Abstract
Brassinosteroids (BRs) regulate various physiological processes, such as tolerance to stresses and root growth. Recently, a connection was reported between BRs and nitric oxide (NO) in plant responses to abiotic stress. Here we present evidence supporting NO functions in BR signaling during root growth process. Arabidopsis seedlings treated with BR 24-epibrassinolide (BL) show increased lateral roots (LR) density, inhibition of primary root (PR) elongation and NO accumulation. Similar effects were observed adding the NO donor GSNO to BR-receptor mutant bri1-1. Furthermore, BL-induced responses in the root were abolished by the specific NO scavenger c-PTIO. The activities of nitrate reductase (NR) and nitric oxide synthase (NOS)-like, two NO generating enzymes were involved in BR signaling. These results demonstrate that BR increases the NO concentration in root cells, which is required for BR-induced changes in root architecture.
Keywords: Arabidopsis, brassinosteroids, nitric oxide, root morphology
Brassinosteroids (BRs) are polyhydroxylated steroid hormones ubiquitously distributed throughout the plant kingdom.1 BRs regulate various physiological processes such as cell elongation, vascular differentiation, responses to light, senescence, root growth and resistance to stresses (for a review see ref. 2). BR-mediated regulation of stress tolerance might be integrated in a cross-talk between BRs and other hormone signaling pathways.3 Moreover, Arabidopsis microarray analyses have identified numerous and diverse BR-regulated genes, suggesting that BR might trigger a complex regulatory network.4,5 Nitric oxide (NO) is a ubiquitous bioactive molecule produced in plants by enzymatic and non-enzymatic routes. The two major enzymatic sources of NO production reported in plants are a nitrate reductase (NR; EC 1.6.6.1) and a nitric oxide synthase (NOS)-like activity.6,7,8,9 NR is the only enzyme whose NO-producing activity has been rigorously confirmed both in vivo and in vitro.10,11 NO synthase (NOS) catalyzes the conversion of L-Arginine to L-citrulline and NO.12 A NOS-like activity has been strongly demonstrated by pharmacological studies in plants using the different substrates or inhibiting of NO production by mammalian NOS inhibitors.13-16
Foresi et al.16 provide compelling evidence for the existence of a canonical NOS enzyme in the unicellular algae Ostreococcus tauri. This work characterizes the NOS gene, the protein structure and activity in vivo and in vitro of the recombinant protein. NO orchestrates a wide range of processes in plants. NO acts as a signal in disease resistance, stimulates germination, confers protection against oxidative stress produced by diquat, drought, salt and UV-B and has been proposed as a broad-spectrum anti-stress molecule.17-21
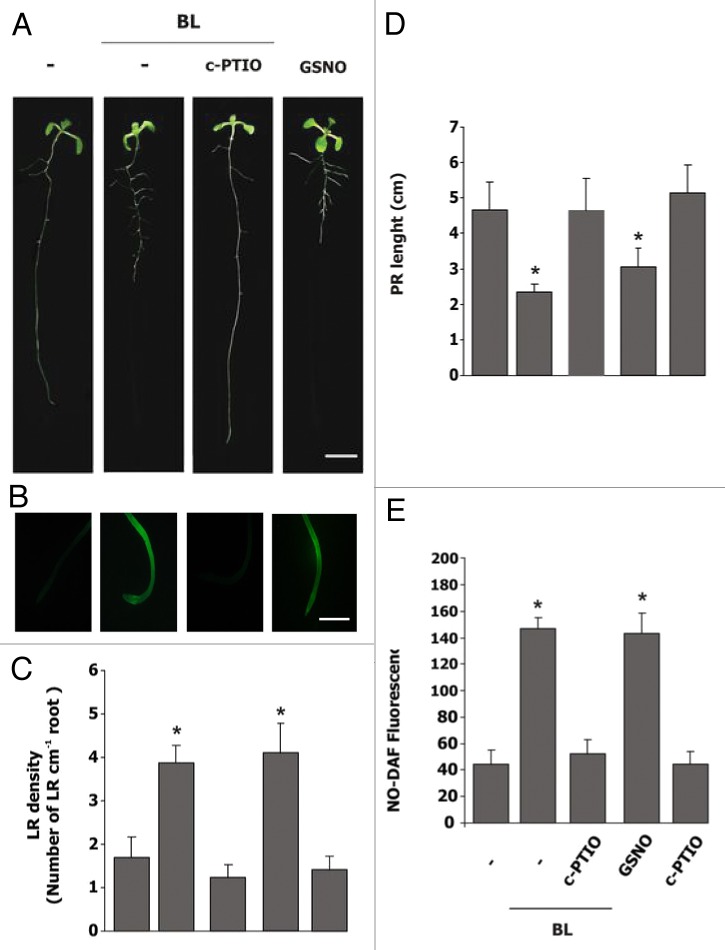
