Abstract
Background
Risks associated with parental separation have received limited attention in research on children of parents with substance use disorders. We examined early substance involvement as a function of parental separation during childhood and parental alcohol and cannabis dependence.
Method
Data were drawn from 1,318 adolescent offspring of monozygotic (MZ) or dizygotic (DZ) Australian twin parents. Cox proportional hazards regression analyses were conducted predicting age at first use of alcohol, first alcohol intoxication, first use and first regular use of cigarettes, and first use of cannabis, from parental separation and both parent and cotwin substance dependence. Parent and cotwin alcohol and cannabis dependence were initially modeled separately, with post-hoc tests for equality of effects.
Results
With few exceptions, risks associated with parental alcohol versus cannabis dependence could be equated, with results largely suggestive of genetic transmission of risk from parental substance (alcohol or cannabis) dependence broadly defined. Controlling for parental substance dependence, parental separation was a strong predictor for all substance use variables, especially through age 13.
Conclusion
Together, findings underscore the importance of parental separation as a risk-factor for early substance involvement over and above both genetic and environmental influences specific to parental alcohol and cannabis dependence.
Keywords: adolescent substance use, parental separation, parental substance dependence, children of twins
1. INTRODUCTION
Compared with children of non-alcoholic parents, children of alcoholics (COAs) report earlier and more frequent use of alcohol as well as tobacco, cannabis, and other illicit drugs, and are at greater risk of alcohol problems during adolescence and adulthood (Lieb et al., 2002; Schuckit and Smith, 1996; Sher et al., 1991). By some reports, COAs are four to six times as likely to develop an alcohol use disorder at some point in their life (Chassin et al., 1991; Russell, 1995). Comparatively less is known about children whose parents abuse other drugs; however, paternal illicit drug use has been linked with earlier tobacco use, and problem use of alcohol, tobacco and illicit drugs (Clark et al., 1998, 1999).
While risks for early and problem substance use associated with parental alcoholism are widely documented, not all COAs initiate use at early ages, and for those offspring who show signs of problem use, many “mature out” during adulthood (Labouvie, 1996; Maisto et al., 2002). Furthermore, COAs experience a range of adversities that often follow from but are not exclusive to parental alcoholism, and many such “non-specific” risks have considerable consequences (Jacob and Johnson, 1997). Parental separation or divorce provides a strong example as alcoholic parents are at increased risk of marital dissolution (Waldron et al., 2013) and compared to children from intact married families, children of divorce also report earlier use of alcohol, tobacco, and cannabis (Hoffman and Su, 1998; Short, 1998), heavier use of these substances (Doherty and Needle, 1991; Hoffman, 1995; Needle et al., 1990), and higher rates of problem use (Fergusson et al., 1994; Hoffman and Johnson, 1998).
Surprisingly, risks to offspring associated with parental separation have received limited attention in research on children of alcoholic or other drug addicted parents. Using a Children-of-Twins (COT) design (Gotteman and Bertelson, 1989; Heath et al., 1985; Nance and Corey, 1976), we examine whether parental separation predicts early substance involvement over and above risks from parental alcohol or cannabis dependence, including genetic risks. Genetic variation has been reported for alcohol abuse and dependence (Heath et al., 1997; McGue, 1994) and a variety of drug use disorders, including cannabis abuse and dependence (Kendler and Prescott, 1998; Lynskey et al., 2002), with genetic variation also observed for initiation, regular use, and problem substance use during adolescence (Maes et al., 1999; McGue et al., 2000; Rhee et al., 2003). Heritable influences on marital status are reported as well, including genetic variation in likelihood of marriage (Trumbetta et al., 2007) and risk of divorce (McGue and Lykken, 1992), with at least one report of genetic covariation between alcohol dependence and both marital timing and survival (Waldron et al., 2011).
In COT studies, genetic and environmental risks are inferred from parent and cotwin history of substance dependence, with outcomes of offspring from a minimum of four groups compared, each with varying degrees of genetic risk and environmental exposure. In the present analysis, these groups include: offspring whose parent is substance dependent (Group 1); offspring of an unaffected parent whose monozygotic (MZ) cotwin is substance dependent (Group 2); offspring of an unaffected parent whose dizygotic (DZ) cotwin is substance dependent (Group 3); and offspring from control families, where neither parent nor cotwin, regardless of zygosity, is substance dependent (Group 4). Hypothesized risks to offspring are summarized in Table 1.
Table 1.
Risk Group by Hypothesized Family Environmental, Genetic, and GxE Risks
| Risk Group | Risk to offspring due to:
|
||
|---|---|---|---|
| Environment | Genes | GxE | |
| 1. Parent affected | high | high | high |
| 2. Parent UN, MZ cotwin affected | low | high | low |
| 3. Parent UN, DZ cotwin affected | low | intermediate | low |
| 4. Parent and cotwin UN | low | low | very low |
Note. UN = unaffected (neither alcohol nor cannabis dependent); GxE = Gene by environment interaction.
Following from quantitative genetic theory, if the association between parental substance dependence and offspring substance involvement results from rearing environment, offspring of affected parents should demonstrate greater risk, compared with unaffected parents (Group 1>Groups 2–4). If the association results from genes shared between parents and their children, i.e., genetic transmission, offspring at high genetic risk should exhibit earlier involvement than offspring at intermediate genetic risk regardless of environmental risk (Groups 1 and 2>Group 3). A pattern consistent with gene-environment interaction (GxE) is evident if offspring reared by an alcoholic or drug dependent parent exhibit greater risk, compared to offspring of unaffected parents, with offspring of an unaffected parent whose cotwin is also unaffected at lowest risk (Group 1>Groups 2–3>Group 4).
