Abstract
Thermobifida fusca o-succinylbenzoate synthase (OSBS), a member of the enolase superfamily that catalyzes a step in menaquinone biosynthesis, shares 22% and 28% amino acid sequence identity with two previously characterized OSBS enzymes from Escherichia coli and Amycolatopsis sp. T-1-60, respectively. These values are considerably lower than typical sequence identities among homologous proteins that have the same function. To determine how such divergent enzymes catalyze the same reaction, we solved the structure of T. fusca OSBS and identified amino acids that are important for ligand binding. We discovered significant differences in structure and conformational flexibility between T. fusca OSBS and other members of the enolase superfamily. In particular, the 20s loop, a flexible loop in the active site that permits ligand binding and release in most enolase superfamily proteins, has a four-amino acid deletion and is well ordered in T. fusca OSBS. Instead, flexibility of a different region allows the substrate to enter from the other side of the active site. T. fusca OSBS was more tolerant of mutations at residues that were critical for activity in E. coli OSBS. Also, replacing active site amino acids found in one protein with the amino acids that occur at the same place in the other protein reduces catalytic efficiency. Thus, the extraordinary divergence between these proteins does not appear to reflect a higher tolerance of mutations. Instead, large deletions outside the active site were accompanied by alteration of active site size and electrostatic interactions, resulting in small but significant differences in ligand binding.
Understanding the natural diversity of enzyme superfamilies is critical for improving functional annotation and protein engineering methods. Toward this end, the enolase superfamily has been extensively studied. Enzymes in the enolase superfamily catalyze over twenty chemical reactions.1, 2 All of these reactions involve the abstraction of a proton from the alpha carbon of a carboxylate and stabilization of the enolate anion intermediate by a divalent cation. The conserved catalytic residues are in an (β/α)7β-barrel, which is connected to an α + β fold domain that forms a cap over the active site. Several recent studies used homology models and computational ligand docking to accurately predict specificity in the dipeptide epimerase family, a family in the enolase superfamily.3–5 Extending this approach to the rest of enolase superfamily requires experimental determination of a representative set of structures in the superfamily to serve as templates for homology models. In addition, characterizing the structural plasticity of proteins in the enolase superfamily offers valuable insight into the designability of the enolase superfamily fold.
Here, we analyze structure-function relationships of Thermobifida fusca o-succinylbenzoate synthase (OSBS), a member of the enolase superfamily. This protein belongs to the large, extremely divergent OSBS family. The biological function of most enzymes in the OSBS family is OSB synthesis, which is a step in menaquinone (Vitamin K) biosynthesis. However, some enzymes in one branch of the family (the NSAR/OSBS subfamily) also catalyze N-succinylamino acid racemization (NSAR; Figure 1).6–8 Members of two subfamilies, E. coli OSBS from the γ-Proteobacteria 1 subfamily and Amycolatopsis NSAR/OSBS from the NSAR/OSBS subfamily, were previously characterized.9–14 T. fusca OSBS belongs to the Actinobacteria OSBS subfamily. Like the γ-Proteobacteria 1 subfamily, genome context indicates that OSBS is the biological function of all members of the Actinobacteria subfamily.6, 7 T. fusca OSBS shares 22% and 28% amino acid sequence identity with E. coli OSBS and Amycolatopsis NSAR/OSBS, respectively. These values are considerably lower than typical sequence identities among homologous proteins whose functions are conserved.15, 16 This could indicate that members of the OSBS family are more tolerant of mutations than typical proteins. In order to understand how such divergent enzymes catalyze the same reaction, we determined the structure of T. fusca OSBS and identified amino acids that are important for ligand binding.
Figure 1.

The OSBS family. A) The o-succinylbenzoate synthase (OSBS) and N-succinylamino acid racemase (NSAR) reactions. The enolate anion intermediate that occurs in all reactions in the enolase superfamily is red; blue atoms are lost or rearranged in the reactions. R = hydrophobic amino acid side chain. B) Phylogenetic tree showing the division of the OSBS family into eight major subfamilies.14 The structure of T. fusca OSBS (PDB: 2OPJ and 2 QVH) from the Actinobacteria subfamily is reported in this work. It is compared to E. coli OSBS (PDB: 1FHV, 1FHU, and 1R6W) from the γ-Proteobacteria 1 subfamily and Amycolatopsis NSAR/OSBS (PDB: 1SJB) from the NSAR/OSBS subfamily.
Our experiments identified significant differences in structure and conformational flexibility between T. fusca OSBS and other members of the enolase superfamily. In particular, the 20s loop, which is a flexible loop from the capping domain that permits ligand binding and release in most enolase superfamily proteins, has a four-amino acid deletion and is well ordered in T. fusca OSBS. Instead, conformational flexibility of a different region allows the substrate to enter from the other side of the active site. Our results also show that T. fusca OSBS was more tolerant of mutations at residues that were critical for activity in E. coli OSBS, even though their molecular functions are conserved.14 Replacing active site amino acids found in one of the proteins with the amino acids that occur at the same place in the other protein reduces catalytic efficiency. Thus, the extraordinary divergence between these proteins does not appear to reflect a heightened ability to tolerate mutations. Instead, the rate of divergence in the OSBS family appears to be dominated by large deletions in the capping domain and loss of quaternary structure (Odokonyero, et al., in preparation). These structural changes occurred along with alteration of active site size and electrostatic interactions, resulting in small but significant differences in ligand binding.
MATERIALS AND METHODS
Protein Production and Crystallization
The target gene for Tfu_1410 from Thermobifida fusca (encoding amino acids 2-317) was obtained via synthesis (Codon Devices), amplified via PCR, cloned into a C-terminal His6-tag expression vector, tested for expression and solubility, fermented at large scale, purified and crystallized using the published methods employed by the NYSGXRC.17 Detailed protocols for effort on this target are publicly available from the NIGMS PSI repository of publicly available protocols, TargetTrack (http://sbkb.org/tt/index.html), by searching on “Project Target ID” NYSGXRC-9312b; the expression clone is available from the PSI Materials Repository (http://dnasu.asu.edu/DNASU/GetCloneDetail.do?cloneid=336395).
Se-Met labeled material was generated by a 1L fermentation of HY medium (Medicilon, Inc.) yielding 43 mg of purified protein. The purity and Se incorporation were confirmed by SDS-PAGE analysis of chromatographic fractions and mass spectrometry (ESI and MALDI) of the final pool. This sample was used for structure determination of the apo and liganded forms of this protein.
