Abstract
Purpose of review
Rho GTPases are key molecular switches controlling the transduction of external signals to cytoplasmic and nuclear effectors. In the last few years, the development of genetic and pharmacological tools has allowed a more precise definition of the specific roles of Rho GTPases in hematopoietic stem cells (HSCs) and progeny of these cells. Rho GTPases are now known to be crucial in HSCs response to hematopoietic microenvironment cues. This article will review the known HSC functions, which are regulated by Rho GTPases.
Recent findings
This review analyzes the latest data on how different Rho GTPases control adhesion, migration, retention, proliferation, survival, senescence and oncogenic transformation of HSCs and relates these new findings to the physiological functions of these cells.
Summary
The development of small molecule inhibitors with ability to interfere Rho GTPase activation by guanine nucleotide exchange factors offers new therapeutic strategies to manipulate the function of HSCs.
Keywords: bone marrow retention, Cdc42, engraftment, hematopoietic stem cells and progenitors, leukemia, Rac, Rho GTPases
Introduction
Hematopoietic stem and progenitor cells (HSC/Ps) reside in the bone marrow cavity during postnatal life and may be localized to specific ‘niches’ within the hematopoietic microenvironment. A tiny fraction of HSCs, of unknown physiological relevance, is found circulating in the blood and the number of these cells in circulation can be increased in a process termed ‘mobilization’. Infusion of HSCs into the blood during bone marrow transplantation procedures leads to engraftment of these cells in the marrow space and subsequent reconstitution of multi-lineage hematopoiesis. On the basis of recent studies, it is clear that signals involved in engraftment and mobilization of HSC/Ps are intimately associated with cytoskeleton rearrangement regulating cell shape, adhesion and migration. These pathways directly or indirectly also affect gene transcription, cell survival, cell cycle progression and may be important in transformation and changes seen in HSCs during aging. These cytoskeleton rearrangements are controlled by members of the Rho GTPase family. This review intends to summarize the most important advances in the knowledge on Rho GTPase functions in HSCs in health and disease.
Rho GTPases
The Rho family of small monomeric GTPases are members of the Ras superfamily and include 22 genes coding for at least 25 proteins [1]. On the basis of sequence identity, domain structure and function, the Rho proteins can be divided into six families: Rac, RhoA-related proteins, Cdc42, TC10 and TCL, Rnd, the Rho BTB subset and the Miro subfamily. This review will focus on Rac and Cdc42, as these are the best-studied Rho GTPases with respect to HSCs functions.
Similar to Ras, Rho proteins are molecular switches that cycle between inactive, guanosine diphosphate (GDP)-bound and active, guanosine triphosphate (GTP)-bound states. In the GTP-bound form, Rho proteins interact with a variety of specific effector or target molecules to trigger diverse cellular responses. Activation of Rho GTPases is induced by a large family of Dbl-family guanine nucleotide exchange factors (GEFs) [2] that are activated by receptor-dependent kinases. Negative regulation of GTPases is achieved by acceleration of the intrinsic GTPase function of the proteins by GTPase activating proteins (GAPs), by sequestering and stabilizing the inactive, GDP-bound protein away from membranes via GDP dissociation inhibitors (GDIs) or by both. Almost all Rho family GTPases influence actin polymerization within the cell via specific or shared effectors and are thereby implicated in reorganization of the cytoskeleton, cell migration and adhesion. However, Rho proteins regulate a multitude of other cellular functions. Among these are apoptosis and survival, cell cycle progression and genomic stability.
Using gene-targeted knockout mice, it has become evident that the Rho family of GTPases play an important role in hematopoietic stem cell (HSC) function. We will summarize the most recent advances in this field.
Role of Rac GTPases in hematopoietic stem and progenitor cells
There are three highly related Rac proteins expressed in mammalian cells, Rac1, Rac2 and Rac3. Rac 1 and 3 are widely expressed, whereas Rac2 expression is hematopoietic specific. Rac activity has been demonstrated to be important for such diverse functions as retention in the bone marrow [3,4], long-term engraftment of HSCs [5] and HSCs mobilization [3,4,6]. Specifically, our group was able to show that Rac2 controls stem cell retention in bone marrow [4] and dissect out the mechanisms of homing depending on Rac1, suggesting that homing and mobilization are not mirror image processes but depend on distinct biochemical mechanisms [3]. Interestingly, in specific conditions, functional dualities were also found in the role of Rac isoforms in proliferation and survival. While Rac2 controls stem cell survival, Rac1 controls HSC proliferation by modifying levels of cyclin D1 and p27kip1 and therefore controlling cell cycle entry [6]. It is noteworthy that Rac is activated in HSC/Ps by ligation of three cell surface receptors known to be important in HSC/Ps adhesion, migration and survival, the receptor tyrosine kinase c-kit, the chemokine receptor chemokine (CXC motif) receptor 4 (CXCR4) and the integrin receptor α4β1. Thus, Rac appears to integrate signaling pathways distinctly important in HSCs engraftment and retention.