To date, a handful of COT studies of alcoholic families have been conducted. For early and problem use of alcohol, evidence of environmental transmission from parental alcoholism has been documented in some but not all reports (Duncan et al., 2006; Jacob et al., 2003; Sartor et al., 2007; Slutske et al., 2008). COT studies based on twin and cotwin history of divorce have been conducted as well, with evidence broadly suggestive of environmental transmission across a range of substance use outcomes (D’Onfrio et al., 2005, 2007). The present study is distinct from earlier work in that we examine timing of alcohol, cigarette and cannabis involvement as a function of parental separation or divorce, employing a COT design to control for genetic and environmental risks from parental substance dependence, including risks from parental cannabis dependence.
2. METHODS
2.1. Participants
Participants were drawn from two studies of Australian children of twins selected from a young adult twin panel born between 1964 and 1971 (Heath et al., 2001; Knopik et al., 2004). Following initial contact by mailed questionnaire in 1989 (thus, the “1989” cohort), twins completed diagnostic telephone interviews during 1997–2002. Pairs where at least one twin had biological children ages 7–24 and one twin met DSM-IV criteria for alcohol use disorder (AUD; operationalized as alcohol dependence (AD) in male twins and either AD or alcohol abuse (AB) in female twins) were subsequently recruited for participation in one of two coordinated follow-up studies: Mothers And Their Children (MATCH) and Parental Alcoholism and Child Environmental Risk (PACER). A random sample of control pairs, where at least one twin had biological children ages 7–24, but neither twin met criteria for AUD, was also recruited. MATCH twins were selected from female same-sex pairs from both the 1989 cohort and an older “1981” cohort described elsewhere (see Heath et al., 1997; Waldron et al., 2009). PACER twins were selected from male same- and opposite-sex pairs from the 1989 cohort only. Assessment of twin parents by telephone interview began in 2000 and 2005 for MATCH and PACER studies, respectively. During the same period, offspring ages 11–24 were invited to complete an interview, also by telephone. From the 1989 cohort, 1,341 offspring completed MATCH or PACER interviews, of whom 23 (<2%) were excluded from analyses because of missing data on twin substance dependence, parental separation, and/or offspring substance involvement, resulting in a final sample of 1,318 offspring. Samples sizes by risk group(s) are shown in Table 2, with sample characteristics provided in Table 3.
Table 2.
Sample Sizes by Risk Group: Combined MATCH and PACER Samples
| Risk Group | ADa
|
CannDb
|
AD or CannD
|
|||
|---|---|---|---|---|---|---|
| ntwins | noffspring | ntwins | noffspring | ntwins | noffspring | |
| 1. Parent affected | 189 | 312 | 79 | 129 | 222 | 362 |
| 2. Parent UN, MZ cotwin affected | 37 | 62 | 33 | 53 | 50 | 83 |
| 3. Parent UN, DZ cotwin affected | 74 | 122 | 47 | 79 | 76 | 131 |
| 4. Parent and cotwin UN | 484 | 822 | 610 | 1031 | 436 | 742 |
Note. AD = alcohol dependence; CannD = cannabis dependence; UN = unaffected (neither alcohol nor cannabis dependent).
coded without regard to cannabis dependence.
coded without regard to alcohol dependence.
Table 3.
Sample Characteristics, by Twin/Parent History of Alcohol and Cannabis Dependence
| AD+/CannD+ noffspring = 79 |
AD+/CannD− noffspring = 233 |
AD−/CannD+ noffspring = 50 |
AD−/CannD− noffspring = 956 |
|
|---|---|---|---|---|
|
|
||||
| Offspring age, M (SD) | 14.48 (3.02) | 14.26 (2.94) | 15.36 (2.94) | 14.23 (2.83) |
| Offspring sex, n (%) female | 43 (54) | 112 (48) | 22 (44) | 480 (50) |
| Twin age*, M (SD) | 30.05 (2.02) | 30.78 (2.24) | 30.92 (2.51) | 30.89 (2.04) |
| Twin sex, n (%) female | 36 (36) | 117 (50) | 25 (50) | 620 (65) |
| Twin education* | ||||
| < 12 years, n (%) | 14 (18) | 42 (18) | 13 (26) | 128 (13) |
| 13+ years, n (%) | 21 (27) | 38 (17) | 8 (16) | 255 (27) |
| Offspring substance use | ||||
| Alcohol, n (%) | 37 (47) | 93 (40) | 34 (68) | 344 (36) |
| Age of onset, M (SD) | 13.59 (2.19) | 14.17 (2.03) | 14.06 (2.42) | 14.23 (1.85) |
| Alcohol intoxication, n (%) | 29 (37) | 66 (29) | 25 (50) | 206 (22) |
| Age of onset, M (SD) | 14.93 (1.49) | 15.47 (1.55) | 15.04 (1.86) | 15.49 (1.73) |
| Cigarettes, n (%) | 29 (37) | 70 (30) | 25 (50) | 200 (21) |
| Age of onset, M (SD) | 13.62 (2.54) | 13.48 (2.17) | 11.88 (3.60) | 13.59 (2.69) |
| Regular smoking, n (%) | 13 (16) | 24 (10) | 6 (12) | 67 (7) |
| Age of onset, M (SD) | 14.54 (2.11) | 15.33 (1.74) | 14.67 (1.37) | 15.13 (1.89) |
| Cannabis, n (%) | 25 (32) | 32 (14) | 11 (22) | 86 (9) |
| Age at first use, M (SD) | 14.72 (2.13) | 15.75 (1.59) | 15.18 (3.16) | 15.58 (1.75) |
| Parental separation, n (%) | 46 (58) | 80 (34) | 26 (52) | 223 (23) |
| Age of onset, M (SD) | 5.83 (4.62) | 6.16 (4.49) | 5.85 (4.17) | 6.77 (4.86) |
Note. AD = alcohol dependence; CannD = cannabis dependence.
as of the 1997–2002 diagnostic interview.