Diffraction quality single crystals of the apo form were obtained by mixing 1 μL of protein at 20 mg/mL with 1 μL of 100 mM Bis-Tris pH 5.5, 25% PEG 3350 and 200 mM ammonium acetate, and equilibrating by vapor diffusion against 100 μL of the same precipitant at room temperature. Crystals of the complex were obtained with a similar procedure using a well solution of 0.1 M Bis-Tris pH 6.5, 25% PEG 3350, 0.2 M ammonium acetate, 10 mM magnesium chloride and 10 mM o-succinylbenzoate. The resulting crystals were cryoprotected and flash frozen in liquid nitrogen.
Data Collection and Structure Determination – OSBS apo structure
Single wavelength anomalous diffraction data from apo crystals, consistent with space group P1 and extending to 1.6 Å resolution, were collected in the vicinity of the selenium anomalous peak wavelength (λ=0.9795 Å) at Brookhaven National Laboratory National Synchrotron Light Source X12C beamline. Matthews coefficient calculations were consistent with the presence of one molecule in the asymmetric unit (ASU).
Two selenium sites were located using the SHELXD program18 and density modified SAD phases were calculated with SHARP19 and SOLOMON.20 Following several rounds of automated and manual model building using CCP421 and Coot22, respectively, refinement using CNS23 converged at Rwork= 20.2% and Rfree= 22.6%.
The final model consists of one chain of Thermobifida fusca Tfu_1410 (2,215 protein atoms; Thr2 to Gln310), 201 waters, and the N-terminal SL cloning artifact. Several segments (Arg101 to Ala106, Asp177 to Leu187, Val311 to Pro317, and the C-terminal cloning artifact EGHHHHHH) were not observed in the electron density presumably due to disorder, consistent with modest flexibility. The coordinates and structure factors have been deposited in the Protein Data Bank as entry 2OPJ.
Data Collection and Structure Determination – OSBS:product structure
Diffraction data from complex crystals, consistent with space group P1 (albeit with a different cell and ASU from the apo, see Table 1) and extending to 1.76 Å resolution, were collected in the vicinity of the selenium anomalous peak wavelength (λ=0.9795 Å) at Brookhaven National Laboratory National Synchrotron Light Source X29A beamline. Matthews coefficient calculations were consistent with the presence of two molecules in the asymmetric unit (ASU).
Table 1. Crystallographic data collection and refinement statistics.
Data collection and refinement statistics for 2OPJ and 2QVH. Values in parentheses are for the highest resolution shell.
| Data Collection | Apo | Product |
|---|---|---|
| PDB Code | 2OPJ | 2QVH |
| Space group | P1 | P1 |
| a = 36.976 | a = 44.543 | |
| b = 37.642 | b = 52.877 | |
| c = 51.990 | c = 61.449 | |
| Cell dimension (Å) | ||
| α =71.28 | α = 70.53 | |
| β =76.13 | β = 87.22 | |
| γ =73.23 | γ = 85.56 | |
| Molecules/ASU | 1 | 2 |
| Wavelength (Å) | 0.9795 | 0.9795 |
| Resolution (Å) | 50 – 1.60 (1.66 – 1.60) | 50.0 – 1.76 (1.82 – 1.76) |
| Unique reflections | 29,912 | 48,053 |
| Completeness (%) | 90.5 (52.7) | 92.0 (95.2) |
| Rsym (%) | 0.037 (0.108) | 0.042 (0.092) |
| <I/II> | 11.3 (2.0) | 23.2 (2.0) |
| Redundancy | 3.9 (3.6) | 3.8 (3.7) |
|
Refinement
| ||
| R/Rfree | 20.2 / 22.6 | 21.8 / 25.1 |
| No. of Atoms | ||
| Protein/Solvent/Ligand | 2,215 / 201 / N/A | 4,698 / 286 / 34 |
| Average B Factor (Å2) | ||
| Protein/Solvent/Ligand | 12.8 / 22.9 / N/A | 19.1 / 24.1 / 14.6 |
| Ramachandran Statistics (%) | ||
| Favored/Allowed/Outlier | 94.0 / 6.0 / 0 | 92.2 / 7.9 / 0 |
| Rms deviation from ideal | ||
| Bonds (Å) | 0.005 | 0.005 |
| Angles (°) | 1.30 | 1.20 |
Two copies of the protein were placed via molecular replacement using MOLREP.24 The model was rebuilt using CCP421 and Coot22. Coordinated Mg2+ ions and o-succinylbenzoate molecules were placed unambiguously prior to the final stages of refinement based on clear features in the difference Fourier synthesis electron density maps. Refinement using CNS23 converged at Rwork= 21.8% and Rfree= 25.1%.
The final model consists of two chains of Thermobifida fusca Tfu_1410 (4,698 protein atoms; chains A and B, Thr2 to Gln310), 2 magnesium ions, 2 o-succinylbenzoate molecules, 286 waters, and both N-terminal SL cloning artifacts. The C-terminal segments (Val311 to Pro317, and the C-terminal cloning artifact EGHHHHHH) were not observed in the electron density presumably due to disorder, consistent with modest flexibility. The coordinates and structure factors have been deposited in the Protein Data Bank as entry 2QVH.
Structure Alignments
The structures of T. fusca OSBS were aligned to E. coli OSBS and Amycolatopsis NSAR/OSBS using the structure matching and alignment tools in UCSF Chimera (Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, supported by NIGMS P41-GM103311).25 The alignment was manually refined based on visual inspection of the structural alignment.
Mutagenesis
Site-directed mutagenesis was performed by the QuikChange Mutagenesis protocol using a 2-stage PCR reaction (see Supporting Information for primers and PCR conditions).26 The templates for mutagenesis were the menC gene from E. coli (gi number 16130196) subcloned into a modified pET15b vector (Novagen) with a 10-histidine N-terminal tag and T. fusca OSBS, which was subcloned into pET15b. Mutations were confirmed by sequencing in both directions (Eton Bioscience, Inc.).
Protein purification for enzymatic assays
All proteins were expressed in the menC− strain BW25113 (menC::kan, DE3) to ensure that the purified proteins would not be contaminated with wild type OSBS.14 Cultures were grown overnight at 37 °C without induction in Luria-Bertani broth supplemented with carbenicillin and kanamycin. Cells were harvested by centrifugation and resuspended in buffer containing 20 mM Tris, pH 8.0, 500 mM NaCl, 5 mM imidazole, 0.02 mg/ml DNAse (Worthington), and 2 μM phenylmethylsulfonyl fluoride (PMSF; Thermo Scientific). Resuspended pellets were lysed by sonication. After centrifugation, the filtered lysate was applied to a 5 ml HisTrap FF column charged with Ni2+ (GE Healthcare). The protein was eluted with a buffer containing 20 mM Tris, pH. 8.0, 500 mM NaCl and 500 mM imidazole using a step to 15% elution buffer followed by a linear gradient to 100% elution buffer. Fractions containing apparently homogenous protein were identified by SDS-PAGE and pooled. Amicon Ultra-15 centrifugal filters (10 kD cutoff) (Millipore) were used to exchange the buffer and concentrate the pooled fractions. Purified proteins were stored in 10 mM Tris, pH. 8.0 and 5 mM MgCl2 supplemented with 25% glycerol for storage at −80 °C.