Two recently published articles reported a connection between BRs and NO in plant responses to abiotic stress. In the first article, Cui and collaborators22 showed that BRs induce NO in a H2O2-dependent manner, alleviating photo-oxidative stress and chilling in cucumber leaves. In the second article, Zhang and collaborators23 reported that BR-induced NO production mediates ABA biosynthesis, which results in the enhancement of tolerance to oxidative damage caused by water stress in maize leaves. Here, we provide evidence that NO also participates in plant growth and developmental processes regulated by BRs. The link between BR and NO was analyzed in Arabidopsis root. It was reported that 10 nM 2,4-epibrassinolide (BL, one of the most potent BRs) inhibits primary root (PR) growth and induces lateral roots (LR) formation in Arabidopsis, increasing the LR density.24,25 Simultaneously, Correa-Aragunde et al.26 reported that NO also induces LR formation and inhibits PR growth in tomato. Thus, we decided to analyze a potential NO requirement for BR regulation of root growth and architecture determination. Figure 1A shows that root phenotype of Col-0 Arabidopsis seedlings treated with 10 nM BL was similar to those produced by 200 µM of NO donor nitrosoglutathione (GSNO). This phenotype was characterized by shortened PR and increased LR number. Notoriously, these responses were completely abolished when BL was applied together with NO scavenger 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide(c-PTIO), suggesting that NO could be participating downstream BL. Figure 1B shows that both BL and GSNO induced a NO burst in the roots, visualized by the cell-permeable fluorescent probe DAF-FM DA. The BL-mediated NO burst was prevented by c-PTIO (Fig. 1B).
Figure 1. 2,4-epibrassinolide induces lateral root (LR) formation and inhibits primary root (PR) elongation in a nitric oxide-dependent process in Arabidopsis. Col-0 Arabidopsis thaliana seedlings were grown vertically on ATS plates for 5 d, treated later with 10 nM BL, 100 µM c-PTIO or 200 µM GSNO. Seedlings were analyzed after 3 d of treatment. (A) Representative images of the Arabidopsis seedlings. Bar: 1 cm. (B) For NO detection, roots were incubated with 15 µM of the fluorescent probe DAF-FMDA and examined by epi-fluorescence (excitation 490 nm; emission 525 nm) in an Eclipse E 200 microscope (Nikon). Bar: 75 min (C) The ratio of LR number/PR length was taken as a measure of LR density. LR number only included those roots that were > 1 mm in length after 3 d of treatment. (D) Seedlings were photographed, and PR length was measured using Image J software (Universal Imaging). (E) For NO quantification, DAF-FM-DA fluorescence was analyzed with the Image J 1.3 software and expressed as arbitrary units (A.U.).Values are the means ± SE of 5 independent experiments (n = 10). Asterisks indicate significant differences at p < 0.05 (t-test).
Figure 1C–E show the quantification of the BL effects on root growth and NO production. The PR was shortened by around 50% and the LR density showed around a 3-fold increase in Arabidopsis seedlings treated with 10 nM BL or 200 µM GSNO (Fig. 1C and D). Figure 1E shows that a 3-fold increase in endogenous NO production was induced by BL in Col-0, attaining the same level as with GSNO. The effects of BL were counteracted by c-PTIO. All together, these results indicate that NO is involved in the BR signaling pathway regulating root development.
