For Healthcare Research And Quality. Agency for Healthcare Research and Quality Web site. Using appropriate price indices for expenditure comparisons. 2013 Mar 22; http://meps.ahrq.gov/about_meps/Price_Index.shtml.
- 77.Congressional Budget Office. CBO’s 2011 Long-Term Budget Outlook. Washington DC: Congress of the United States, Congressional Budget Office; 2011. [Google Scholar]
- 78.Expectancy Data. The dollar value of a day: 2009 dollar valuation. Shawnee Mission, KS: John Ward Economics; 2010. [Google Scholar]
- 79.US Bureau of Labor Statistics. The Employment Situation: January 2009. 1. Vol. 56. Washington, DC: Bureau of Labor Statistics; 2009. Persons not in the labor force by desire and availability for work, age and sex. 2009. [Google Scholar]
- 80.US Department of Commerce. Table p-37 full-time, year-round workers by mean income and sex: 1955 to 2008. Washington, DC: US Bureau of the Census; 2009. Historical income tables--people. [Google Scholar]
- 81.Heron M, Hoyert DL, Murphy SL, Xu J, Murphy S, Xu J, Kochanek K, Tejada-Vera B. National Vital Statistics Reports. 14. Vol. 57. Hyattsville, MD: National Center for Health Statistics; 2009. Deaths: final data for 2006. [PubMed] [Google Scholar]
- 82.Congressional Budget Office. The Long-Term Budget Outlook, June 2009. Washington DC: Congress of the United States, Congressional Budget Office; 2009. [Google Scholar]