OSBS activity assay
Wild type and mutant OSBS enzymes were assayed with varying concentrations of 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate (SHCHC) in 50 mM Tris, pH 8.0, 0.1 mM MnCl2 at 25°C. SHCHC was synthesized as described previously.9, 14 The assays were performed by quantifying the decrease in absorbance at 310 nm (ΔΔ= −2400 M−1 cm−1), as previously described.9, 10 Initial rates were calculated using VisionPro (Thermo Scientific) and fit to the Michaelis-Menten equation using Kaleidagraph (Synergy Software).
RESULTS
Structure of T. fusca OSBS
The structure of T. fusca OSBS exhibits some unexpected differences from other members of the enolase superfamily. Like E. coli OSBS, it is monomeric, which is atypical in the enolase superfamily (Odokonyero et al., in preparation). T. fusca and E. coli OSBSs are ~40 amino acids shorter than average enolase superfamily members, at 319 and 320 amino acids, respectively, although deletions occur at different sites (Figure 2A; Odokonyero et al., in preparation). Surprisingly, T. fusca OSBS has a 4-amino acid deletion in the 20s loop, which is around position 20 in the capping domain. In other members of the enolase superfamily, the 20s loop helps close the active site when substrate or product is bound.11, 27–30 In Amycolatopsis NSAR/OSBS, the 20s loop completely closes the active site (Figure 2B).13 However, the active site is partially open in both T. fusca and E. coli OSBSs.11 In T. fusca OSBS, the 20s loop closes one side of the active site by contacting the barrel domain, but there is an opening on the other side of the active site due to the deletion. In contrast, the active site in E. coli OSBS is a deep, open crevice because the 20s loop does not contact the barrel domain.11
Figure 2.
Comparison of OSBS enzymes from Thermobifida fusca (2QVH), Escherichia coli (1FHV), and Amycolatopsis sp. T-1-60 (1SJB).11, 13 A) Insertions and deletions. Regions in red are deleted in one of the other enzymes. Regions in blue are insertions in that enzyme. Regions in orange have insertions and deletions in all three enzymes and cannot be aligned to each other. OSB is shown in black, and the magnesium ion is shown in lime green. The 20s loops and 50s loops are marked with a star and a circle, respectively. The three α-helices of the capping domain are labeled. B) Active site accessibility. Space-filling models have been rotated 90° toward the right (top) or left (bottom) relative to panel A. The 20s loop is cyan, the 50s loop is pink, the rest of the capping domain is light gray, and the barrel domain is green. Another subunit of the Amycolatopsis NSAR/OSBS octamer is shown in dark grey to illustrate subunit interactions. OSB is shown in black.
The other unexpected finding was that the first two helices of the capping domain, which are found in almost all other members of the enolase superfamily, have been deleted in T. fusca OSBS. The deletion begins after the second active site loop (the 50s loop), where the chain takes a short-cut to α-helix 3 of the capping domain. This deletion is conserved in all members of the Actinobacteria subfamily. The second α-helix of the capping domain is also truncated or deleted in E. coli and many other OSBSs (Odokonyero et al., in preparation). These helices are not in the active site, but they are at the interface between subunits in the octomeric Amycolatopsis NSAR/OSBS and most other multimeric enolase superfamily members.13, 30–36 T. fusca OSBS appears to compensate for the loss of these helices with an extra helix appended to the C-terminus that crosses over the top of the capping domain. This helix is not present in any other enolase superfamily members.
Conformation changes between apo- and product-bound forms
In most structurally characterized enolase superfamily proteins, including E. coli OSBS, the 20s loop is disordered in the absence of substrate or product.11, 27–30, 37 Although the 20s loop helps determine specificity by making hydrogen bonds with the substrate in some enolase superfamily members5, 30, substitutions and deletions in the 20s loop of E. coli OSBS and Pseudomonas putida mandelate racemase suggest that it serves primarily as a “flexible flap” that partially closes the active site to limit substrate dissociation.14, 38
In contrast, the 20s loop of T. fusca OSBS is ordered in both the presence and absence of bound OSB. Instead, two other regions are disordered in the apo structure. The first is the loop after β-strand 2 in the barrel domain (post-β2), which has a 4-amino acid insertion (Figure 3). This insertion is only present in the Actinobacteria OSBS subfamily. When product is bound, some atoms of post-β2 are within 5 Å of the 20s loop, but there are no hydrogen bonds between the highly polar post-β2 loop (sequence ERGQSEA) and the 20s loop. Post-β2 is also >13 Å from the deeply buried substrate. While it might help restrict entrance or exit of the ligand from its position at the mouth of the active site, its distance from the 20s loop and OSB suggest that post-β2 becomes ordered due to greater overall protein stability when ligand is bound, as observed in other proteins.39, 40
Figure 3.

Comparison of conformation changes upon ligand binding. Apo- and ligand-bound structures were aligned by superimposing the conserved catalytic motifs in the barrel domain. The apo-structure is in dark grey, and the ligand-bound structure is light grey. Regions that become ordered upon ligand binding are red, and residues whose alpha-carbons shift by >2 Å are in yellow. A) T. fusca OSBS in the presence and absence of OSB (2QVH and 2OPJ, respectively). B) E. coli OSBS in the absence (1FHU) and presence of OSB (1FHV) or SHCHC (1R6W).11, 12
The second region which becomes ordered when OSB is bound is a loop following β-strand 5 (post-β5) in the barrel domain. One of the metal-binding residues is in this loop. No metal ion is bound to the apoprotein because magnesium chloride was not present in the crystallization conditions. However, flexibility of this loop is probably not a crystallization artifact, because in the structure bound to OSB and Mg2+, B-factors of atoms in residues 184-190 of this loop are in the top 10%. Ordering this loop causes a small conformation change that propagates through the capping domain. When product is bound, post-β5 collides with the 50s loop. Because the 50s loop contacts the 20s loop and the C-terminal helix that crosses over the top of the capping domain, they also move ≥2 Å toward the left in Figure 3A. Consequently, ordering the post-β5 loop does not completely close the entrance to the active site. Nevertheless, partial occlusion of the active site by post-β5 only when ligand is bound suggests that post-β5, instead of the 20s loop, provides an entry portal for substrate binding. Thus, ordering this loop assists catalysis by forming the metal-binding site and sterically limiting substrate dissociation.