Although the three Rac proteins share a high degree of protein homology, each appears to subserve unique as well as some overlapping roles in HSC/Ps. Initial studies in Rac2−/− mice demonstrated that Rac2 is important for HSC/Ps adhesion and migration, and Rac2−/− mice showed increased numbers of circulating HSC/Ps, suggesting its importance in retaining cells in the hematopoietic microenvironment. Rac2 activity was also important in maintaining normal Rac1 andCdc42 function, confirming in primary cells ‘crosstalk’ between Rac2 and other Rho GTPases that is important in regulating HSC/Ps migration, particularly uropod retraction, in vivo [4,5].
Although Rac2 is more than 95% homologous to Rac1, gene-targeting studies found that Rac2 and Rac1 regulate distinct HSC functions. Rac1-deficient HSC/Ps stimulated with stem cell factor (SCF) demonstrated defective proliferative signaling via the c-kit receptor tyrosine kinase in vitro [6]. In contrast, loss of Rac2 activity leads to a proapoptotic phenotype in both mast cells and HSC/Ps in the presence of SCF [6–8]. Rac1Δ/Δ and Rac1Δ/Δ/Rac2−/− double-deficient mice were generated by genetic crosses and the use of conditional deletion alleles. In contrast to Rac2, Rac1 was required for engraftment of HSC/Ps into the bone marrow [6] (Fig. 1).However, the deletion of both Rac1 and Rac2 led to the massive mobilization of stem cells from the bone marrow due to decreased adhesion and defective retention in the marrow, suggesting overlapping functions [3,6]. In addition, Rac1Δ/Δ/Rac2−/− HSC/Ps showed a combination of reduced proliferation and increased apoptosis [6].
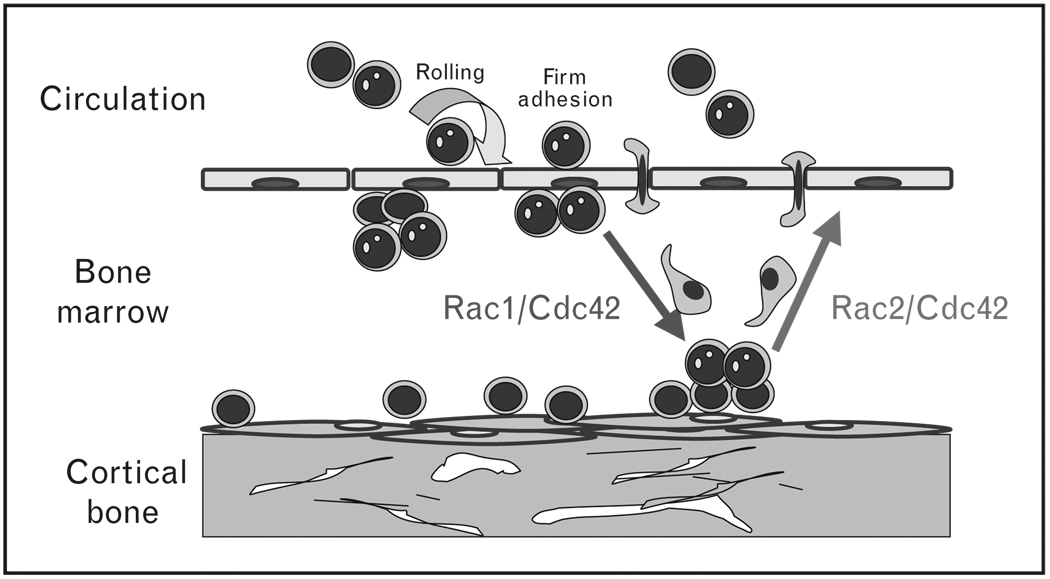
Figure 1. Normal hematopoietic stem and progenitor cells migrate in and out from the bone marrow niches.