2.2. Measures
Twins completed telephone adaptations of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994; Hesselbrock et al., 1999). Much of the SSAGA was retained at reinterview, with additional items from the Family History Assessment Module (FHAM; Rice et al., 1995) included to assess biological coparent psychopathology. To assess offspring psychopathology, parent-report items from the Diagnostic Interview for Children and Adolescents (DICA; Herjanic and Reich, 1982) and the Child SSAGA (C-SSAGA-P; Kuperman et al., 2001) were also incorporated. Offspring completed either the child (ages 11–14) or adolescent (ages 15+) version of the SSAGA, also adapted for telephone administration.
2.2.1. Offspring substance involvement
Lifetime use and age at first use of alcohol, cigarettes, and cannabis were assessed in the offspring interview. History of alcohol intoxication and regular smoking were also assessed. Onset of regular smoking was coded from age at first regular use of cigarettes, the latter defined as a) having smoked 100 or more cigarettes, or b) smoking between 20–99 cigarettes and having smoked at least once per week for a period of two months or more.
2.2.2. Parental substance dependence
DSM-IV AD was directly assessed in the 1997–2002 interview, with new onsets coded from parent interviews. An abbreviated assessment of cannabis dependence was included in the 1997–2002 interview only. Based on published sensitivity analyses (Lynskey et al., 2002), cannabis dependence was defined as 2 or more of 4 symptoms assessed (use of larger amount/over longer period than intended; tolerance; continued use despite problems; persistent desire to cut down) within a 12-month period. We computed separately for alcohol and cannabis dependence, three dummy variables corresponding to hypothesized genetic and environmental risks, i.e., Groups 1–3, with control families (Group 4) comprising the reference group.
Biological coparent history of substance use or disorder was also coded as offspring phenotypes depend on behavior of both parents even when mating is random (Eaves et al., 2005). Consistent with research documenting strong within-family agreement (Waldron et al., 2012), coparent alcoholism was coded positive by twin, coparent or offspring report. In MATCH, AD symptoms experienced by biological coparents were assessed without regard to temporal clustering; thus, a probable dependence diagnosis was coded. In PACER, twins were asked only whether “drinking ever caused the biological (mother/father) of (child1/2/3) to have problems with health, family, job or police, or other problems,” an item that originated in the Family History Research Diagnostic Criteria assessment (FHRDC; Andreasen et al., 1977), and whether they ever felt that the coparent was an “excessive drinker.” In both MATCH and PACER, offspring ages 15 and older were asked similar questions, that is, whether “drinking ever caused your biological (mother/father) to have problems…,” and whether they were an “excessive drinker.” Endorsement of both problem and excessive drinking was required to code a coparent positive by twin report (in PACER) or offspring report. Neither MATCH nor PACER interviews included assessment of cannabis dependence symptoms experienced by biological coparents; instead, coparent recurrent use was coded from twin or coparent report, defined as lifetime use of cannabis on 11 or more occasions. Parental cannabis use was not assessed by offspring report. Given 15% missingness, two dummy variables were coded to distinguish coparent recurrent use from missing coparent cannabis data, with coparents having never used or used on less than 11 occasions comprising the reference group.
2.2.3. Parental separation
History of biological parent separation or divorce prior to offspring age 18 was coded from parent and offspring interviews. Separations for non-relationship reasons (for example, one parent working overseas or incarcerated) were not coded, nor were subsequent separations between a biological parent and stepparent, where assessment was limited. Offspring age at parental separation was computed from parent report of year marriage or marriage-like relationship or, if missing, age offspring last lived with both biological parents assessed of both parents and offspring.
2.2.4. Control variables
To ensure specificity of effects, a number of demographic, familial and individual-level risks were included as control variables. In addition to offspring sex and age at interview, twin sex, and twin age and education as of the 1997–2002 interview were included among demographic control variables. Dummy variables for having not finished high school and completion of any tertiary education were computed, with high school only comprising the reference group. Control variables for parent comorbid psychopathology include twin and biological coparent history of DSM-IV major depressive disorder (MDD) and a nondiagnostic measure of antisocial personality disorder (ASP). MDD and ASP were assessed of twins in the 1997–2002 interview and both twins and coparents in parent interview. For coparent MDD and ASP, two dummy variables were coded to distinguish affected coparents from those with missing data (11% each for MDD and ASP). Twin history of regular smoking was also assessed in the 1997–2002 interview, with coparent regular smoking queried in parent and offspring interviews. Control variables for offspring comorbid psychopathology include DSM-IV conduct disorder, MDD, social phobia, generalized anxiety disorder, and suicidality (ideation, plan, or attempt), assessed in the offspring interview. Offspring DSM-IV inattention, hyperactivity and oppositional defiant disorder were queried in parent interviews, with two dummy variables coded to distinguish affected offspring from those with missing parent-report data (8% each).
2.3. Analytic strategy
Analyses were performed in STATA version 12 (StataCorp, 2011), with the Huber-White robust variance estimator used to compute standard errors and confidence intervals adjusted for non-independence of twin-family data. Time-to-event data were analyzed using survival analysis to assess likelihood as well as timing of onset. Cox proportional hazards (PH) regression was conducted predicting onset of substance involvement from parental separation and parent and cotwin substance dependence. Parent and cotwin alcohol and cannabis dependence were initially modeled separately, with post-hoc tests for equality. Where no significant differences were observed, twin alcohol and cannabis dependence were examined as a combined phenotype, that is, either alcohol or cannabis dependence. Parental separation was modeled as a time-varying covariate to ensure onset prior to or during the same year as initiation. Offspring from intact families were right-censored at age at last interview if younger than 18 years. In the case of parental death, intact families were right-censored at offspring age when their parent(s) died. Post-hoc tests of interactions between parental separation and parental substance dependence were also conducted. The Efron approximation (Efron, 1977) was used for survival ties. To examine potential violation of the PH assumption, the Grambsch and Therneau test of Schoenfeld residuals (Grambsch and Therneau 1994) was employed, with age-interactions modeled to correct observed violations (Cleves et al., 2004).
