Abstract
Advances in molecular-genetic tools for labeling neuronal subtypes, and the emerging development of robust genetic probes for neural activity, are likely to revolutionize our understanding of the functional organization of neural circuits. In principle, these tools should be able to detect activity at cellular resolution for large ensembles of identified neuron types as they participate in specific behaviors. This review describes the use of genetically encoded calcium indicators (GECI's), combined with two-photon microscopy, to characterize V1 interneurons, known to be critical for setting the duration of the step cycle. All V1 interneurons arise from a common precursor population and express Engrailed-1 (En1). Our data show that although neighboring interneurons arising from the same developmental lineages often share features, such as projection patterns and neurotransmitter profiles, they are not irrevocably committed to have the same pattern of activity.
Keywords: Engrailed-1, GCaMP3, two-photon
Introduction
In the nervous system, the interconnected activity of neurons comprising a circuit is transformed into a coherent output that drives behavior. A complete understanding of information processing in any mammalian neural circuit has proved difficult because it requires the integration of many disparate perspectives. Approaches used to piece together circuits include tracing techniques to define synaptic connectivity, electrophysiological recordings to investigate the properties of specific synapses, and biophysical experiments to examine how neurons compute their outputs. Nevertheless, there remains a large gap in our understanding of the relationship between activity at the cellular and circuit levels. Filling this gap requires recording the activity of many neurons simultaneously so that circuit level activity can be related to a behavioral output. Two emerging techniques that are being applied in this way are multi-electrode array recordings and neuronal activity imaging.
Extracellular recordings from electrode arrays have the advantage of high temporal resolution, although it is difficult to extract detailed information about the identities of the recorded neurons. Likewise, optical imaging with non-specific voltage or calcium dyes can be difficult to correlate with cell types and suffers further from weak signals and/or slow temporal responses of the dye. The gap in relating neuronal function to neuronal subtypes has long been acknowledged and is being addressed using molecular genetics to link gene expression patterns with cell types. In genetically-modifiable systems, such as that in the zebrafish and mouse, it is now possible to use genetically-encoded reporters expressed in neuron types to anchor the relationship between neuronal identity, connectivity, biophysical properties, and function. This approach has reached a new level of potential with the emergence of sensitive types of genetically-encoded calcium indicators (GECIs), such as the GCaMPs, which simplify the task of establishing the relationship between neuronal subtype identity and cellular activity within the context of a defined circuit.
The locomotor circuitry comprising the central pattern generator within the lumbar spinal cord is a well-suited system for understanding the cellular basis of circuit function. Because spinal motor circuits directly link the central nervous system (CNS) to behavior, the significance of motor activity is relatively easy to interpret. An intensive characterization of spinal cord development, gene expression profiling, and the development of genetic tools, such as Cre lines for labeling and toxins for ablating specific cell types in the spinal cord, has led to the identification of neurons that are required for locomotor function, and the creation of genetic tools that can be leveraged to study the cellular constituents of locomotor circuitry.1
Two-photon imaging with GECIs
Optical detection of neuronal activity at a cellular resolution has been achieved with a variety of indicators for either voltage or calcium.2 Of the available indicators, calcium-binding molecules generate the largest changes in fluorescent signals. When paired with the deep tissue imaging potential of fast scanning two-photon (2P) microscopes, calcium imaging offers a powerful compromise between spatial and temporal resolution, and sensitivity. Cellular resolution imaging studies of mammalian spinal locomotor circuits have used 2P calcium imaging to monitor the activity of neuron subtypes using genetically-encoded fluorescent reporters, which allow labeling of molecularly-defined neurons in combination with non-specific bolus loading of calcium dyes. This approach has been used to characterize the activity of Hb9-expressing interneurons, Chx10-expressing V2a excitatory interneurons, and lamina V/VI gamma aminobutyric acid (GABA)-ergic interneurons during fictive locomotion.3–6
While these early experiments have mainly been used to complement electrophysiological observations and to explore technical aspects of imaging and data analysis, their observations highlight the promise of imaging locomotor network activity. One common observation emerging from imaging studies is that even very small regions of the spinal cord seem to contain neurons oscillating in a variety of phases. For example, neighboring Chx10-expressing V2a interneurons, as well as unidentified lamina VII/VIII interneurons, can be rhythmically active but in distinct phases relative to motor output.5,7 A major advantage of imaging is that oscillations in neighboring neurons can be readily compared. Studies that imaged groups of Hb9-expressing interneurons during successive bouts of fictive locomotion found that individual Hb9 interneurons are active only in a subset of locomotor cycles.3 Although Hb9 interneuron oscillations were sparse at the level of single and small groups of cells, it remains to be determined how Hb9 interneuron activity at a population level relates to motor output.