In addition to these large-scale movements, the side chain of R17, a residue in the 20s loop, moves 5.1 Å between the apo- and product-bound forms of T. fusca OSBS. R17 moves from the center of the active site where it interacts with one of the Mg2+-binding residues in the apo-structure, to a cation-π interaction with F39 in the 50s loop of the capping domain when OSB binds. From this position, it can also form a salt bridge with E41 and a hydrogen bond with Y42 (Figure 4A). R17, F39, E41, and Y42 are conserved in nearly all members of the Actinobacteria subfamily.14 The δ-carbon of R17, F39, A229 and V230 form a hydrophobic pocket for the cyclohexyl ring of the substrate (Figure 4B).
Figure 4.

Conformation changes in the active site. A) In T. fusca OSBS, R17 forms a salt bridge with one of the metal-binding residues in the absence of OSB and Mg2+ (dark grey; 2OPJ). When OSB and Mg2+ are bound, R17 interacts with three residues from the fifties loop instead (light grey; 2QVH). B) In T. fusca OSBS bound to OSB (2QVH; grey), R17, F39, V230, and A229 form a pocket for binding the top of OSB (black). The structure is rotated ~90° toward the left relative to panel A. In E. coli OSBS bound to OSB (1FHV), the 20s and 50s loops interact via R20 and F51. The Mg2+ ion is shown as a lime sphere. C) In E. coli OSBS, L109 and S262 form a slot where the succinyl tail of the substrate and product bind. The Apo-structure (1FHU) is dark grey, the product-bound structure is light blue (1FHV), and the substrate-bound structure is light grey (1R6W).11, 12 OSB and SHCHC are black.
We compared apo- and ligand-bound structures of T. fusca OSBS to E. coli OSBS, which has also been solved in the apo- and product-bound forms.11 In addition, a substrate-bound enzyme was solved by mutating the catalytic lysine (K133) to arginine.12 Two regions of E. coli OSBS undergo disorder-order transitions upon ligand binding, the 20s loop and the first α-helix of the barrel. When substrate or product is bound, the 20s loop and connected β-strands in the capping domain rotate backwards in Figure 3B. The ordered 20s loop collides with the 50s loop, rotating it and the following helices backwards and to the right, in the opposite direction of the conformation change in T. fusca OSBS.
In E. coli OSBS, structural transitions of the first α-helix of the barrel are associated with β-strand 1 of the barrel moving slightly closer to β-strand 7 on the opposite side of the barrel (Figure 4C). Two residues on these strands, L109 and S262, form a slot where the succinyl tail of the substrate and product bind. The distance is widest in the product bound structure, with 9.8 Å between the δ-carbon of L109 and the hydroxyl of S262. This distance shrinks to 8.5 Å when substrate binds. Although mutating S262 to glycine had little effect, mutating L109 to alanine reduced efficiency 24-fold.14 Together, the structural and mutational data suggest that small movements of β-strand 1 could be important for substrate binding and product release.
In summary, the conformation changes between apo- and ligand-bound structures of T. fusca OSBS and E. coli OSBS are different. E. coli OSBS is more similar to other members of the enolase superfamily, in that flexibility of the 20s loop allows ligand binding and release.11, 27–30, 37 This is accompanied by flexibility of the first α-helix and β-strand of the barrel. In contrast, both the 20s loop and first β-strand of the barrel are well-structured in the apo- and product-bound forms of T. fusca OSBS. Instead, a deletion in the 20s loop and a disordered region in the post-β5 loop suggest that substrate binding and product release occur on the opposite side of the active site. This is especially noteworthy because this side of the active site is at the interface between subunits in Amycolatopsis NSAR/OSBS and most other multimeric enolase superfamily members.13, 30–36
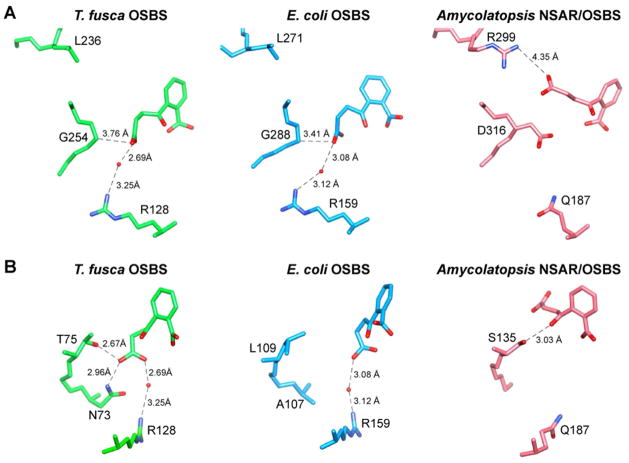
Comparison of ligand binding
The product OSB binds E. coli OSBS and Amycolatopsis NSAR/OSBS in different conformations (Figure 5).11, 13 In E. coli OSBS, mutating either G288 or R159 interferes with substrate binding in the correct conformation, decreasing catalytic efficiency >200-fold.14 Except for the NSAR/OSBS subfamily, glycine and arginine are conserved at these positions in most other OSBSs, suggesting that most of the OSBS family binds OSB in the same conformation as E. coli OSBS. Supporting this idea, mutating this glycine (G295A) in Thermosynechococcus elongatus OSBS, a member of the Cyanobacteria 1 subfamily, reduces the enzyme’s efficiency ~100-fold.
Figure 5.
Comparison of ligand contacts in the active site. A) Position of the ligand relative to R128, L236, and G254 of T. fusca OSBS and related enzymes.11, 12 B) Interaction between the ligand and β-strand 1 of the barrel domain. The images are rotated ~90° toward the left relative to panel A, so that L236 and G254 would be behind the ligand.
As predicted, T. fusca OSBS binds OSB in the same conformation as E. coli OSBS (Figure 5A). However, mutating the conserved glycine (G254A) or arginine (R128M) in T. fusca OSBS only reduces its catalytic efficiency ~3-fold (Table 2). Replacing G254 with threonine had a larger effect, reducing catalytic efficiency 34-fold. In both enzymes, replacing this glycine with larger amino acids should create a steric conflict with the substrate. According to the CASTp database, the active site volume of T. fusca OSBS is larger than E. coli’s (1441 Å3 vs 1223 Å3, respectively), which might permit the substrate to changes its position in the G254A or G254T mutants.41 However, differences in the calculated active site volumes could be due to differences in determining the boundaries of the binding pocket in these relatively open active sites. Alternatively, T. fusca OSBS could be more tolerant of these mutations due to compensation by other protein-ligand interactions (such as the first β-strand in the barrel, see below), mechanistic differences between the enzymes, or higher stability.
Table 2.