3. RESULTS
There were no differences in offspring age or sex by parental AD or cannabis dependence. Offspring of separated parents were slightly older (r=0.19, p<0.0001), with no differences in offspring sex by parental separation. Twin parents with a history of AD or cannabis dependence were slightly younger at the 1997–2002 assessment (r=−0.06 and −0.07, p<0.05, respectively), with fewer female twins than male meeting criteria for either disorder [OR=0.54 (95%CI: 0.42–0.70) and OR=0.55 (95%CI: 0.38–0.80), respectively]. Differences by parental separation in twin age or sex were nonsignificant. Compared to unaffected twins, rates of parental separation were higher for twins with a history of AD [OR=2.06 (95%CI: 1.58–2.69)] or cannabis dependence [OR=3.69 (95%CI: 2.55–3.85)]. Twin AD and cannabis dependence were moderately correlated (polychoric r=0.55, p<0.0001).
3.1. Survival Analyses
Results from three survival models for each substance use variable are presented in Tables 4–8, all of which include risk group based on parent and cotwin substance dependence (AD, cannabis dependence or a combined phenotype). In Model I, risk group only is modeled. In Model II, parental separation is included as an additional predictor, with control variables including coparent substance use/disorder added in Model III. Below we provide detailed summary for alcohol use, with results of Model III only summarized for alcohol intoxication, smoking, regular smoking, and cannabis use.
Table 4.
Hazard Ratios and (95% Confidence Intervals) from Cox Proportional Hazards Regression Models of First Alcohol Use
| Predictor (risk period) | Model Ia | Model IIb | Model IIIc |
|---|---|---|---|
| Risk Group | |||
| 1. Parent AD+ or CannD+ | 1.43 (1.18 – 1.74) | 1.29 (1.03 – 1.60) | 1.14 (0.88 – 1.47) |
| 2a. Parent AD−, MZ cotwin AD+ (< 14) | 2.82 (1.69 – 4.72) | 2.68 (1.60 – 4.50) |
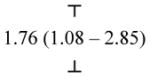
|
| 2b. Parent CannD−, MZ cotwin CannD+ (< 14) | 1.11 (0.62 – 2.01) | 0.93 (0.47 – 1.82) | |
| 2c. Parent UN, MZ cotwin AD+ or CannD+ (≥ 14) | 1.05 (0.54 – 2.01) | 0.93 (0.40 – 2.16) | |
| 3. Parent UN, DZ cotwin AD+ or CannD+ | 1.18 (0.88 – 1.58) | 1.22 (0.89 – 1.67) | 1.04 (0.72 – 1.50) |
| Parental separation (< 11) | -- | 14.86 (3.94 – 56.04) | 13.04 (2.84 – 59.95) |
| Parental separation (11 – 13) | -- | 2.76 (1.99 – 3.82) | 2.22 (1.58 – 3.14) |
| Parental separation (≥ 14) | -- | 1.49 (1.16 – 1.90) | 1.41 (1.06 – 1.88) |
Note. AD = alcohol dependence; CannD = cannabis dependence; UN = unaffected (neither alcohol nor cannabis dependent). Where brackets are shown, reported risks (HRs) are equivalent across risk periods. Post-hoc tests of interactions between parental separation and parental substance dependence were nonsignificant with one exception—through age 10, risks were further increased for offspring of separated alcoholic or cannabis dependent parents [HRModel II = 5.32 (95%CI: 1.11 – 25.48); HRModel III = 8.35 (95%CI: 1.22 – 57.19)].
Risk Groups 2a > 1, 2b, 2c and 3, p < 0.05.
controlling for demographic characteristics, coparent substance use/dependence, and psychiatric comorbidities of parents and offspring, Risk Groups 1=2=3, p > 0.10.
Table 8.
Hazard Ratios and (95% Confidence Intervals) from Cox Proportional Hazards Regression Models of First Cannabis Use
| Predictor (risk period) | Model Ia | Model IIb | Model IIIc |
|---|---|---|---|
| Risk Group | |||
| 1. Parent AD+ or CannD+ | 2.33 (1.63 – 3.33) | 1.97 (1.38 – 2.83) | 1.45 (0.92 – 2.29) |
| 2. Parent UN, MZ cotwin AD+ or CannD+ | 3.04 (1.68 – 5.52) | 2.64 (1.52 – 4.59) | 3.03 (1.64 – 1.61) |
| 3. Parent UN, DZ cotwin AD+ or CannD+ | 1.01 (0.50 – 2.01) | 1.01 (0.52 – 1.98) | 0.64 (0.29 – 1.44) |
| Parental separation (< 14) | -- | 5.09 (2.18 – 11.87) | 3.25 (1.36 – 7.73) |
| Parental separation (14 – 17) | -- | 1.67 (1.15 – 2.44) |
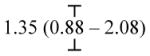
|
| Parental separation (≥ 18) | -- | 0.80 (0.31 – 2.10) | |
Note. AD = alcohol dependence; CannD = cannabis dependence; UN = unaffected (neither alcohol nor cannabis dependent). Where brackets are shown, reported risks (HRs) are equivalent across risk periods. Post-hoc tests of interactions between parental separation and parental substance dependence were nonsignificant with one exception—through age 13, risks were further increased for offspring of separated alcoholic or cannabis dependent parents [HRModel III = 5.53 (95%CI: 1.89 – 16.12)].