Despite success using 2P microscopy to image single cells deep within the spinal cord, this approach also has drawbacks that limit the detailed characterization of locomotor circuitry. Key among these are the difficulty in labeling all of the relevant neurons using non-specific bolus loading of dyes, and the trade-offs between field of view and frame rate inherent in laser scanning microscopy. For these reasons, we have explored the use of fast scanning 2P imaging in combination with GECIs.
A new generation of promising GECIs has become available during the last few years. Genetic sensors of neuronal activity have long been sought as a tool by neuroscientists, but have required multiple iterations of development to produce GECIs that detect calcium at physiological levels without being toxic, while producing fluorescent signals that are easily separated from noise. The early sensors were based on fusing the calcium-binding protein, calmodulin, between two different fluorescent proteins whose fluorescence resonance energy transfer efficiency changed when calcium was bound.8,9 New, more sensitive, forms of GECIs have been created by fusing calmodulin to circular permutations of fluorescent proteins, such as green fluorescent protein (GFP), to create a series of probes with different sensitivities and spectral qualities. Structure-guided evolution of these probes continues to produce variants with improved kinetics and fluorescence changes on calcium binding.10 For example, the widely adopted GECI, GCaMP3, reliably reports even small groups of two to three spikes in vitro and in vivo.11 GCaMP3-based experiments have allowed examination of neural activity over long periods,12 and large areas13 of the vertebrate brain. The ongoing development of GCaMP sensors recently lead to the description of a family of improved GCaMP5 sensors.10 As the development of GECI molecules continues, neuroscientists will have access to indicators with spectral, affinity, and kinetic properties tailored to a specific experiment. A variety of vehicles may be employed to deliver GECIs into neurons including viruses, transgenic mice, and recombinase-dependent systems, which allow flexible integration of GECI expression across the gamut of experimental designs. Moreover, GECI's may be readily utilized in animals carrying other mutations that alter locomotor activity or to study wild type locomotion.
A primary advantage of GECIs is that they allow specific, reproducible, population-wide labeling with the reporter. In circuit studies, the genetic markers used to identify a neuron of interest may or may not have any direct functional bearing on the cell; nevertheless, they serve as a proxy for studying the labeled neuron. For example, Engrailed-1 (En1) has been used extensively as a tool to label and study spinal interneurons arising from the V1 cell class.14,15 While En1 is dispensable for studying many properties of V1 interneurons,16 it has been invaluable for characterizing the development and connectivity of the ancestrally-related V1 population. The advent of GECIs with En1 gene drivers now provides a means to study neuronal activity of the V1 population during locomotion. For example, mice expressing Cre recombinase under the control of the En1 promoter may be crossed with a Cre-dependent GCaMP3 mouse available to the research community17 (ROSA:Lox-STOP-Lox-GCaMP3) to allow GCaMP3 expression to be maintained specifically in V1 interneurons from embryonic development into adulthood (abbreviated En1:GCaMP3 mice). Viral-based GECI expression strategies may also be used to image specific interneuron subsets. However, these approaches necessitate the invasive injection of a virus into the animal several days prior to imaging, complicating early postnatal experiments. Furthermore, much like the bolus loading of cell permeable dyes, viral-driven expression of GECIs will lead to variable indicator expression in a small area of the CNS surrounding the injection site. For these reasons, we have focused on a genetic GECI expression system to stably and reproducibly express the indicator throughout the CNS.