Effect of mutations on OSBS activity. Mutations in the same row are located at the same positions in the structures of the two proteins.
| Variant | kcat (s−1) | KM (μM) | kcat/KM (M−1 s−1) | Relative kcat/KM | Variant | kcat (s−1) | KM (μM) | kcat/KM (M−1 s−1) | Relative kcat/KM |
|---|---|---|---|---|---|---|---|---|---|
| T. fusca OSBS | E. coli OSBS | ||||||||
| WT | 122 ± 4.6 | 182 ± 22 | 6.7 × 105 | 1.0 | WT a | 24 ± 0.8 | 12 ± 1.8 | 2.0 × 106 | 1.0 |
| 20s del b | 0.1 ± 0.005 | 54 ± 8 | 1.9 × 103 | 0.003 | 20s del a, c | 0.2 ± 0.003 | 29 ± 1.3 | 6.9 × 103 | 0.0034 |
| F16A | 4.4 ± 1 | 36 ± 1 | 1.2 × 105 | 0.18 | L19A a | 3.9 ± 0.1 | 13 ± 2.4 | 3.0 × 105 | 0.15 |
| R17A | 0.6 ± 0.02 | 7 ± 1.4 | 8.6 × 104 | 0.13 | R20S | 0.5 ± 0.1 | 22 ± 0.1 | 2.3 × 104 | 0.01 |
| F39A | 163 ± 19 | 666 ± 151 | 2.5 × 105 | 0.37 | L48A a | 14 ± 0.8 | 111 ± 17 | 1.3 × 105 | 0.063 |
| Y42A | 210 ± 13 | 588 ± 74 | 3.6 × 105 | 0.5 | F51A a | 49 ± 2.6 | 122 ± 21 | 4.0 × 105 | 0.20 |
| R17A/F39A | 0.2 ± 0.01 | 48 ± 6 | 4.2 × 103 | 0.006 | |||||
| R17A/Y42A | 0.36 ± 0.01 | 38 ± 4.2 | 9.5 × 103 | 0.014 | R20S/F51A | 0.616 ± 0.03 | 144 ± 19 | 4.3 × 103 | 0.002 |
| N73A | 10 ± 0.7 | 322 ± 56 | 3.1 × 104 | 0.05 | A107N | 32 ± 1.9 | 109 ± 17 | 2.9 × 105 | 0.13 |
| T75A | 55 ± 1.5 | 154 ± 15 | 3.6 × 105 | 0.53 | L109A a | 2.9 ± 0.2 | 36 ± 8 | 8.0 × 104 | 0.042 |
| T75L | 18 ± 0.5 | 57 ± 5 | 3.2 × 105 | 0.47 | L109T | 3.6 ± 0.3 | 83 ± 17 | 4.3 × 104 | 0.022 |
| L109S | 5.8 ± 0.2 | 78 ± 9 | 7.4 × 104 | 0.037 | |||||
| N73A/T75A | n.a. d | n.a. | n.a. | n.a. | |||||
| N73L/T75V | 0.18 ± 0.01 | 300 ± 63 | 6.1 × 102 | 0.0009 | |||||
| N73A/T75L | 5.1 ± 0.4 | 256 ± 51 | 2.0 × 104 | 0.03 | A107N/L109T | 2.4 ± 0.2 | 456 ± 85 | 5.3 × 103 | 0.0026 |
| R128M | 104 ± 4 | 470 ± 42 | 2.2 × 105 | 0.33 | R159M a | 0.9 ± 0.05 | 80 ± 15 | 1.1 × 104 | 0.0056 |
| G254A | 16 ± 0.3 | 86 ± 6 | 1.9 × 105 | 0.28 | G288A a | n.d.e | n.d. | 3.8 × 103 | 0.0019 |
| G254T | 13 ± 0.7 | 668 ± 72 | 1.9 × 104 | 0.03 | |||||
| V230L | 29 ± 1.6 | 145 ± 22 | 2.0 × 105 | 0.30 | I265A a | 6 ± 0.2 | 10 ± 1.5 | 5.8 × 105 | 0.30 |
| T. elongatus OSBS | |||||||||
| WT | 80 ± 4 | 78 ± 17 | 1.0 × 106 | 1.0 | |||||
| G295A f | 4 ± 0.4 | 304 ± 82 | 1.3 × 104 | 0.013 | |||||
ref 14.
The 20s loop in T. fusca OSBS was truncated by replacing residues R15, F16, R17, G18 and I19 with glycine
The 20s loop in E. coli OSBS was truncated by deleting residues V17,V18,L19,R20,D21,R22,R23, and L24.
No activity detected.
Not determined because substrate saturation could not be achieved.
At the same position in the structure as G288 in E. coli OSBS and G254 in T fusca OSBS.
Stronger interactions between the ligand and β-strand 1 in the barrel might help T. fusca OSBS tolerate the G254 and R128 mutations. N73 and T75 form hydrogen bonds with the succinyl carboxylate in T. fusca OSBS, while A107 and L109 are at the corresponding positions in E. coli OSBS (Figure 5B). Mutating T75 to alanine had little effect on catalytic efficiency, but changing N73 to alanine reduced efficiency 20-fold. The double mutation N73A/T75A had no detectable activity. Soluble protein expression of this mutant was severely reduced, suggesting that these mutations decrease protein stability. Consequently, we constructed an N73L/T75V variant which retained the shape of the amino acids and yielded the same amount of soluble protein as the wild type. This mutation reduced efficiency 1000-fold. In contrast, replacing N73 and T75 with the residues found in E. coli OSBS, alanine and leucine, only reduced activity 33-fold. Thus, N73 and T75 together make important interactions with the succinyl carboxylate. Loss of these hydrogen bonds can be partially tolerated if the residues are mutated to amino acids found in a related OSBS.
In E. coli OSBS, mutating L109 to alanine, serine, or threonine reduced efficiency 24–45-fold. Mutating A107, which is >5 Å from the ligand, to asparagine only reduced efficiency 10-fold. The effect of the double mutation A107N/L109T was additive, reducing efficiency 385-fold. Together, mutations in E. coli and T. fusca OSBSs demonstrate that amino acids on β-strand 1 make significant contributions to the reaction, and the amino acids are not interchangeable between different OSBSs.
Given that its truncated 20s loop is an unusual feature of T. fusca OSBS, we also constructed mutations to assess its role in catalysis. Deleting an additional five residues to remove the rest of the loop reduced efficiency 300-fold, identical to the effect of deleting the 20s loop in E. coli OSBS.14 Likewise, mutating individual residues that are closest to the ligand (F16 and R17 in T. fusca OSBS and L19 in E. coli OSBS), reduced efficiency <10-fold. As noted above, R17 interacts with F39 and Y42 in the 50s loop when OSB is bound. Mutating either F39 or Y42 had a small effect, but the double mutants R17A/F39A and R17A/Y42A reduced efficiency 160- and 71-fold, respectively.