Rac1 controls proliferation, endosteal localization and engraftment, whereas Rac2 controls survival and bone marrow retention. Cdc42 controls endosteal localization, engraftment and retention as well as aging and self-renewal of HSC/Ps. HSC/Ps, hematopoietic stem and progenitor cells.  stem cell;
stem cell;  , hematopoietic progenitor;
, hematopoietic progenitor;  , migrating cell;
, migrating cell;  , endosteal niche;
, endosteal niche;  , vascular niche.
, vascular niche.
Rac1, but not Rac2, is also required for HSC/Ps localization to the required supportive stem cell niche in the endosteal space of the medullary cavity [3]. In myeloid cells (and presumably HSC/Ps), specificity of function of Rac1 versus Rac2 maps to the carboxy terminus and the regulation of intracellular GTPase localization indicating that, at least, some of the functional dualities of Rac isoforms depend on their distinct intracellular location [9]. Therefore, although some overlap exists in the functions, Rac1 appears to predominantly regulate HSC/Ps cell cycle progression, homing and bone marrow microlocalization, whereas Rac2 mostly regulates survival and retention of HSC/Ps in the bone marrow. Of note, these data strongly suggest that HSC/Ps homing and retention in the bone marrow are not mirror image processes biochemically.
During fetal hematopoiesis, Rac1, but not Rac2, also appears to play a crucial role in HSC/Ps trafficking [10•]. In the absence of Rac1, circulating HSC/Ps are decreased in the blood of E10.5 embryos, whereas yolk sac definitive hematopoiesis was quantitatively normal. Intraembryonic hematopoiesis was significantly impaired in Rac1-deficient embryos, including by absence of intraaortic clusters, and near complete absence of aortogonadal-mesenchymal (AGM) and fetal liver hematopoiesis. These in-vivo phenotypes were associated with decreased HSC/Ps transwell migration and impaired interaction with the microenvironment-derived stromal cells in migration-dependent assays. Interestingly, these data support the long-controversial observations by Moore and Metcalf [11] (and later data by Palis et al. [12]) that definitive intraembryonic hematopoiesis is ‘seeded’ from the yolk sac.
Downstream effectors of Rac, p21-activated kinase (PAK), POR1 and signal transducer and activator of transcription 5 (STAT5) have been implicated in Rac effector functions in primary hematopoietic cells. Depending on the specific lineage and agonist, Rac can activate p42/p44 and p38 extracellular signal regulated kinases (ERKs), c-Jun-activated kinase (JNK) and Akt kinases [3,4,6,8,13,14]. Wnt/β-catenin signaling has been proposed as a key signal in maintenance of HSCs quiescence [15–17]. Wu et al. [18••] found that Rac1 complexes with Jnk2 and β-catenin. In this complex, Rac1 through Jnk2 kinase is able to phosphorylate β-catenin at Ser191 and Ser605 residues, inducing nuclear translocation via the canonical Wntdependent β-catenin activation pathway. Genetic ablation of Rac1 in the mouse embryonic limb bud ectoderm disrupts canonical Wnt signaling and phenocopies deletion of β-catenin in causing severe truncations of the limb development. Interestingly, Rac and McgRacGAP also are required for nuclear localization of Stat5a [19], suggesting that Rac proteins directly or indirectly regulate nuclear localization of factors involved in gene regulation.
Cdc42 in hematopoiesis
Cdc42 has been previously implicated in cellular gradient sensing and filopodia formation [20]. Until very recently, most work in hematopoietic cells utilized macrophage cell lines or examined the role of Cdc42 in lymphocytes. Interest in lymphocytes is derived in part because of the association of the Cdc42 effector target Wiskott–Aldrich Syndrome protein (WASp), which is defective in the human immunodeficiency disease Wiskott–Aldrich Syndrome [21].