Genetic labeling strategies can take advantage of the wide variety of Cre lines driving expression in specific sets of neurons. One important starting point are Cre drivers for the major ventral interneuron domains: V0 Dbx1:Cre, V1 En1:Cre, V2a Chx10:Cre, VMN Hb9:Cre, Isl1:Cre, and V3 Sim1:Cre. Genetic strategies may also take advantage of expression patterns defined by many other genes, including those of neurotransmitters, axon guidance and cell adhesion molecules, as well as transcription factors not directly related to the developmental classes of spinal neurons.1,18 A variety of exploratory online tools such as the Gensat project (www.gensat.org/cre.jsp), Allen Institute gene expression atlas (mouse.brain-map.org/), and a comprehensive list of Cre lines in published research (www.informatics.jax.org/recombinase.shtml) are available to facilitate the development of mouse genetic expression strategies for cell types of interest.
From photons to physiology
The use of imaging to monitor neuronal activity in large numbers of identified cells offers unprecedented opportunities, but these types of experiments generate large multidimensional data sets that introduce new technical challenges. Analysis of calcium imaging data generally begins with choosing regions of interest (ROIs). Manually drawing ROIs can introduce biases into the sampling and becomes tedious with large cell populations. A solution is to design algorithms that automatically identify ROIs based on neuron morphology.19 We have found methods based on grouping pixels with similar intensity fluctuations over time to be particularly powerful for extracting interesting signals from our data sets.20–23
Cell type–specific GECI labeling facilitates automated morphological approaches by restricting labeling to particular neural classes, increasing the contrast between neurons of interest and the surrounding neuropil. In cases where neurons are recruited sparsely within a population, ROI detection by neuronal morphology may be advantageous. By segmenting images independent of time series, information from morphology-based approaches will similarly detect silent and active neurons. In contrast, segmentation methods based on time series information generally rely on the presence of non-noise signals recorded on particular pixels. For example, silent neurons may be grouped with background noise. Correlated activity between pixels or procedures, such as independent component analysis, can group pixels with related activity patterns. Because time series–based methods are designed to detect similar patterns of activity from pixels, individual neurons with similar activity profiles are often grouped, requiring a final morphology-based approach to break cell groups into individual somata.17 While it is intuitive to segment imaging data into individual neurons, it is also important to characterize groups of neurons with similar activity patterns. Our preliminary results indicate that a combination of principal component analysis and k-means clustering may be used to detect groups of spinal motoneurons with related activity, bypassing the need to segment images into single cells.23
Once imaging data is segmented into specific ROIs, further analyses can be performed comparing the detected regions. To date, spinal cord imaging has focused on identifying rhythmically-active neurons coupled to particular phases of motor output. Methods based on continuous wavelet transforms24 and coherence analysis7 can be used to automate detection of common frequencies and to identify oscillation phase relationships between single neuron calcium signals and electrically-recorded motoneuron firing.
Because calcium signals area a reflection of neuronal spiking,11,25 their waveforms can be used to infer important information about the firing patterns of neurons. While waveform analysis of GECI signals may be complicated by slow kinetics, non-linear responses, and the difficulty of comparing signal amplitudes between neurons, a variety of information may be extracted from these neural activity–derived signals. Because the slow decay kinetics of sensors results in summing of fluorescence when a train of spikes occurs, the relative position of a calcium oscillation peak is related to the timing of the final spike in a burst of firing in the imaged neuron. Likewise, despite the difficulty comparing signal amplitudes between neurons, it remains possible to extract novel information about a single neuron's circuit integration by correlating its signal amplitude over time with other neurons (Fig. 1D).