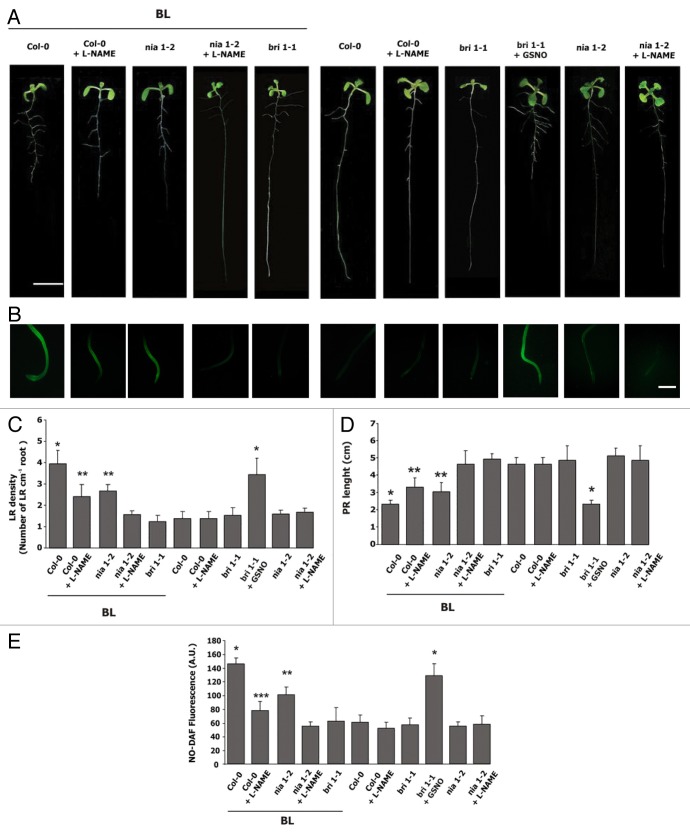
Pharmacological and genetic approaches were used to study the enzymatic source of the NO burst involved in the BR regulation of root morphology. If NR is responsible for the NO burst, BL should have no effect on the (NR)-null mutant nia1-2 27. Figure 2A–C show that BL effect on root growth was only partially reduced in nia1-2. PR was shortened 33% and LR density had a 2-fold increase in nia1-2 mutant, a week response compared with BL-treated Col0. 2-fold increase in NO (Fig. 2B and E) indicates that NR has a partial participation in the NO-mediated BL effect. Similar results were obtained when the NOS inhibitor L-NAME was applied together with BL in wild type plants (Fig. 2A–D). This result indicates that the NOS-like activity also has a partial contribution to the NO-mediated BL effect.
Figure 2. Characterization of NO signaling operating downstream BL in Arabidopsis roots. Arabidopsis thaliana Col-0, nia1-2 and bri1-1 mutant lines were grown on ATS plates for 5 d, treated later with 10 nM BL, 100 µM L-NAME or 200 M GSNO. Seedlings were analyzed after 3 d of treatment. (A) Representative images of the Arabidopsis seedlings. Bar: 1 cm. (B) For NO detection, roots were incubated with 15 µM of the fluorescent probe DAF-FMDA, and examined by epi-fluorescence. Bar: 75 min. LR density (C), PR length (D) and NO (E) were quantified as indicated in Figure 1. Values are the means ± SE of five independent experiments (n = 10). Asterisks indicate significant differences at p < 0.05 (t-test).
Interestingly, Figure 2 also shows that the addition of L-NAME to the nia1-2 seedlings completely abolished the effect of BL, indicating that both NR and NOS-like activities are responsible of the NO production during the BL-induced effects on root architecture. This result is coincident with that reported by Cui et al.22 where both NOS-like and NR were responsible for the BL-induced NO production in response to abiotic stresses. Arabidopsis mutant bri1-1 (mutated in the BR receptor)28 was insensitive to BL treatment for both changes in LR density and NO increase (Fig. 2A–E), thus supporting that BL action occurs through its receptor. However, if the bri1-1 mutants were supplied with GSNO, the LR density reached similar level to that observed in BL-treated Col-0, confirming that NO is downstream BR in the signaling pathway (Fig. 2A–D).
Altogether, these results demonstrate that BR promotes an increase in endogenous NO concentration, which in turn is required for changes in root morphology. For full NO production, a functional BR receptor, and both NR and NOS-like activities are necessary.