Various gene-targeted knockout or conditional allele mice have helped to clarify the role of Cdc42 in HSC/Ps. Loss of the Cdc42 GEF PIXa leads to defective G-coupled receptor signaling, PAK activation and reduced migration [22]. Gene-targeted mice deficient in the Cdc42 GAP protein exhibit increased Cdc42 activity in bone marrow cells and increased apoptosis in HSC/P populations [23]. Neutrophils from gene-targeted Cdc42-deficient mice show increased random motility but reduced directed migration associated with reduced podosome-like structures at the leading edge of the cells through activation of different mitogen-acitvated protein kinases (MAPKs)[24]. Cdc42Δ/Δ neutrophils show increased lateral and tail membrane protrusions. Directed migration appears associated with defective p38 MAPK activity, which is apparently required for antagonizing the lateral extensions of these ‘filopodia-like’ structures.HSC/Ps from Cdc42Δ/Δ mice have a higher frequency of cells in an active cell cycle, resulting in a significantly initially increased number and frequency of the HSC/Ps in the bone marrow followed by exhaustion of the stem cell pool which correlates with decreased expression of p21cip1, β1-integrin and N-cadherin and increased c-myc expression. HSC/Ps from Cdc42Δ/Δ mice also show defective migration, adhesion, engraftment, homing and retention associated with abnormal F-actin assembly, resulting in increased numbers of circulating HSCs (Fig. 1) [25]. These animals display erythroid cell development and myelopoiesis blockade, including the development of a fatal myeloproliferative disorder [26].
Changes in HSC/Ps seen in aging mice have also been related to Cdc42 activity. Adhesion of HSCs to the hematopoietic microenvironment appears to be reduced, whereas granulocyte-colony stimulating factor-induced mobilization is increased in aged HSCs compared with young HSCs and this difference may be, at least partly, a consequence of the increased activity of the small Rho GTPase Cdc42 in aged HSCs [27].
WASp is the best characterized Cdc42 effector. WASp activation depends on the specific interaction with GTP-loaded Cdc42, which is mediated through a Cdc42 and Rac-interactive binding domain [28–30]. WASp and the isoform N-WASp activate Arp 2/3, which regulates polymerization of actin from the barbed and branching filaments. WASp is expressed in a hematopoietic-specific fashion. A spectrum of clinical disease is seen that correlates with mutations in specific domains of the WASp protein. ‘Classic’ WAS patients express no WASp and have severely defective immune function that is characterized by aberrant polarization and defective directed migration of hematopoietic cells. WASp-deficient mice have been generated and also show significant hematopoietic defects. Serial stem cell transplantation and competitive repopulation studies in mice have confirmed a selective homing and engraftment advantage for normal compared with WASp-deficient HSC/Ps, and hematopoiesis established by means of engraftment of chimeric fetal liver populations results in dominance of normal HSC/Ps over WASp-deficient hematopoiesis [31].
Rho GTPases in leukemia: Rho GTPases in chronic myelogenous leukemia
Chronic myelogenous leukemia (CML) is a hematological malignancy that is characterized by an uncontrolled expansion of immature myeloid cells and their premature release into the circulation. CML is caused by the expression of the fusion oncoprotein p210-BCR-ABL, a constitutively active tyrosine kinase that regulates a variety of signaling cascades, including Ras, ERK, Akt, JNK, p38, CrkL, STAT5, and nuclear factor kappa B [32]. Expression of p210-BCR-ABL confers a proliferative advantage to cells and induces abnormal adhesion and migration of hematopoietic progenitor cells [33,34]. These effects can be suppressed by the tyrosine kinase inhibitor, imatinib mesylate. Imatinib is a highly effective drug in CML treatment, but tyrosine kinase inhibition resistance due to mutations in p210-BCR-ABL and other causes of HSC resistance to drug treatment have increased the interest in better defining signaling pathways activated by p210-BCR-ABL [35].