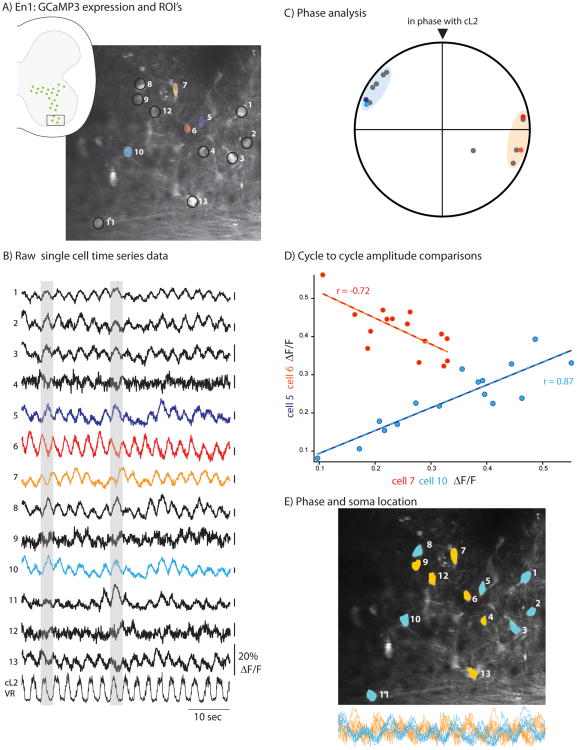
Figure 1.
Rhythmic activity and heterogeneity among neighboring V1 interneurons. (A) Fluorescence image of upper lumbar, ventral horn En1:GCaMP3 neurons imaged through the ventral white matter in a P1 mouse. Thirteen neurons were circled as ROI in this 300 × 300 mm field of view. Lateral is up. Inset illustrates En1:GCaMP3 expression in upper lumbar spinal cord. Box indicates approximate location of imaged neurons within the ventral horn ∼50 μm deep within the ventral grey matter. (B) Time series traces of fluorescence intensity fluctuations from ROIs defined in A. Cells 5, 6, 7, and 10 are highlighted in panels C and D. During neurochemically-induced fictive locomotion, 12/13 interneurons in this field of view showed rhythmic activity significantly phase locked to electrically-recorded ventral root output (bottom trace). Oscillation amplitudes varied between 10–60% ΔF/F. Grey shading highlights two arbitrary cycles of activity to clarify phase relationships between the imaged neurons. (C) The phase of V1 interneuron bursts identified in 1B cluster into two groups. Phase relationships relative to the ventral root were calculated from smoothed imaging traces. Each group contains similar numbers of cells and are ∼180 degrees out of phase. In this example, 7/13 neurons are approximately in phase with the cyan neuron (blue shading), whereas the remaining six neurons are out of phase with the cyan neuron (orange shading). (D) Pair-wise cell comparisons of relative burst amplitudes. Trough to peak amplitudes were calculated for each locomotor cycle in each cell and plotted against one another; linear regressions determined whether correlated amplitude fluctuations were present between neurons. A high amplitude oscillation in neuron 10 (cyan) predicts a high amplitude oscillation in neuron five (blue, R = 0.87). In contrast, high amplitude oscillations in neuron seven (orange) predict a low amplitude oscillation in neuron six (red, R = -0.72). (E) Neighboring V1 interneurons are not spatially segregated by phase. ROIs were color coded according to the phase groups identified in C. Neighboring V1 interneurons were often active in opposite phases (e.g., cells five and six) such that no clear spatial segregation was noticed. Superimposed, expanded traces from all 13 neurons in the field of view are shown below the image. Traces are color coded according to their phase group from C, highlighting the two alternating cell groups in the field of view. Imaging trace amplitudes scaled arbitrarily, scale bar 10 s.
