Abstract
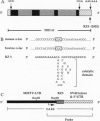
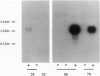
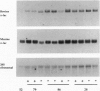
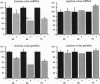
Transgenic mice carrying a bovine alpha-lactalbumin (alpha-lac) specific ribozyme gene under the transcriptional control of the mouse mammary tumor virus long terminal repeat were generated and cross-bred with animals that highly express a bovine alpha-lac transgene (0.4 mg of alpha-lac/ml(-1) of milk). The ribozyme contains the hammerhead catalytic domain, flanked by 12-nt sequences complementary to the 3' untranslated region of bovine alpha-lac transcript. High-level expression of the ribozyme gene was detected by Northern blot analysis in the mammary gland of 7-8 day lactating transgenic mice, from 3 of 12 lines analyzed. Heterozygous expression of the ribozyme resulted in a reduction in the levels of the target mRNA to 78, 58, and 50% of that observed in the nonribozyme transgenic littermate controls for three independent lines. The ribozyme-mediated reduction in the levels of the bovine protein paralleled that observed for the mRNA, and was positively correlated with the level of expression of the ribozyme. In nonribozyme expressing transgenic mice, the level of bovine alpha-lac mRNA and protein was not affected. The specificity of this activity is demonstrated by the absence of a reduction in the levels of the endogenous murine alpha-lac mRNA or protein. These results demonstrate the feasibility of ribozyme-mediated down-regulation of highly-expressed transcripts in transgenic animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbu V., Dautry F. Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res. 1989 Sep 12;17(17):7115–7115. doi: 10.1093/nar/17.17.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. J., Cullimore J. V. Selective cleavage of closely-related mRNAs by synthetic ribozymes. Nucleic Acids Res. 1992 Feb 25;20(4):831–837. doi: 10.1093/nar/20.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E., Pictet R., Grange T. Can hammerhead ribozymes be efficient tools to inactivate gene function? Nucleic Acids Res. 1994 Feb 11;22(3):293–300. doi: 10.1093/nar/22.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cameron F. H., Jennings P. A. Specific gene suppression by engineered ribozymes in monkey cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9139–9143. doi: 10.1073/pnas.86.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor G. H., McElwain T. F., Birkebak T. A., Palmer G. H. Ribozyme cleaves rex/tax mRNA and inhibits bovine leukemia virus expression. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10932–10936. doi: 10.1073/pnas.90.23.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisell P., Thompson S., James W. Inhibition of HIV-1 replication by ribozymes that show poor activity in vitro. Nucleic Acids Res. 1993 Nov 11;21(22):5251–5255. doi: 10.1093/nar/21.22.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubayko F., Riegel A. T., Wellstein A. Ribozyme-targeting elucidates a direct role of pleiotrophin in tumor growth. J Biol Chem. 1994 Aug 19;269(33):21358–21363. [PubMed] [Google Scholar]
- Denman R. B., Smedman M., Ju W., Rubenstein R., Potempska A., Miller D. L. Ribozyme mediated degradation of beta-amyloid peptide precursor mRNA in COS-7 cells. Nucleic Acids Res. 1994 Jun 25;22(12):2375–2382. doi: 10.1093/nar/22.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorai T., Kobayashi H., Holland J. F., Ohnuma T. Modulation of platelet-derived growth factor-beta mRNA expression and cell growth in a human mesothelioma cell line by a hammerhead ribozyme. Mol Pharmacol. 1994 Sep;46(3):437–444. [PubMed] [Google Scholar]
- Efrat S., Leiser M., Wu Y. J., Fusco-DeMane D., Emran O. A., Surana M., Jetton T. L., Magnuson M. A., Weir G., Fleischer N. Ribozyme-mediated attenuation of pancreatic beta-cell glucokinase expression in transgenic mice results in impaired glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2051–2055. doi: 10.1073/pnas.91.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J., Kim K. H. Inhibition of fatty acid synthesis by expression of an acetyl-CoA carboxylase-specific ribozyme gene. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9951–9955. doi: 10.1073/pnas.91.21.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Homann M., Tabler M., Tzortzakaki S., Sczakiel G. Extension of helix II of an HIV-1-directed hammerhead ribozyme with long antisense flanks does not alter kinetic parameters in vitro but causes loss of the inhibitory potential in living cells. Nucleic Acids Res. 1994 Sep 25;22(19):3951–3957. doi: 10.1093/nar/22.19.