p210-BCR-ABL contains additional functional domains of interest. In particular, a Dbl homology domain with GEF that can activate Rho GTPases and a Srchomology3 domain, which can recruit other proteins with GEF activity as well as Rac protein, have been shown to activate a variety of signaling molecules that coincide with known downstream targets of p210-BCR-ABL [36,37]. In this regard, hyperactivation of Rac1 and Rac2 and, to a lesser extent, Rac3 in HSC/Ps isolated from chronic phase CML patients has recently been demonstrated [38]. In an experimental model of CML in mice, Rac GTPases were also shown to be hyperactivated in primary HSC/Ps expressing p210-BCR-ABL after retrovirus-mediated gene transfer [38]. Although Rac1 deficiency did not modify the median survival of p210-BCR-ABL-expressing mice, the median survival of p210-BCR-ABL-expressing Rac2-deficient mice was significantly increased (from 21 to 43 days), and the median survival of p210-BCR-ABL-expressing Rac1/Rac2-deficient mice was even more strikingly increased (to 92 days). Expression of p210-BCR-ABL in Rac1/Rac2-deficient HSC/Ps also led to an altered disease phenotype, with mice showing oligoclonal leukemias of myeloid, lymphoid or bi-lineage immunophenotypes. In this murine model, ERK, JNK, p38, Akt, STAT5 and CrkL signaling were all attenuated in splenocytes harvested from p210-BCR-ABL-expressing Rac2-deficient leukemic recipients and almost completely abrogated in the Rac1/Rac2-deficient cells. These alterations in signaling correlated with the overall survival that was observed in animals from each of these genotypes. The decreased activation of downstream pathways was not due to decreased ABL tyrosine kinase activity, as autophosphorylation of p210-BCR-ABL was still noted in these cells [38]. STAT5 phosphorylation was also detectable in leukemic cells regardless of the presence or absence of Rac1 and Rac2 GTPase activity. Although activation of CrkL, which has been suggested to be an effector that binds directly to p210-BCR-ABL [39], was decreased in Rac2-deficient and practically abrogated in Rac1/Rac2-deficient leukemias, suggesting that CrkL activation is dependent on a other proteins as well. These data suggested that Rac1 and Rac2 are critical for p210-BCR-ABL transformation and myeloproliferative disease (MPD) development in vivo (Fig. 2).
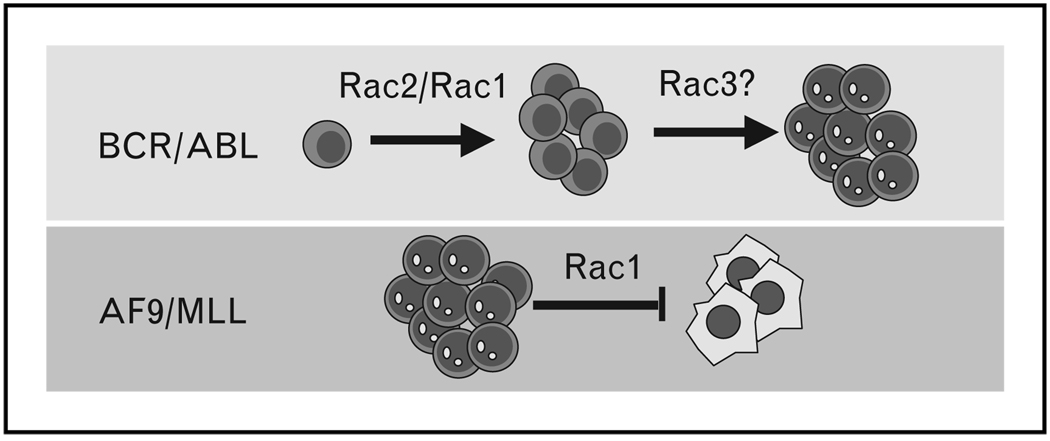
Figure 2. Leukemic hematopoietic stem and progenitor cells proliferation and survival depends on Rac activation.
p210-BCR-ABL leukemic proliferation in MPD depends on Rac activation, especially Rac2. Rac1 and Rac3 also play a role in leukemic proliferation induced by p210-BCR-ABL, and Rac3 may be involved in blastic transformation induced by both p210-BCR-ABL and p190-BCR-ABL. Rac1 prevents apoptosis of AF9/MLL leukemic cells. MLL, mixed-lineage leukemia; MPD, myeloproliferative disease.  , Leukemic blast;
, Leukemic blast;  , myeloproliferative disease;
, myeloproliferative disease;  , apoptotic cell.
, apoptotic cell.
Interestingly, Rac3 appeared hyperactivated in splenocytes derived after long latency in Rac1/Rac2-deficient animals. Rac3 was originally discovered by screening the p210-BCR-ABL-expressing erythroid blastic-phase CML cell line K562 [40]. Rac3 activation has been demonstrated in p190-BCR-ABL-expressing malignant precursor B-lineage lymphoblasts [41], suggesting that Rac3 hyperactivation could play a specific role in cancer development and invasiveness (Fig. 2). These data, along with the observed differences in survival mediated by Rac1 versus Rac2-deficient HSCs, support the hypothesis that individual Rac GTPases play unique roles in the development of p210-BCR-ABL-mediated disease.