Rhythmic activity in the V1 interneuron population
The cardinal progenitor cell domains, pV0–pV3, generate multiple ventral neuron subtypes, including V0, V1, V2a, V2b, and V3 interneurons. We have examined the ensemble activity of En1+ V1 interneurons, which, although they arise from a common progenitor population, represent a mixture of incompletely defined interneuron subtypes that include at least two cell groups first identified in classic studies using electrophysiology: Renshaw cells and 1A inhibitory interneurons,26,27 both of which provide monosynaptic inhibitory inputs onto motor neurons. Renshaw cells receive collateral inputs from motor neurons, and dampen activity in homonymous and synergistic motor neurons. Likewise, 1A inhibitory interneurons dampen motor activity, but target antagonistic motor pools as part of a simple reflex circuit that includes 1A sensory afferents. The precise contribution of Renshaw cells and 1A inhibitory interneurons to locomotor network activity and behavior remains to be determined, but it is clear that the burst duration of the step cycle is elongated when the function of the entire V1 cell population is disrupted, or when cholinergic feedback from motor neurons to Renshaw cells is prevented.14,28 Thus, the activity of V1 cells influences the rate of fictive stepping.
To investigate the activity of En1-expressing interneurons, the expression of GCaMP3 was targeted to the V1 lineage using the En1:GCaMP3 mouse described above. We have found that the expression of GCaMP3 driven from the ROSA locus did not cause obvious fictive locomotor phenotypes in animals where recombination was activated in V1 interneurons (data not shown). Likewise, we have generated CMV:GCaMP3 transgenic mice that express high levels of GCaMP3 throughout all tissues in embryos and adults, and did not observe fictive locomotor phenotypes as a byproduct of widespread expression of this calcium binding sensor (unpublished observations, K. Hilde, C.A. Hinckley, and S.L. Pfaff).
We have noticed fewer labeled neurons with the ROSA:Lox-STOP-Lox-GCaMP3 allele compared to similar ROSA-targeted conditional reporter strains (e.g., ROSA:Lox-STOP-Lox-tdTom) when crossed to a variety of cell-specific Cre lines labeling spinal neurons (data not shown). A between-animal comparison of GCaMP3 and tdTomato recombination efficiency with a robust Cre driver suggested that 50–75% of the neurons observed with tdTomato would clearly express GCaMP3. Whether these differences reflect low expression of GCaMP3 in a subset of neurons or subtle differences in the recombination efficiencies of the ROSA-targeted transgenes remains unknown. Consistent with previous studies,14,29 we have not observed any clear patterns of bias or a lack of specificity in En1:Cre–driven GCaMP3 recombination, suggesting that Cre-dependent expression of GCaMP3 is a powerful tool to examine the activity of defined neuron populations with cellular resolution. Even though baseline GCaMP3 fluorescence is dim relative to GFP, we have generally been able to identify individual GCaMP3-expressing En1+ neurons in the quiescent spinal cord. Although it was necessary to use Ti:sapphire laser powers several fold higher than those for GFP to image GCaMP3 fluorescence, we routinely image individual fields of view for 100 s without significant bleaching or photo damage.
We investigated the activity heterogeneity of upper lumbar (L2) V1 interneurons during neurochemically-induced fictive locomotion. The activity of individual En1:GCaMP3 neurons in the Renshaw cell area16 (Fig. 1A inset) were imaged through the ventral surface of isolated spinal cord at postnatal day 0–2 using 2P microscopy. Following pharmacological induction of fictive locomotor activity with N-methyl-D,L-aspartate (NMA) and serotonin-generated robust GCaMP3 fluorescence, oscillations in V1 interneurons routinely exceeded 30% ΔF/F, similar to other reports using the conditional CGaMP3 reporter mouse17 (Fig. 1A and 1B). These observations suggest that GCaMP3 has the required sensitivity and kinetics to resolve locomotor oscillations with single cell resolution within an en bloc tissue preparation.