Risk Groups 1, 2 > 3, p < 0.05.
controlling for demographic, coparent substance use/dependence, and psychiatric comorbidities of parents and offspring, Risk Groups 2 > 1 > 3, p < 0.05.
3.1.1. Alcohol use
Results for first alcohol use are shown in Table 4. For all models, parent and cotwin alcohol and cannabis dependence could be equated in Groups 1 and 3, and Group 2 from age 14 onwards. In Model I, offspring of alcoholic or cannabis dependent parents (Group 1) were at 1.43 times higher risk of early drinking, compared to controls (Group 4). Through age 13, offspring of unaffected parents with an alcoholic identical cotwin (Group 2a) were at nearly three times greater risk of early drinking (HR=2.82), with little to no risk observed of offspring of unaffected parents with a cannabis dependent identical cotwin (Group 2b), nor offspring of unaffected parents with an identical cotwin who is either alcohol or cannabis dependent (Group 2c), the latter from age 14 onwards. Risk to Group 3 was also small and nonsignificant. In post-hoc tests, risk to Group 2a was greater than Groups 1, 2b, 2c, and 3, with nonsignificant differences among Groups 1, 2b, 2c and 3.
A similar pattern was observed when parental separation was included in Model II, with risks associated with parent or cotwin substance dependence reducing slightly. As with Model I, risk was higher for Group 2a than Groups 1, 2b, 2c and 3, with nonsignificant differences among Groups 1, 2b, 2c and 3. Through age 10, parental separation was associated with 14.86 times higher likelihood of early drinking. From ages 11–13, risks from parental separation predicted 2.76 times greater risk, and from age 14 onwards, approximately 50% greater risk.
In Model III, controlling for demographic characteristics, coparent substance use/dependence, and psychiatric comorbidities of both parents and offspring, risk to Groups 2a, 2b and 2c could be equated. While offspring of unaffected parents with an alcoholic or cannabis dependent identical cotwin were at 1.76 times higher risk of early drinking, and without age interaction, risk to Groups 1, 2 and 3 did not significantly differ in post-hoc tests. Parental separation remained a strong predictor of early drinking in Model III, associated with 13.04 times higher likelihood of drinking through age 10, and 2.22 times higher likelihood from ages 11–13; from age 14 onwards, parental separation was associated with 41% higher likelihood of drinking.
3.1.2. Alcohol intoxication
Results for first alcohol intoxication are shown in Table 5. For all models, and across risk group, parent and cotwin alcohol and cannabis dependence could be equated. In the fully adjusted Model III, risk to Group 2 was significant through age 13, with offspring of unaffected parents with a substance dependent identical cotwin (Group 2a) at 7.39 times greater risk of early intoxication, compared to controls (Group 4). From age 14 onwards, risk to Group 2 was elevated, but nonsignificant. Through age 13, parental separation was uniquely associated with 3.58 times higher likelihood of early intoxication, and from age 14 onwards, 1.56 times higher likelihood.
Table 5.
Hazard Ratios and (95% Confidence Intervals) from Cox Proportional Hazards Regression Models of First Alcohol Intoxication
| Predictor (risk period) | Model Ia | Model IIb | Model IIIc |
|---|---|---|---|
| Risk Group | |||
| 1. Parent AD+ or CannD+ | 1.76 (1.39 – 2.24) | 1.52 (1.20 – 1.93) | 1.32 (0.95 – 1.83) |
| 2a. Parent UN, MZ cotwin AD+ or CannD+ (< 14) | 7.62 (3.51 – 16.56) | 6.27 (3.08 – 12.79) | 7.39 (3.19 – 17.14) |
| 2b. Parent UN, MZ cotwin AD+ or CannD+ (≥ 14) | 1.45 (0.80 – 2.62) | 1.37 (0.73 – 2.55) | 1.70 (0.96 – 2.99) |
| 3. Parent UN, DZ cotwin AD+ or CannD+ | 1.23 (0.80 – 1.90) | 1.23 (0.81 – 1.86) | 1.04 (0.60 – 1.80) |
| Parental separation (< 14) | -- | 4.59 (2.37 – 8.90) | 3.58 (1.72 – 7.45) |
| Parental separation (≥ 14) | -- | 1.51 (1.21 – 1.89) | 1.56 (1.14 – 2.13) |
Note. AD = alcohol dependence; CannD = cannabis dependence; UN = unaffected (neither alcohol nor cannabis dependent). Post-hoc tests of interactions between parental separation and parental substance dependence were nonsignificant.
Risk Groups 2a > 1, 2b and 3, p < 0.05, Risk Groups 1 > 2b and 3, p < 0.10.
Risk Groups 2a > 1, 2b and 3, p < 0.05.
controlling for demographic characteristics, coparent substance use/dependence, and psychiatric comorbidities of parents and offspring, Risk Groups 2a > 1, 2b and 3, p < 0.05.
3.1.3. Cigarette use
Results for first cigarette use are shown in Table 6. For all models, parent and cotwin alcohol and cannabis dependence could be equated in Groups 2 and 3, and in Group 1 from age 9 onwards. In the fully adjusted Model III, through age 8, offspring of cannabis dependent parents (Group 1b) were at 7.30 times increased risk of early smoking, compared to controls (Group 4). However, offspring of alcoholic parents (Group 1a), were at 74% reduced risk of early smoking, also through age 8. From age 9 onwards, risk to offspring of alcoholic or cannabis dependent parents (Group 1c) was nonsignificant. Offspring of unaffected parents with a substance dependent identical cotwin (Group 2) were at 2.22 times greater risk of early smoking, with offspring of unaffected parents with a fraternal cotwin who is substance dependent (Group 3) at little to no risk relative to controls. Parental separation uniquely predicted 77% higher likelihood of early smoking without significant age interaction.