On the basis of these genetic data, the effect of a Rac inhibitor NSC23766 on p210-BCR-ABL-induced transformation was examined. NSC23766 is a first-generation, Rac-specific small molecule inhibitor [42] that was developed based on the GEF–Rac1 GTPase complex and computer-assisted virtual screening. NSC23766 was found to fit into a shallow surface groove of Rac1 that has been shown to be critical for GEF specification. NSC23766 was shown to effectively inhibit Rac protein binding and activation by the Rac-specific GEFs TrioN or Tiam1 in a dose-dependent manner. In contrast, NSC23766 did not interfere with the binding or activation of Cdc42 or RhoA by their respective GEFs. NSC23766 induced decreased Rac-dependent PAK activation and mobilization of normal HSCs in mouse studies [3]. In either the murine model of p210-BCR-ABL-induced MPD (described above) or in a human xenogeneic transplantation assay, NSC23766 inhibited Rac GTPase activity and impaired leukemogenesis [38]. These studies define Rac as an attractive molecular target in p210-BCR-ABL transformed HSCs.
Rho GTPases in acute leukemia with rearrangements of the gene mixed-lineage leukemia
The mixed-lineage leukemia (MLL) gene on chromosome 11q23 is rearranged in both acute myelogenous leukemia (AML) and ALL and constitutes a group of leukemias associated to poor prognosis. These MLL gene rearrangements consist of reciprocal translocations that fuse the amino terminus of MLL to a diverse group of partner genes [43,44]. In a fashion similar to p210-BCR-ABL CML, the leukemia-initiating cell is a HSC or progenitor with HSC-like characteristics [45] and MLL rearrangements can be found in up to 7–10% of acute leukemias. In AML, the most common partner gene for MLL is AF9 on chromosome 9p22. Krivtsov et al. [45] showed that leukemia stem cells in the context of AF9/MLL leukemias maintain their progenitor identity from which they arise and activate a self-renewal-associated program. More recently, Somervaille and Cleary [46] demonstrated that AML AF9/MLL stem cell-like cells show increased chemotactic response to chemokine (CXC motif) ligand 12 and increased Rac1 and Cdc42 activity, suggesting that these signaling pathways can be targeted [46]. In a xenograft model of MLL–AF9 leukemia, Wei et al. [47••] targeted the Rac1 signaling pathway pharmacologically or by gene silencing, which resulted in rapid apoptosis of MLL–AF9-expressing cells (Fig. 2). Confirming these data, in a panel of acute myelogenous leukemic cell lines, Muller et al. [48] demonstrated that the MLL gene-rearranged cell lines ML-2 and THP-1 displayed a profound dependence on Rac signaling and treatment with NSC23766 inhibited the growth of these cells in vitro and in an xenograft model in vivo.
Conclusion
Rho GTPases appear to play crucial roles in key HSC functions related to the cytoskeleton, including adhesion, migration, retention in the bone marrow and engraftment. Using gene-targeted mice, which allows more precise studies in primary cells, Rho GTPases appear also to regulate proliferation, survival, transformation and senescence of HSCs. Genetic and pharmacological approaches have been developed to dissect out the unique and overlapping roles of different Rho GTPases in HSC/P populations. As a result, specific molecular mechanisms of action of Rac and Cdc42 have begun to be clarified in these cells. However, the role of specific agonist and cell-specific GEFs in activation and function of Rho GTPases in HSCs has not yet been fully elucidated. In addition, stoichiometric and functional relationships between Rho GTPases and their downstream effectors are yet to be completely understood. This is particularly relevant as the number of GEFs and effectors are far in excess of the number of Rho GTPases and many Rho GTPases show promiscuity with respect to these interactions.
Rho GTPases appear to be key regulators of the localization of HSCs within the hematopoietic microenvironment and the interaction of HSCs with cellular niches. Further research into the roles of Rho GTPases in the microenvironment in relationship with the osteoblastic and vascular HSC niches is needed, and studies of the role of the related GTPase, RhoA, are now underway [49]. Finally, the development of small molecule inhibitors, although difficult due to the affinity of the interactions of GEFs with Rho GTPases, offers new opportunities for therapeutic intervention at the level of HSCs in cell, gene and cancer therapies.