Preliminary analyses have extensively characterized the activity heterogeneity among 13 GCaMP3+ V1 interneurons present in a single 300 × 300 μm optical section, ∼50 μm deep in the ventral grey matter, scanned at 13–24 frames/s. Similar types of heterogeneity were evident in a set of experiments (n = 4; see below) where ROIs were manually drawn in image J, while subsequent phase and amplitude measurements were performed on 0.1–1.0 Hz band pass filtered traces by custom written scripts in IGOR Pro (WaveMetrics, Oregon). Changes in rhythmic fluorescence intensity, phase locked to ventral root bursting, were detected (P < 0.01 for r > 0.6, Rayleighs test) in 12 out of 13 cells (92%) in the presence of locomotor-evoking drugs (Fig. 1B, supplementary video). While the amplitude of oscillations varied among these neighboring V1 interneurons, even V1 interneurons with low signal-to-noise oscillations (e.g., cells four and nine) retained tight coupling to electrically-recorded ventral root activity (r = 0.87 and 0.89, respectively).
Next, we examined the phase relationship of the rhythmic V1 interneuron oscillations and found two dominant patterns. Seven cells were clustered around a similar bursting phase to cells five and 10 (Fig. 1C, blue shading), whereas six cells displayed bursting patterns that were ∼180 degrees out of phase compared to this grouping (Fig.1C, orange shading), a bi-phasic distribution of bursts observed with single-cell recordings of Renshaw cell activity during fictive locomotion.30 This preliminary example suggests that GECI-based imaging can recapitulate phase measurements from whole cell recordings;30 however, further analysis will be necessary to determine whether all fields of view contain similar heterogeneity. In these studies, the cells were distributed in nearly equal numbers in the two phasegroups. One interpretation is that the Renshaw cells, and more generally, the V1 interneuron population studied here, are linked to the opposing flexor-extensor activity and therefore fall into two phase groups.
Although this interpretation is a strong possibility, there are several observations that suggest that this bi-phasic pattern of activity may be associated with a different layer of CPG regulation. First, the finding that the number of L2 V1 interneurons is evenly distributed between the two phases seems inconsistent with the flexor-dominated L2 motor output. Second, genetic ablation of V1 interneuron function altered the bursting rate of the CPG and failed to cause defects in flexor-extensor coordination.14 It will be intriguing to monitor the pattern of V1 interneuron activity in mutants that lack flexor-extensor control in order to determine whether V1 activity can be dissociated from the circuitry that controls flexion and extension.
Waveform analysis: circuit integration from GECI imaging
It is possible that additional heterogeneity could be identified among V1 interneurons by extending our analysis beyond the characterization of phase relationships. For example, the examination of GECI waveforms might help identify neurons similarly integrated into locomotor circuits because neurons with common sources of synaptic drive will likely have highly correlated activity. To investigate this possibility, the cycle-to-cycle fluctuations in the GCaMP3 signal amplitude were analyzed, which were thought to vary depending on the amount of synaptically-driven spiking during a cycle. Pair-wise comparisons were performed between this set of 13 interneurons and examples were found of cells whose bursts were strongly correlated in amplitude, either positively or negatively (Fig. 1D). Interestingly, cells with in-phase activity could have positive or negative correlations. Future experiments will determine the precise relationship between spike numbers and GCaMP3 signals. The most parsimonious explanation for these findings is that spike numbers numbers/rates may be modulated independently of oscillation phase. Because the V1 interneurons sharing the same burst phase could be further divided into groups that were positively, negatively, and non-significantly correlated with burst amplitudes, it is clear that the activity patterns of V1 cells are more heterogeneous than would be suggested by phase comparisons alone.
Finally, this 300 × 300 μm field of view was examined for spatial organization among V1 interneurons that were active during the two main phases. In contrast to the organization of motoneurons in functionally-related homonymous motor pools, initial analyses suggest that V1 interneurons active in different phases were intermingled (Fig. 1E). Amplitude correlations may provide another measure of network-driven activity that could be used to examine whether neighboring neurons have similar activity (Fig. 1D). For example, neurons with highly correlated cycle-to-cycle amplitude fluctuations may spatially segregate from uncorrelated neurons.