Table 6.
Hazard Ratios and (95% Confidence Intervals) from Cox Proportional Hazards Regression Models of First Cigarette Use
| Predictor (risk period) | Model Ia | Model IIb | Model IIIc |
|---|---|---|---|
| Risk Group | |||
| 1a. Parent AD+ (< 9) | 0.09 (0.01 – 0.89) | 0.82 (0.01 – 0.80) | 0.26 (0.09 – 0.82) |
| 1b. Parent CannD+ (< 9) | 9.64 (3.24 – 28.66) | 7.55 (2.53 – 22.50) | 7.30 (2.78 – 19.16) |
| 1c. Parent AD+ or CannD+ (≥ 9) | 1.83 (1.39 – 2.42) | 1.53 (1.13 – 2.07) | 1.26 (0.85 – 1.86) |
| 2. Parent UN, MZ cotwin AD+ or CannD+ | 2.14 (1.37 – 3.35) | 1.95 (1.27 – 2.99) | 2.22 (1.35 – 3.67) |
| 3. Parent UN, DZ cotwin AD+ or CannD+ | 1.00 (0.61 – 1.62) | 1.00 (0.62 – 1.61) | 1.10 (0.67 – 1.81) |
| Parental separation (< 14) | -- | 3.13 (2.21 – 4.43) |
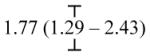
|
| Parental separation (≥ 14) | -- | 1.66 (1.13 – 2.41) | |
Note. AD = alcohol dependence; CannD = cannabis dependence; UN = unaffected (neither alcohol nor cannabis dependent). Where brackets are shown, reported risks (HRs) are equivalent across risk periods. Post-hoc tests of interactions between parental separation and parental substance dependence were nonsignificant.
Risk Groups 1b > 1a, 1c, 2 and 3, p < 0.05, and Risk Groups 1c, 2 > 3, p < 0.05.
controlling for demographic characteristics, coparent substance use/dependence, and psychiatric comorbidities of parents and offspring, Risk Groups 1b > 1a, 1c, 2 and 3, p < 0.05.
3.1.4. Regular smoking
Results for onset of regular smoking are shown in Table 7. For all models, and across risk group, parent and cotwin alcohol and cannabis dependence could be equated. In Model III, Group 2 offspring of unaffected parents with a substance dependent identical cotwin were at 2.72 times higher risk of regular smoking, compared to controls (Group 4). Parental separation was uniquely associated with 3.37 times higher likelihood of regular smoking through age 13 only.
Table 7.
Hazard Ratios and (95% Confidence Intervals) from Cox Proportional Hazards Regression Models of First Regular Smoking
| Predictor (risk period) | Model Ia | Model IIb | Model IIIc |
|---|---|---|---|
| Risk Group | |||
| 1. Parent AD+ or CannD+ | 1.88 (1.22 – 2.90) | 1.56 (1.00 – 2.45) | 1.16 (0.68 – 1.99) |
| 2. Parent UN, MZ cotwin AD+ or CannD+ | 3.03 (1.66 – 5.54) | 2.57 (1.48 – 4.47) | 2.72 (1.30 – 5.68) |
| 3. Parent UN, DZ cotwin AD+ or CannD+ | 1.29 (0.54 – 3.08) | 1.31 (0.56 – 3.06) | 1.15 (0.45 – 2.93) |
| Parental separation (< 14) | -- | 6.49 (2.30 – 18.38) | 3.37 (1.11 – 10.25) |
| Parental separation (≥ 14) | -- | 1.47 (0.93 – 2.33) | 0.98 (0.51 – 1.86) |
Note. AD = alcohol dependence; CannD = cannabis dependence; UN = unaffected (neither alcohol nor cannabis dependent). Post-hoc tests of interactions between parental separation and parental substance dependence were nonsignificant.
Risk Groups 1=2=3, p > 0.10.
controlling for demographic characteristics, coparent substance use/dependence, and psychiatric comorbidities of parents and offspring,, Risk Groups 2 > 1, p < 0.05.
3.1.5. Cannabis use
Results for first cannabis use are shown in Table 8. For all models, and across risk group, parent and cotwin alcohol and cannabis dependence could be equated. In the fully adjusted Model III, risk to Group 2 only was significant, with offspring of unaffected parents with a substance dependent identical cotwin at 3.03 times higher risk of early cannabis use, compared to controls (Group 4). Parental separation was uniquely associated with over three times higher likelihood of early cannabis use through age 13 (HR=3.25).
4. DISCUSSION
Despite well-documented associations between problem substance use and relationship instability, risks to COAs associated with parental separation or divorce have received limited empirical attention, and this is especially true of children of parents with other drug dependence. In the present study, we examined initiation of substance involvement as a function of parental separation using a Children-of-Twins (COT) design to control for both genetic and environmental risks associated with parental substance dependence. Although parental alcohol and cannabis dependence were initially modeled separately, with few exceptions, effects could be equated. Thus, for most models we subsequently examined risks from parental substance dependence broadly defined, i.e., parental history of alcohol or cannabis dependence.
Having a substance dependent parent was generally predictive of earlier onset alcohol use, drinking to intoxication, smoking, regular smoking, and cannabis use. However, across most substance use variables, offspring of unaffected parents with a substance dependent identical cotwin exhibited greater risk of early involvement than offspring of substance dependent parents. Risk to offspring of unaffected parents with a substance dependent fraternal cotwin was on average small and nonsignificant. While suggestive of genetic versus environmental transmission from parental substance dependence, a somewhat different pattern was observed for cigarette use. Risk to offspring of unaffected parents with a substance dependent identical (but not fraternal) cotwin was elevated in models of early smoking; however, risk was much greater for offspring of cannabis dependent parents, especially through age 8, than for offspring of alcoholic parents or unaffected parents. Thus, for very early use of cigarettes, results suggest an environmental mode of transmission from parental cannabis versus alcohol dependence, perhaps involving modeling of smoking behavior. Relatively permissive attitudes regarding underage substance use, particularly smoking, might also play a role.