Re-examining the structure of E. coli OSBS revealed a similar cation-π interaction between R20 in the 20s loop and F51 in the 50s loop.11 F51 is between the bound OSB and R20, which is at the surface of the protein and does not contact the ligand (Fig. 4B). R20S and F51A mutations reduced efficiency 100-fold and 5-fold, respectively, and an R20S/F51A double mutant reduced efficiency nearly 500-fold.
As mentioned above, residues from the 20s and 50s loops of T. fusca OSBS, along with A229 and V230, form a hydrophobic pocket for binding the top of the cyclohexyl ring of the substrate (Figure 4B). Residues at structurally equivalent positions in E. coli OSBS (L19, L48, F51, S264, and I265) form a similar pocket. In both enzymes, single mutations at these positions tend to have little impact. It is only when we introduced mutations that disrupt interactions between the 20s and 50s loop that enzyme efficiency plummeted. Thus, the 20s and 50s loops interact in similar ways in T. fusca and E. coli OSBSs. This interaction is critical for forming the hydrophobic binding pocket, even though the identity of the hydrophobic amino acids in the pocket appears to be less important.
DISCUSSION
Analysis of structure-function relationships in T. fusca OSBS offers new insight into the structural diversity of the enolase superfamily. The most striking difference between T. fusca OSBS and most other structurally characterized members of the enolase superfamily is the site of substrate entry. In many enolase superfamily members, such as E. coli OSBS and Pseudomonas putida mandelate racemase, the 20s loop is a flexible flap that assists catalysis by closing when ligand is bound.11, 14, 27–30, 37, 38, 42 In contrast, the 20s loop of T. fusca OSBS is ordered in the absence of both the substrate and metal ion, and it is truncated by four amino acids. Instead, the post-β5 loop becomes ordered when substrate binds, which also positions the third side chain that coordinates the metal ion. This side of the active site is blocked by other subunits in many multimeric enolase superfamily proteins.2, 13, 30, 33 T. fusca OSBS, however, is a monomer.
The only other known protein whose substrate enters from the 50s loop side of the active site is yeast enolase (PDB: 1EBG).37 In yeast enolase, the 20s loop is deleted, but this surface of the protein is the subunit interface of the homodimer. Instead, the 50s loop is extended, forming a flexible loop that closes the entrance to the active site.
The 20s loop is also truncated in several other members of the enolase superfamily. Novosphingobium aromaticivorans mannonate dehydratase (PDB:2QJN) and several uncharacterized members of the enolase superfamily (PDB:2GL5, 2QQ6, and 2OO6) compensate for the loss of the 20s loop with insertions after the first and/or second β-strands of the barrel domain.33 As another variation on the theme, the truncated 20s loop of Oceanobacillus iheyensis galactarate dehydratase (PDB:3HPF), is compensated by a C-terminal extension that closes the active site when the ligand is bound.2
We also identified a cation-π interaction between the 20s and 50s loops that is important for forming a hydrophobic binding pocket in both T. fusca and E. coli OSBSs. This interaction is not conserved in the whole OSBS family. Some OSBSs lack aromatic residues in the 50s loop or basic residues in the part of the 20s loop that forms the hydrophobic binding pocket.14 For example, the 20s loop of Amycolatopsis NSAR/OSBS interacts with the 50s loop only through hydrophobic interactions.13 Instead, polar and basic residues in the 20s loop interact with the barrel domain, which would be too far away in T. fusca and E. coli OSBSs.
Finally, structural analysis and mutagenesis of T. fusca OSBS, E. coli OSBS, and Amycolatopsis NSAR/OSBS help to explain how proteins that share < 30% amino acid sequence identity catalyze the same reaction. Sequences in the OSBS family diverged much more rapidly than other families in the enolase superfamily. For example, the average pairwise amino acid sequence identity of OSBSs from 66 species is 26%, while enolase enzymes from the same species average 56% amino acid sequence identity.7 Other work from our laboratory shows that this divergence correlates with a large number of deletions and lack of quaternary structure in the OSBS family (Odokonyero, et al., in preparation). Our results here show how this divergence is manifested in the active site. Ligand conformation differs between the NSAR/OSBS subfamily and other OSBS subfamilies.11, 13 Even though the ligand conformation is the same in T. fusca and E. coli OSBSs, different amino acids are important for binding and orienting the substrate for catalysis. Two amino acids were critical for activity in E. coli OSBS (R159 and G288), but mutation of the homologous residues in T. fusca OSBS (R128 and G254) had a modest effect. This could be due to stronger interactions with β-strand 1 of the barrel via hydrogen bonds in T. fusca OSBS. Alternatively, effects of these mutations might be masked by higher thermostability of T. fusca OSBS, which is from a thermophile.
A potential explanation for the rapid divergence of the OSBS family was that it is more tolerant of mutations than related families, possibly due to the lower energetic cost of aromatic ring formation in the OSBS reaction compared to other reactions initiated by abstracting a proton from the alpha carbon adjacent to a carboxylate.43 If this were true, many of the amino acid substitutions between T. fusca and E. coli OSBSs would be neutral. However, we discovered that mutating residues on β-strand 1 of the barrel in one protein to the amino acids found in the other protein reduced kcat/KM 33-fold in T. fusca OSBS and 385-fold in E. coli OSBS. Thus, although these mutations appear neutral when considering the overall reaction catalyzed by these enzymes, they do not have neutral effects on their molecular mechanisms. This incompatibility is probably due to subtle changes to the conformation and dynamics of the active site caused by amino acid substitutions at other positions. These results add support to an emerging theme in protein evolution, in which epistatic mutations (silent mutations that do not alter a protein’s activity) place constraints on the amino acids that are permitted at other positions, making evolutionary pathways irreversible.44, 45
Supplementary Material
Acknowledgments
Funding Statement: This work was supported by grant A-1758 from the Robert A. Welch Foundation (Principle Investigator: M.E. Glasner). The NYSGXRC was supported by NIH Grant U54 GM074945 (Principal Investigator: S.K. Burley). The NYSGRC is supported by NIH Grant U54 GM094662 (Principal Investigator: S.C. Almo). The Center for Synchrotron Biosciences, where diffraction data were collected, was supported by grant P30-EB-009998 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB). Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.
We acknowledge the efforts of all NYSGXRC and NYSGRC personnel who contributed to the structure determination and manuscript preparation. We thank Chenxi Wang, Wan Wen Zhu, and Christine Jones for assistance with site directed mutagenesis and Dr. Andrew McMillan for insightful comments on the manuscript.