Because only small fields of view (300 × 300 μm) have been examined, the large-scale organization among V1 interneurons remains unknown. Motoneurons, the ultimate targets of locomotor networks, are highly spatially organized, segregating into stereotyped columns and pools that spread rostro-caudally over multiple spinal segments.31,32 It would be reasonable to predict that any spatial organization among spinal interneurons would reflect this rostro-caudal columnar pattern. Therefore, reconstructing neural activity over multi-segment regions of the spinal cord is a likely prerequisite to uncover any spatial organization in the network.
Conclusion
The preliminary work presented here is intended to illustrate our approach to analyzing cell type–specific imaging data. A surprising degree of heterogeneity has been detected in the activity of V1 interneurons by comparing the phase of rhythmic oscillations and correlations of amplitude between neighboring interneurons. In the same way that differences in oscillation phase can be attributed to differences in synaptic drive, we hypothesize that differences in amplitude modulation and imaging signal waveforms are likely to reflect distinct patterns of network integration. If this interpretation is correct, these novel measures provide an activity-based readout of the heterogeneity within a cell population. The observation of many distinct functionally-related groups within a defined neural class would suggest that the spinal locomotor network is built up from numerous semi-independent modules.
Our preliminary experiments indicate that neurons from a common genetically-defined lineage are specified such that even neighboring neurons can differentially integrate into locomotor networks. Our observation that similar numbers of neurons fall into two distinct phase groups may suggest a tightly regulated developmental process producing equal numbers of functionally defined V1 subtypes in a specific area, similar to the balanced production of V2a and V2b subtypes from a common progenitor domain.33 This result is perhaps most surprising because, based on their ventral location, many of the V1 neurons sampled are likely Renshaw cells, which are generally believed to be synaptically driven by nearby motoneurons. Given that the fictive locomotor output from L2 motoneurons is dominated by a single phase of motoneuron activity, local motoneuron input seems unable to drive two equal anti-phase populations of V1 Renshaw cells. Alternatively, this heterogeneity could reflect spatial intermixing between distinct subtypes of V1 interneurons. Our experiments point to the promise of GECI-based imaging to uncover functional heterogeneity within molecularly-defined cell populations. Future experiments will systematically examine many aspects of V1 interneuron activity over larger areas of the lumbar spinal cord, potentially providing clues about how these neurons contribute to locomotor networks.
Supplementary Material
Raw and processed images from the cells analyzed in Figure 1 replayed at 5× speed. Top Left: Raw images from data acquisition. Top Right: Raw images filtered with the 3D hybrid median filter in image J. This filter uses adjacent averaging to reduce spatial and temporal noise. Bottom Left: Pixel by pixel 1Hz low pass filter on raw images. This image approximates the preprocessing used to extract phase and amplitude information. Bottom Right: Same 1Hz low pass filtration as bottom left with each pixel's minimum intensity value set to zero by subtraction.
Acknowledgments
We thank Ariel Levine for helpful comments. Conditional GCaMP3 mice were provided by Hongkui Zeng, The Allen Institute for Brain Science; Engrailed-1 Cre mice are available from Jackson Labs, line 007916. C.A.H. is supported by an NINDS NRSA fellowship and S.L.P. is Benjamin H. Lewis Chair in Neuroscience and Investigator with the Howard Hughes Medical Institute.