Controlling for both genetic and environmental risks from parental substance dependence, parental separation was associated with early initiation across substance classes, with effects of separation most pronounced through ages 10 or 13. Controlling for parental separation only, a slight reduction in risks from parental substance dependence was observed, suggesting partial mediation at best. A greater reduction was observed with additional adjustment for demographic characteristics, coparent substance use/dependence, and psychiatric comorbidities of both parents and offspring. In most models, having a substance dependent parent was no longer predictive, except for onset of smoking as a function of parental cannabis dependence, described above. In contrast, effects of parental separation remained strong, suggesting parental separation confers unique risks beyond demographic, familial and individual-level influences highly correlated with parental substance dependence.
There are a number of limitations to our study suggesting cautious interpretation of findings, including incomplete assessment of major domains of risk. For example, we do not know why parents separated, and reason for relationship dissolution may have important implications. While post-hoc tests of interactions were largely nonsignificant, if substance dependence was the primary cause of separation and removal from the home of the alcoholic or other drug-addicted parent worked to reduce offspring risk, our understanding of parental separation as a risk-factor would need reconsidering. Data on prior separations is also limited, leaving open the possibility that some parents may have separated before final dissolution, and that parents in families coded as intact previously separated and since reconciled.
Regarding parental substance use or disorder, we assessed lifetime history of alcohol and cannabis dependence approximately ten years prior to offspring assessment. Because some parents may no longer meet criteria during childrearing years, our results likely underestimate risks from ongoing, chronic alcohol or cannabis use by twin parents. Additionally, we examined recurrent use of cannabis by coparents, defined as having used cannabis on 11 or more occasions lifetime, which may or may not reflect problem use, and may or may not be ongoing. Given that onset or remission data are not available for all twins, nor any coparents, measures of parenting behavior will be especially informative. Unfortunately, a direct measure of environmental exposure, or parenting “under the influence,” was not administered.
There are also a range of risks, both genetic and environmental, correlated with parental separation that remain unmeasured. It will be important for future research to examine more proximal risks from both parental substance dependence and separation in samples of sufficient size to parse potentially mediated or moderated effects. In the present study, statistical power was limited, including power to examine separately maternal versus paternal substance dependence and, in some cases, alcohol versus cannabis dependence. Lastly, participants are almost exclusively European ancestry, reflecting the predominantly Caucasian population from which twin parents were ascertained. Replication in a more diverse sample is critical, as is cross-national replication.
Despite these limitations, our findings underscore the importance of parental separation as a risk-factor for substance involvement independent of parental alcohol or cannabis dependence while highlighting very early adolescence as a particularly vulnerable period for children whose parents are separated, and, thus, a potential focus of targeted substance abuse prevention beyond family history.
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIAAA grants AA011998, AA07535, AA07729, AA0120249, AA015210, AA017688, NIDA grants DA023696 and DA14363, and NICHD grant HD049024; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Richard Parker and Monica DeNooyer for their assistance with data collection, and the twin families who participated in the studies.
Footnotes
Contributors
Authors Heath, Bucholz and Martin designed the study and Authors Heath, Bucholz, Glowinski and Waldron wrote the protocol. Author Waldron managed the literature searches and summaries of previous related work. Authors Waldron undertook the statistical analysis, and author Waldron wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. Family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chassin L, Rogosch F, Barrera M. Substance use and symptomatology among adolescent children of alcoholics. J Abnorm Psychol. 1991;100:449–463. doi: 10.1037//0021-843x.100.4.449. [DOI] [PubMed] [Google Scholar]
- Cleves M, Gould WW, Gutierrez RG. Introduction to Survival Data Analysis with Stata. Stata Press; College Station, TX: 2004. Revised Edition. [Google Scholar]
- Clark DB, Kirisci L, Moss HB. Early adolescent gateway drug use in sons of fathers with substance use disorders. Addict Behav. 1998;23:561–566. doi: 10.1016/s0306-4603(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Clark DB, Parker AM, Lynch KG. Psychopathology, substance use and substance related problems. J Clin Child Psychol. 1999;28:333–341. doi: 10.1207/S15374424jccp280305. [DOI] [PubMed] [Google Scholar]
- Doherty WJ, Needle RH. Psychological adjustment and substance use among adolescents before and after a parental divorce. Child Dev. 1991;62:328–337. doi: 10.1111/j.1467-8624.1991.tb01534.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Emery RE, Slutske WS, Heath AC, Madden PA, Martin NG. A genetically informed study of marital instability and its association with offspring psychopathology. J Abnorm Psychol. 2005;114:570–586. doi: 10.1037/0021-843X.114.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Emery RE, Maes HH, Silberg J, Eaves LJ. A Children of Twins Study of parental divorce and offspring psychopathology. J Child Psychol Psychiatry. 2007;48:667–675. doi: 10.1111/j.1469-7610.2007.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AE, Scherrer JS, Fu Q, Bucholz KK, Heath AC, True WR, Haber JR, Howell D, Jacob T. Exposure to paternal alcoholism does not predict development of alcohol-use disorders in offspring: Evidence from an offspring-of-twins study. J Stud Alcohol. 2006;67:649–656. doi: 10.15288/jsa.2006.67.649. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Maes HH. Revisiting the children of twins: Can they be used to resolve the environmental effects of dyadic parental treatment on child behavior? Twin Res Hum Genet. 2005;8:283–290. doi: 10.1375/1832427054936736. [DOI] [PubMed] [Google Scholar]