Acknowledgements
We thank Megan Smith for administrative assistance and members of our laboratories for helpful discussions. Supported by Department of Defense (CM-064050, J.A.C), Alex’s Lemonade Stand Foundation (J.A.C.), National Blood Foundation (J.A.C.) and the Leukemia and Lymphoma Society National Institutes of Health (DK062757, CA113969, D.A.W.; HL087159, J.A.C.).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 317).
- 1.Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–337. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 3.Cancelas JA, Lee AW, Prabhakar R, et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 4.Yang FC, Atkinson SJ, Gu Y, et al. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci U S A. 2001;98:5614–5618. doi: 10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen M, Yang FC, Cancelas JA, et al. Rac2-deficient hematopoietic stem cells show defective interaction with the hematopoietic microenvironment and long-term engraftment failure. Stem Cells. 2005;23:335–346. doi: 10.1634/stemcells.2004-0216. [DOI] [PubMed] [Google Scholar]
- 6.Gu Y, Filippi MD, Cancelas JA, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Jia B, Yang FC, et al. Biochemical and biological characterization of a human Rac2 GTPase mutant associated with phagocytic immunodeficiency. J Biol Chem. 2001;276:15929–15938. doi: 10.1074/jbc.M010445200. [DOI] [PubMed] [Google Scholar]
- 8.Yang FC, Kapur R, King AJ, et al. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity. 2000;12:557–568. doi: 10.1016/s1074-7613(00)80207-1. [DOI] [PubMed] [Google Scholar]
- 9.Filippi MD, Harris CE, Meller J, et al. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol. 2004;5:744–751. doi: 10.1038/ni1081. [DOI] [PubMed] [Google Scholar]
- 10. Ghiaur G, Ferkowicz MJ, Milsom MD, et al. Rac1 is essential for intra-embryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells. Blood. 2008;111:3313–3321. doi: 10.1182/blood-2007-08-110114. This study demonstrates the role of Rac1 in fetal HSC/Ps trafficking. Rac1 deficiency induces absence of fetal liver and AGM, whereas earlier stages of yolk sac definitive hematopoiesis remain normal. These data support the hypothesis that definitive hematopoiesis initiates in the yolk sac and migrates to AGM and fetal liver.
- 11.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 12.Palis J, Chan RJ, Koniski A, et al. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc Natl Acad Sci U S A. 2001;98:4528–4533. doi: 10.1073/pnas.071002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carstanjen D, Yamauchi A, Koornneef A, et al. Rac2 regulates neutrophil chemotaxis, superoxide production, and myeloid colony formation through multiple distinct effector pathways. J Immunol. 2005;174:4613–4620. doi: 10.4049/jimmunol.174.8.4613. [DOI] [PubMed] [Google Scholar]
- 14.Roberts AW, Kim C, Zhen L, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 15.Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 17.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 18. Wu X, Tu X, Joeng KS, et al. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. This study demonstrates that Rac1 activation controls Wnt/β-catenin signaling through the canonical signaling pathway. Rac1 forms a multiprotein complex with JNK2 kinase and β-catenin. In this complex, Rac1 activates JNK2 kinase leading to β-catenin activation and nuclear localization. This mechanism, although untested in HSCs, may represent a major pathway by which Rac1 controls HSCs proliferation with the context of the hematopoietic microenvironment.