References
- 1.Alaynick WA, Jessell TM, Pfaff SL. SnapShot: spinal cord development. Cell. 2011;146:178–178.el. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knöpfel T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nature reviews Neuroscience. 2012;13:687–700. doi: 10.1038/nrn3293. [DOI] [PubMed] [Google Scholar]
- 3.Kwan AC, Dietz SB, Webb WW, Harris-Warrick RM. Activity of Hb9 interneurons during fictive locomotion in mouse spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:11601–13. doi: 10.1523/JNEUROSCI.1612-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson JM, Dombeck DA, Díaz-Ríos M, Harris-Warrick RM, Brownstone RM. Two-photon calcium imaging of network activity in XFP-expressing neurons in the mouse. Journal of neurophysiology. 2007;97:3118–25. doi: 10.1152/jn.01207.2006. [DOI] [PubMed] [Google Scholar]
- 5.Zhong G, et al. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:170–82. doi: 10.1523/JNEUROSCI.4849-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson JM, Blagovechtchenski E, Brownstone RM. Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:1137–48. doi: 10.1523/JNEUROSCI.1401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwan AC, Dietz SB, Zhong G, Harris-Warrick RM, Webb WW. Spatiotemporal dynamics of rhythmic spinal interneurons measured with two-photon calcium imaging and coherence analysis. Journal of neurophysiology. 2010;104:3323–33. doi: 10.1152/jn.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romoser VA, Hinkle PM, Persechini A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. The Journal of biological chemistry. 1997;272:13270–4. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- 9.Miyawaki A, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–7. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 10.Akerboom J, et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. Journal of Neuroscience. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber D, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–8. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahrens MB, et al. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 2012;485:471–7. doi: 10.1038/nature11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosgnach S, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–9. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 15.Benito-Gonzalez A, Alvarez FJ. Renshaw cells and Ia inhibitory interneurons are generated at different times from p1 progenitors and differentiate shortly after exiting the cell cycle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:1156–70. doi: 10.1523/JNEUROSCI.3630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapir T, et al. Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1255–64. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zariwala HA, et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:3131–41. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Campmany L, Stam FJ, Goulding M. From circuits to behaviour: motor networks in vertebrates. Current opinion in neurobiology. 2010;20:116–25. doi: 10.1016/j.conb.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 20.Ozden I, Lee HM, Sullivan MR, Wang SSH. Identification and clustering of event patterns from in vivo multiphoton optical recordings of neuronal ensembles. Journal of neurophysiology. 2008;100:495–503. doi: 10.1152/jn.01310.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miri A, Daie K, Burdine RD, Aksay E, Tank DW. Regression-based identification of behavior-encoding neurons during large-scale optical imaging of neural activity at cellular resolution. Journal of neurophysiology. 2011;105:964–80. doi: 10.1152/jn.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–60. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinckley C, Driscoll S, Pfaff S. Large scale imaging and reconstruction of spinal motoneuron activity. Soc Neurosci Abstract. 2012;788.23 [Google Scholar]
- 24.Gallarda BW, Sharpee TO, Pfaff SL, Alaynick WA. Defining rhythmic locomotor burst patterns using a continuous wavelet transform. Annals of the New York Academy of Sciences. 2010;1198:133–9. doi: 10.1111/j.1749-6632.2010.05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smetters D, Majewska A, Yuste R. Detecting action potentials in neuronal populations with calcium imaging. Methods (San Diego, Calif) 1999;18:215–21. doi: 10.1006/meth.1999.0774. [DOI] [PubMed] [Google Scholar]
- 26.Renshaw B. Influence of Discharge of Motoneurons Upon Excitation of Neighboring. Journal of neurophysiology. 1941;4:167–183. [Google Scholar]
- 27.Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. The Journal of physiology. 1954;126:524–62. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers CP, et al. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron. 2005;46:37–49. doi: 10.1016/j.neuron.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Zariwala HA, et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:3131–41. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimaru H, Restrepo CE, Kiehn O. Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:5320–8. doi: 10.1523/JNEUROSCI.5127-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanes GJ. The motor cell columns of the lumbo-sacral spinal cord of the cat. The Journal of comparative neurology. 1951;94:313–63. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- 32.McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- 33.Peng CY, et al. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53:813–27. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw and processed images from the cells analyzed in Figure 1 replayed at 5× speed. Top Left: Raw images from data acquisition. Top Right: Raw images filtered with the 3D hybrid median filter in image J. This filter uses adjacent averaging to reduce spatial and temporal noise. Bottom Left: Pixel by pixel 1Hz low pass filter on raw images. This image approximates the preprocessing used to extract phase and amplitude information. Bottom Right: Same 1Hz low pass filtration as bottom left with each pixel's minimum intensity value set to zero by subtraction.