- Efron B. The efficiency of Cox’s likelihood function for censored data. J Am Stat Assoc. 1977;72:557–565. [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Parental separation, adolescent psychopathology, and problem behaviors. J Am Acad Child Adolesc Psychiatry. 1994;33:1122–1131. doi: 10.1097/00004583-199410000-00008. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Bertelsen A. Confirming unexpressed genotypes for schizophrenia. Arch Gen Psychiatry. 1989;46:867–872. doi: 10.1001/archpsyc.1989.01810100009002. [DOI] [PubMed] [Google Scholar]
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: Informativeness of different relationships. Behav Genet. 1985;15:439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Kirk KM, Madden PAF, Bucholz KK, Nelson EC, Slutske WS, Statham DJ, Martin NG. Predictors of non-response to a questionnaire survey of a volunteer twin panel: findings from the Australian 1989 twin cohort. Twin Res. 2001;4:73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- Herjanic B, Reich W. Development of a structured psychiatric interview for children: agreement between child and parent on individual symptoms. J Abnormal Child Psychol. 1982;10:307–324. doi: 10.1007/BF00912324. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA: a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann JP. The effects of family-structure and family-relations on adolescent marijuana use. Int J Addict. 1995;30:1207–1241. doi: 10.3109/10826089509105131. [DOI] [PubMed] [Google Scholar]
- Hoffman JP, Su SS. Parental substance use disorder, mediating variables and adolescent drug use: a non-recursive model. Addiction. 1998;93:1352–1364. doi: 10.1046/j.1360-0443.1998.93913516.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann JP, Johnson RA. A national portrait of family structure and adolescent drug use. J Marital Fam. 1998;60:633–645. [Google Scholar]
- Jacob T, Johnson S. Parenting influences on the development of alcohol abuse and dependence. Alcohol Health Res World. 2002;21:204–209. [PMC free article] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Jacob T, Waterman B, Heath A, True W, Bucholz KK, Haber R, Scherrer J, Fu Q. Genetic and environmental effects on offspring alcoholism: new insights using an offspring-of-twins design. Arch Gen Psychiatry. 2003;60:1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Overview of Key Findings, 2011. Institute for Social Research, The University of Michigan; Ann Arbor: 2012. Monitoring the Future National Results on Adolescent Drug Use. [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Walters EE, Forthofer MS. The social consequences of psychiatric disorders, III: Probability of marital stability. Am J Psychiatry. 1998;155:1092–1096. doi: 10.1176/ajp.155.8.1092. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Waldron M, Martin NG. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychol Med. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Risk domains associated with an adolescent alcohol dependence diagnosis. Addiction. 2001;96:629–636. doi: 10.1046/j.1360-0443.2001.96462911.x. [DOI] [PubMed] [Google Scholar]
- Labouvie E. Maturing out of substance use: selection and self-correction. J Drug Issues. 1996;26:457–476. [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Rutter M, Simonoff E, Pickles A, Carbonneau R, Neale MC, Eaves LJ. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Martin CS, Pollock NK, Cornelius JR, Chung TA. Nonproblem drinking outcomes in adolescents treated for alcohol use disorders. Exp Clin Psychopharmacol. 2002;10:324–331. doi: 10.1037//1064-1297.10.3.324. [DOI] [PubMed] [Google Scholar]
- McGue M. Genes, Environment and the Etiology of Alcoholism. National Institute on Alcohol Abuse and Alcoholism; Washington, DC: 1994. [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Nance WE, Corey LA. Genetic models for the analysis of data from the families of identical twins. Genetics. 1976;83:811–826. doi: 10.1093/genetics/83.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needle RH, Su SS, Doherty WJ. Divorce, remarriage, and adolescent substance use: a prospective longitudinal study. J Marriage Fam. 1990;52:157–169. [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Russell M. Prevalence of alcoholism among children of alcoholics. In: Windle M, Searles JS, editors. Children of Alcoholics: Critical Perspectives. Guilford Press; New York: 1990. pp. 9–38. [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Short J. Predictors of substance use and mental health of children of divorce: a prospective analysis. J Divorce Remarriage. 1998;29:147–166. [Google Scholar]
- Slutske WS, D’Onofrio BM, Turkheimer E, Emery RE, Harden KP, Heath AC, Martin NG. Searching for an environmental effect of parental alcoholism on offspring alcohol use disorder: a genetically informed study of children of alcoholics. J Abnorm Psychol. 2008;117:534–551. doi: 10.1037/a0012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. StataCorp LP; College Station, TX: 2011. [Google Scholar]
- Waldron M, Bucholz KK, Lynskey MT, Madden PAF, Heath AC. Alcoholism and timing of separation in parents: findings in a Midwestern birth cohort. J Stud Alcohol Drugs. 2013;74:337–348. doi: 10.15288/jsad.2013.74.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron M, Madden PAF, Nelseon EC, Knopik VS, Glowinski AL, Grant JG, Lynskey MT, Jacob T, Sher KJ, Bucholz KK, Heath AC. Interpretability of family history reports of alcoholism is general community samples: findings in a Midewestern U.S. twin birth cohort. Alcohol Clin Exp Res. 2012;36:1091–1098. doi: 10.1111/j.1530-0277.2011.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron M, Heath AC, Bucholz KK, Madden PAF, Martin NG. Alcoholic marriage: later start, sooner end. Alcohol Clin Exp Res. 2011;35:632–642. doi: 10.1111/j.1530-0277.2010.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron M, Martin N, Heath AC. Parental alcoholism and offspring behavior problems: findings in Australian children of twins. Twin Res Hum Genet. 2009;12:433–440. doi: 10.1375/twin.12.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