Abbreviations
- OSBS
o-succinylbenzoate synthase
- NSAR
N-succinylamino acid racemase
- SHCHC
2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate
- PMSF
phenylmethylsulfonyl fluoride
Footnotes
SUPPORTING INFORMATION AVAILABLE
Detailed methods, primers, and templates used for site directed mutagenesis are available as supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gerlt JA, Babbitt PC, Rayment I. Divergent evolution in the enolase superfamily: the interplay of mechanism and specificity. Arch Biochem Biophys. 2005;433:59–70. doi: 10.1016/j.abb.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 2.Rakus JF, Kalyanaraman C, Fedorov AA, Fedorov EV, Mills-Groninger FP, Toro R, Bonanno J, Bain K, Sauder JM, Burley SK, Almo SC, Jacobson MP, Gerlt JA. Computation-facilitated assignment of the function in the enolase superfamily: a regiochemically distinct galactarate dehydratase from Oceanobacillus iheyensis. Biochemistry. 2009;48:11546–11558. doi: 10.1021/bi901731c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song L, Kalyanaraman C, Fedorov AA, Fedorov EV, Glasner ME, Brown S, Imker HJ, Babbitt PC, Almo SC, Jacobson MP, Gerlt JA. Prediction and assignment of function for a divergent N-succinyl amino acid racemase. Nat Chem Biol. 2007;3:486–491. doi: 10.1038/nchembio.2007.11. [DOI] [PubMed] [Google Scholar]
- 4.Kalyanaraman C, Imker HJ, Fedorov AA, Fedorov EV, Glasner ME, Babbitt PC, Almo SC, Gerlt JA, Jacobson MP. Discovery of a dipeptide epimerase enzymatic function guided by homology modeling and virtual screening. Structure. 2008;16:1668–1677. doi: 10.1016/j.str.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukk T, Sakai A, Kalyanaraman C, Brown SD, Imker HJ, Song L, Fedorov AA, Fedorov EV, Toro R, Hillerich B, Seidel R, Patskovsky Y, Vetting MW, Nair SK, Babbitt PC, Almo SC, Gerlt JA, Jacobson MP. Homology models guide discovery of diverse enzyme specificities among dipeptide epimerases in the enolase superfamily. Proc Natl Acad Sci U S A. 2012;109:4122–4127. doi: 10.1073/pnas.1112081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meganathan R. Biosynthesis of menaquinone (vitamin K2) and ubiquinone (coenzyme Q): a perspective on enzymatic mechanisms. Vitam Horm. 2001;61:173–218. doi: 10.1016/s0083-6729(01)61006-9. [DOI] [PubMed] [Google Scholar]
- 7.Glasner ME, Fayazmanesh N, Chiang RA, Sakai A, Jacobson MP, Gerlt JA, Babbitt PC. Evolution of structure and function in the o-succinylbenzoate synthase/N-acylamino acid racemase family of the enolase superfamily. J Mol Biol. 2006;360:228–250. doi: 10.1016/j.jmb.2006.04.055. [DOI] [PubMed] [Google Scholar]
- 8.Sakai A, Xiang DF, Xu C, Song L, Yew WS, Raushel FM, Gerlt JA. Evolution of enzymatic activities in the enolase superfamily: N-succinylamino acid racemase and a new pathway for the irreversible conversion of D- to L-Amino Acids. Biochemistry. 2006;45:4455–4462. doi: 10.1021/bi060230b. [DOI] [PubMed] [Google Scholar]
- 9.Palmer DR, Garrett JB, Sharma V, Meganathan R, Babbitt PC, Gerlt JA. Unexpected divergence of enzyme function and sequence: “N-acylamino acid racemase” is o-succinylbenzoate synthase. Biochemistry. 1999;38:4252–4258. doi: 10.1021/bi990140p. [DOI] [PubMed] [Google Scholar]
- 10.Taylor Ringia EA, Garrett JB, Thoden JB, Holden HM, Rayment I, Gerlt JA. Evolution of enzymatic activity in the enolase superfamily: functional studies of the promiscuous o-succinylbenzoate synthase from Amycolatopsis. Biochemistry. 2004;43:224–229. doi: 10.1021/bi035815+. [DOI] [PubMed] [Google Scholar]
- 11.Thompson TB, Garrett JB, Taylor EA, Meganathan R, Gerlt JA, Rayment I. Evolution of enzymatic activity in the enolase superfamily: structure of o-succinylbenzoate synthase from Escherichia coli in complex with Mg2+ and o-succinylbenzoate. Biochemistry. 2000;39:10662–10676. doi: 10.1021/bi000855o. [DOI] [PubMed] [Google Scholar]
- 12.Klenchin VA, Taylor Ringia EA, Gerlt JA, Rayment I. Evolution of enzymatic activity in the enolase superfamily: structural and mutagenic studies of the mechanism of the reaction catalyzed by o-succinylbenzoate synthase from Escherichia coli. Biochemistry. 2003;42:14427–14433. doi: 10.1021/bi035545v. [DOI] [PubMed] [Google Scholar]
- 13.Thoden JB, Taylor Ringia EA, Garrett JB, Gerlt JA, Holden HM, Rayment I. Evolution of enzymatic activity in the enolase superfamily: structural studies of the promiscuous o-succinylbenzoate synthase from Amycolatopsis. Biochemistry. 2004;43:5716–5727. doi: 10.1021/bi0497897. [DOI] [PubMed] [Google Scholar]
- 14.Zhu WW, Wang C, Jipp J, Ferguson L, Lucas SN, Hicks MA, Glasner ME. Residues required for activity in Escherichia coli o-succinylbenzoate synthase (OSBS) are not conserved in all OSBS enzymes. Biochemistry. 2012;51:6171–6181. doi: 10.1021/bi300753j. [DOI] [PubMed] [Google Scholar]
- 15.Rost B. Enzyme function less conserved than anticipated. J Mol Biol. 2002;318:595–608. doi: 10.1016/S0022-2836(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 16.Tian W, Skolnick J. How well is enzyme function conserved as a function of pairwise sequence identity? J Mol Biol. 2003;333:863–882. doi: 10.1016/j.jmb.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 17.Sauder MJ, Rutter ME, Bain K, Rooney I, Gheyi T, Atwell S, Thompson DA, Emtage S, Burley SK. High throughput protein production and crystallization at NYSGXRC. Methods in molecular biology. 2008;426:561–575. doi: 10.1007/978-1-60327-058-8_37. [DOI] [PubMed] [Google Scholar]
- 18.Sheldrick GM. A short history of SHELX. Acta crystallographica Section A, Foundations of crystallography. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 19.Bricogne G, Vonrhein C, Flensburg C, Schiltz M, Paciorek W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta crystallographica Section D, Biological crystallography. 2003;59:2023–2030. doi: 10.1107/s0907444903017694. [DOI] [PubMed] [Google Scholar]