- 19.Kawashima T, Kitamura T. Rac and nuclear translocation of signal transducers and activators of transcription factors. Methods Enzymol. 2008;439:171–180. doi: 10.1016/S0076-6879(07)00413-2. [DOI] [PubMed] [Google Scholar]
- 20.Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 21.Symons M, Derry JM, Karlak B, et al. Wiskott–Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Hannigan M, Mo Z, et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Yang L, Filippi MD, et al. Genetic deletion of Cdc42GAP reveals a role of Cdc42 in erythropoiesis and hematopoietic stem/progenitor cell survival, adhesion, and engraftment. Blood. 2006;107:98–105. doi: 10.1182/blood-2005-05-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczur K, Xu H, Atkinson S, et al. Rho GTPase CDC42 regulates directionality and random movement via distinct MAPK pathways in neutrophils. Blood. 2006;108:4205–4213. doi: 10.1182/blood-2006-03-013789. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Wang L, Geiger H, et al. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc Natl Acad Sci U S A. 2007;104:5091–5096. doi: 10.1073/pnas.0610819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Wang L, Kalfa TA, et al. Cdc42 critically regulates the balance between myelopoiesis and erythropoiesis. Blood. 2007;110:3853–3861. doi: 10.1182/blood-2007-03-079582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Z, Ryan MA, Daria D, et al. Increased hematopoietic stem cell mobilization in aged mice. Blood. 2006;108:2190–2197. doi: 10.1182/blood-2005-12-010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdul-Manan N, Aghazadeh B, Liu GA, et al. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott–Aldrich syndrome’ protein. Nature. 1999;399:379–383. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- 29.Miki H, Takenawa T. Direct binding of the verprolin-homology domain in N-WASP to actin is essential for cytoskeletal reorganization. Biochem Biophys Res Commun. 1998;243:73–78. doi: 10.1006/bbrc.1997.8064. [DOI] [PubMed] [Google Scholar]
- 30.Rohatgi R, Ma L, Miki H, et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 31.Lacout C, Haddad E, Sabri S, et al. A defect in hematopoietic stem cell migration explains the nonrandom X-chromosome inactivation in carriers of Wiskott–Aldrich syndrome. Blood. 2003;102:1282–1289. doi: 10.1182/blood-2002-07-2099. [DOI] [PubMed] [Google Scholar]
- 32.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev. 2005;5:172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 33.Ramaraj P, Singh H, Niu N, et al. Effect of mutational inactivation of tyrosine kinase activity on BCR/ABL-induced abnormalities in cell growth and adhesion in human hematopoietic progenitors. Cancer Res. 2004;64:5322–5331. doi: 10.1158/0008-5472.CAN-03-3656. [DOI] [PubMed] [Google Scholar]
- 34.Zhao RC, Jiang Y, Verfaillie CM. A model of human p210(bcr/ABL)-mediated chronic myelogenous leukemia by transduction of primary normal human CD34(+) cells with a BCR/ABL-containing retroviral vector. Blood. 2001;97:2406–2412. doi: 10.1182/blood.v97.8.2406. [DOI] [PubMed] [Google Scholar]
- 35.Deininger M. Resistance and relapse with imatinib in CML: causes and consequences. J Natl Compr Canc Netw. 2008;6(Suppl 2):S11–S21. [PubMed] [Google Scholar]
- 36.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz M. Rho signalling at a glance. J Cell Sci. 2004;117:5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 38.Thomas EK, Cancelas JA, Chae HD, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007;12:467–478. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Oda T, Heaney C, Hagopian JR, et al. Crkl is the major tyrosine-phosphorylated protein in neutrophils from patients with chronic myelogenous leukemia. J Biol Chem. 1994;269:22925–22928. [PubMed] [Google Scholar]
- 40.Haataja L, Groffen J, Heisterkamp N. Characterization of RAC3, a novel member of the Rho family. J Biol Chem. 1997;272:20384–20388. doi: 10.1074/jbc.272.33.20384. [DOI] [PubMed] [Google Scholar]
- 41.Cho YJ, Zhang B, Kaartinen V, et al. Generation of rac3 null mutant mice: role of Rac3 in Bcr/Abl-caused lymphoblastic leukemia. Mol Cell Biol. 2005;25:5777–5785. doi: 10.1128/MCB.25.13.5777-5785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y, Dickerson JB, Guo F, et al. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Braekeleer M, Morel F, Le Bris MJ, et al. The MLL gene and translocations involving chromosomal band 11q23 in acute leukemia. Anticancer Res. 2005;25:1931–1944. [PubMed] [Google Scholar]
- 44.Dimartino JF, Cleary ML. MLL rearrangements in haematological malignancies: lessons from clinical and biological studies. Br J Haematol. 1999;106:614–626. doi: 10.1046/j.1365-2141.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 45.Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 46.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 47. Wei J, Wunderlich M, Fox C, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. This is the first study of intervention on Rac activation in the context of AF9/MLL leukemia. Rac1 expression interference induces apoptosis of microenvironment-dependent leukemic cells.
- 48.Muller LU, Schore RJ, Zheng Y, et al. Rac guanosine triphosphatases represent a potential target in AML. Leukemia. 2008;22:1803–1806. doi: 10.1038/leu.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghiaur G, Lee A, Bailey J, et al. Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment. Blood. 2006;108:2087–2094. doi: 10.1182/blood-2006-02-001560. [DOI] [PubMed] [Google Scholar]