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehntopf M., Brach M. A., Licht T., Petschauer S., Karawajew L., Kirschning C., Herrmann F. Ribozyme-mediated cleavage of the MDR-1 transcript restores chemosensitivity in previously resistant cancer cells. EMBO J. 1994 Oct 3;13(19):4645–4652. doi: 10.1002/j.1460-2075.1994.tb06787.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- L'Huillier P. J., Davis S. R., Bellamy A. R. Cytoplasmic delivery of ribozymes leads to efficient reduction in alpha-lactalbumin mRNA levels in C127I mouse cells. EMBO J. 1992 Dec;11(12):4411–4418. doi: 10.1002/j.1460-2075.1992.tb05541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange W., Cantin E. M., Finke J., Dölken G. In vitro and in vivo effects of synthetic ribozymes targeted against BCR/ABL mRNA. Leukemia. 1993 Nov;7(11):1786–1794. [PubMed] [Google Scholar]
- Laptev A. V., Lu Z., Colige A., Prockop D. J. Specific inhibition of expression of a human collagen gene (COL1A1) with modified antisense oligonucleotides. The most effective target sites are clustered in double-stranded regions of the predicted secondary structure for the mRNA. Biochemistry. 1994 Sep 13;33(36):11033–11039. doi: 10.1021/bi00202a024. [DOI] [PubMed] [Google Scholar]
- Larsson S., Hotchkiss G., Andäng M., Nyholm T., Inzunza J., Jansson I., Ahrlund-Richter L. Reduced beta 2-microglobulin mRNA levels in transgenic mice expressing a designed hammerhead ribozyme. Nucleic Acids Res. 1994 Jun 25;22(12):2242–2248. doi: 10.1093/nar/22.12.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Halter S. A., Holt J. T., Hogan B. L., Coffey R. J. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990 Jun 15;61(6):1147–1155. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- Ross S. R., Hsu C. L., Choi Y., Mok E., Dudley J. P. Negative regulation in correct tissue-specific expression of mouse mammary tumor virus in transgenic mice. Mol Cell Biol. 1990 Nov;10(11):5822–5829. doi: 10.1128/mcb.10.11.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon K. J., Ishida H., Kashani-Sabet M. Ribozyme-mediated reversal of the multidrug-resistant phenotype. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11123–11127. doi: 10.1073/pnas.91.23.11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M. Interaction between tumour necrosis factor alpha ribozyme and cellular proteins. Involvement in ribozyme stability and activity. J Mol Biol. 1994 Oct 7;242(5):619–629. doi: 10.1006/jmbi.1994.1612. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Stinnakre M. G., Vilotte J. L., Soulier S., Mercier J. C. Creation and phenotypic analysis of alpha-lactalbumin-deficient mice. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6544–6548. doi: 10.1073/pnas.91.14.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Huarte J., Belin D., Vassalli A., Rickles R. J., Vassalli J. D. Antisense RNA directed against the 3' noncoding region prevents dormant mRNA activation in mouse oocytes. Science. 1988 Aug 5;241(4866):680–684. doi: 10.1126/science.2456615. [DOI] [PubMed] [Google Scholar]
- Sun L. Q., Warrilow D., Wang L., Witherington C., Macpherson J., Symonds G. Ribozyme-mediated suppression of Moloney murine leukemia virus and human immunodeficiency virus type I replication in permissive cell lines. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9715–9719. doi: 10.1073/pnas.91.21.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Self-cleavage of RNA in the replication of small pathogens of plants and animals. Trends Biochem Sci. 1989 Nov;14(11):445–450. doi: 10.1016/0968-0004(89)90103-5. [DOI] [PubMed] [Google Scholar]
- Tang X. B., Hobom G., Luo D. Ribozyme mediated destruction of influenza A virus in vitro and in vivo. J Med Virol. 1994 Apr;42(4):385–395. doi: 10.1002/jmv.1890420411. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Vilotte J. L., Soulier S., Stinnakre M. G., Massoud M., Mercier J. C. Efficient tissue-specific expression of bovine alpha-lactalbumin in transgenic mice. Eur J Biochem. 1989 Dec 8;186(1-2):43–48. doi: 10.1111/j.1432-1033.1989.tb15175.x. [DOI] [PubMed] [Google Scholar]
- Westneat D. F., Noon W. A., Reeve H. K., Aquadro C. F. Improved hybridization conditions for DNA 'fingerprints' probed with M13. Nucleic Acids Res. 1988 May 11;16(9):4161–4161. doi: 10.1093/nar/16.9.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Ojwang J., Yamada O., Hampel A., Rapapport J., Looney D., Wong-Staal F. A hairpin ribozyme inhibits expression of diverse strains of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6340–6344. doi: 10.1073/pnas.90.13.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. J., Pick L. Generating loss-of-function phenotypes of the fushi tarazu gene with a targeted ribozyme in Drosophila. Nature. 1993 Sep 30;365(6445):448–451. doi: 10.1038/365448a0. [DOI] [PubMed] [Google Scholar]