- 20.Abrahams JP, Leslie AG. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta crystallographica Section D, Biological crystallography. 1996;52:30–42. doi: 10.1107/S0907444995008754. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta crystallographica Section D, Biological crystallography. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 22.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta crystallographica Section D, Biological crystallography. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 23.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta crystallographica Section D, Biological crystallography. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 24.Vagin A, Teplyakov A. MOLREP: an Automated Program for Molecular Replacement. Journal of Applied Crystallography. 1997;30:1022–1025. [Google Scholar]
- 25.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Malcolm BA. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. Biotechniques. 1999;26:680–682. doi: 10.2144/99264st03. [DOI] [PubMed] [Google Scholar]
- 27.Neidhart DJ, Howell PL, Petsko GA, Powers VM, Li RS, Kenyon GL, Gerlt JA. Mechanism of the reaction catalyzed by mandelate racemase. 2. Crystal structure of mandelate racemase at 2.5-Å resolution: identification of the active site and possible catalytic residues. Biochemistry. 1991;30:9264–9273. doi: 10.1021/bi00102a019. [DOI] [PubMed] [Google Scholar]
- 28.Landro JA, Gerlt JA, Kozarich JW, Koo CW, Shah VJ, Kenyon GL, Neidhart DJ, Fujita S, Petsko GA. The role of lysine 166 in the mechanism of mandelate racemase from Pseudomonas putida: mechanistic and crystallographic evidence for stereospecific alkylation by (R)-alpha-phenylglycidate. Biochemistry. 1994;33:635–643. doi: 10.1021/bi00169a003. [DOI] [PubMed] [Google Scholar]
- 29.Gulick AM, Hubbard BK, Gerlt JA, Rayment I. Evolution of enzymatic activities in the enolase superfamily: crystallographic and mutagenesis studies of the reaction catalyzed by D-glucarate dehydratase from Escherichia coli. Biochemistry. 2000;39:4590–4602. doi: 10.1021/bi992782i. [DOI] [PubMed] [Google Scholar]
- 30.Klenchin VA, Schmidt DM, Gerlt JA, Rayment I. Evolution of enzymatic activities in the enolase superfamily: structure of a substrate-liganded complex of the L-Ala-D/L-Glu epimerase from Bacillus subtilis. Biochemistry. 2004;43:10370–10378. doi: 10.1021/bi049197o. [DOI] [PubMed] [Google Scholar]
- 31.Yew WS, Fedorov AA, Fedorov EV, Rakus JF, Pierce RW, Almo SC, Gerlt JA. Evolution of enzymatic activities in the enolase superfamily: L-fuconate dehydratase from Xanthomonas campestris. Biochemistry. 2006;45:14582–14597. doi: 10.1021/bi061687o. [DOI] [PubMed] [Google Scholar]
- 32.Yew WS, Fedorov AA, Fedorov EV, Wood BM, Almo SC, Gerlt JA. Evolution of enzymatic activities in the enolase superfamily: D-tartrate dehydratase from Bradyrhizobium japonicum. Biochemistry. 2006;45:14598–14608. doi: 10.1021/bi061688g. [DOI] [PubMed] [Google Scholar]
- 33.Rakus JF, Fedorov AA, Fedorov EV, Glasner ME, Vick JE, Babbitt PC, Almo SC, Gerlt JA. Evolution of enzymatic activities in the enolase superfamily: D-Mannonate dehydratase from Novosphingobium aromaticivorans. Biochemistry. 2007;46:12896–12908. doi: 10.1021/bi701703w. [DOI] [PubMed] [Google Scholar]
- 34.Rakus JF, Fedorov AA, Fedorov EV, Glasner ME, Hubbard BK, Delli JD, Babbitt PC, Almo SC, Gerlt JA. Evolution of enzymatic activities in the enolase superfamily: L-rhamnonate dehydratase. Biochemistry. 2008;47:9944–9954. doi: 10.1021/bi800914r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai A, Fedorov AA, Fedorov EV, Schnoes AM, Glasner ME, Brown S, Rutter ME, Bain K, Chang S, Gheyi T, Sauder JM, Burley SK, Babbitt PC, Almo SC, Gerlt JA. Evolution of enzymatic activities in the enolase superfamily: stereochemically distinct mechanisms in two families of cis,cis-muconate lactonizing enzymes. Biochemistry. 2009;48:1445–1453. doi: 10.1021/bi802277h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lietzan AD, Nagar M, Pellmann EA, Bourque JR, Bearne SL, St Maurice M. Structure of mandelate racemase with bound intermediate analogues benzohydroxamate and cupferron. Biochemistry. 2012;51:1160–1170. doi: 10.1021/bi2018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedekind JE, Poyner RR, Reed GH, Rayment I. Chelation of serine 39 to Mg2+ latches a gate at the active site of enolase: structure of the bis(Mg2+) complex of yeast enolase and the intermediate analog phosphonoacetohydroxamate at 2.1-Å resolution. Biochemistry. 1994;33:9333–9342. doi: 10.1021/bi00197a038. [DOI] [PubMed] [Google Scholar]
- 38.Bourque JR, Bearne SL. Mutational analysis of the active site flap (20s loop) of mandelate racemase. Biochemistry. 2008;47:566–578. doi: 10.1021/bi7015525. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara T, Kuwajima K, Sugai S. Folding of Staphylococcal Nuclease A Studied by Equilibrium and Kinetic Circular-Dichroism Spectra. Biochemistry. 1991;30:2698–2706. doi: 10.1021/bi00224a018. [DOI] [PubMed] [Google Scholar]
- 40.Berka K, Anzenbacherova E, Hendrychova T, Lange R, Masek V, Anzenbacher P, Otyepka M. Binding of quinidine radically increases the stability and decreases the flexibility of the cytochrome P450 2D6 active site. J Inorg Biochem. 2012;110:46–50. doi: 10.1016/j.jinorgbio.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Research. 2006;34:W116–W118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebioda L, Stec B. Crystal structure of enolase indicates that enolase and pyruvate kinase evolved from a common ancestor. Nature. 1988;333:683–686. doi: 10.1038/333683a0. [DOI] [PubMed] [Google Scholar]
- 43.Taylor EA, Palmer DR, Gerlt JA. The lesser “burden borne” by o-succinylbenzoate synthase: an “easy” reaction involving a carboxylate carbon acid. J Am Chem Soc. 2001;123:5824–5825. doi: 10.1021/ja010882h. [DOI] [PubMed] [Google Scholar]
- 44.Bridgham JT, Ortlund EA, Thornton JW. An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature. 2009;461:515–519. doi: 10.1038/nature08249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong LI, Suchard MA, Bloom JD. Stability-mediated epistasis constrains the evolution of an influenza protein. eLife. 2013;2:e00631. doi: 10.7554/eLife.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.