Acknowledgments
We wish to thank Dr G. Pagnussat for critical reading of this manuscript and helpful comments. This work was financed by Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Agencia Nacional para Promoción de Ciencia y Tecnología (ANPCyT) and Universidad Nacional de Mar del Plata. L.L. and R.C. are permanent members of the Research Career of CONICET. V.T. is a post-doctoral fellow of CONICET.
Glossary
Abbreviations:
- BRs
Brassinosteroids
- NO
nitric oxide
- BL
BR 24-epibrassinolide
- LR
lateral roots
- PR
primary root
- NR
nitrate reductase
- NOS
nitric oxide synthase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24712
References
- 1.Bajguz A, Tretyn A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry. 2003;62:1027–46. doi: 10.1016/S0031-9422(02)00656-8. [DOI] [PubMed] [Google Scholar]
- 2.Clouse SD, Sasse JM. BRASSINOSTEROIDS: Essential Regulators of Plant Growth and Development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–51. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- 3.Divi UK, Rahman T, Krishna P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010;10:151. doi: 10.1186/1471-2229-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vert G, Nemhauser JL, Geldner N, Hong F, Chory J. Molecular mechanisms of steroid hormone signaling in plants. Annu Rev Cell Dev Biol. 2005;21:177–201. doi: 10.1146/annurev.cellbio.21.090704.151241. [DOI] [PubMed] [Google Scholar]
- 5.Li J. Regulation of the nuclear activities of brassinosteroid signaling. Curr Opin Plant Biol. 2010;13:540–7. doi: 10.1016/j.pbi.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci. 1999;4:128–9. doi: 10.1016/S1360-1385(99)01393-X. [DOI] [PubMed] [Google Scholar]
- 7.Neill S, Bright J, Desikan R, Hancock J, Harrison J, Wilson I. Nitric oxide evolution and perception. J Exp Bot. 2007;59:25–35. doi: 10.1093/jxb/erm218. [DOI] [PubMed] [Google Scholar]
- 8.Corpas FJ, Palma JM, del Río LA, Barroso JB. Evidence supporting the existence of L-arginine-dependent nitric oxide synthase activity in plants. New Phytol. 2009;184:9–14. doi: 10.1111/j.1469-8137.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT. On the origins of nitric oxide. Trends Plant Sci. 2011;16:160–8. doi: 10.1016/j.tplants.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Corpas FJ, Barroso JB, Carreras A, Quirós M, León AM, Romero-Puertas MC, et al. Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol. 2004;136:2722–33. doi: 10.1104/pp.104.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamasaki H, Cohen MF. NO signal at the crossroads: polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci. 2006;11:522–4. doi: 10.1016/j.tplants.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Rand MJ, Li CG, Li CG. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–82. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- 13.Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, et al. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008;49:1711–22. doi: 10.1093/pcp/pcn144. [DOI] [PubMed] [Google Scholar]
- 14.Jasid S, Simontacchi M, Bartoli CG, Puntarulo S. Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol. 2006;142:1246–55. doi: 10.1104/pp.106.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian QY, Sun DH, Zhao MG, Zhang WH. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol. 2007;174:322–31. doi: 10.1111/j.1469-8137.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- 16.Foresi N, Correa-Aragunde N, Parisi G, Caló G, Salerno G, Lamattina L. Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell. 2010;22:3816–30. doi: 10.1105/tpc.109.073510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beligni MV, Lamattina L. Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants. Nitric Oxide. 1999;3:199–208. doi: 10.1006/niox.1999.0222. [DOI] [PubMed] [Google Scholar]
- 18.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–36. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W. Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta. 2006;224:545–55. doi: 10.1007/s00425-006-0242-z. [DOI] [PubMed] [Google Scholar]
- 20.Tossi V, Lamattina L, Cassia R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009;181:871–9. doi: 10.1111/j.1469-8137.2008.02722.x. [DOI] [PubMed] [Google Scholar]
- 21.Tossi V, Amenta M, Lamattina L, Cassia R. Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ. 2011;34:909–21. doi: 10.1111/j.1365-3040.2011.02289.x. [DOI] [PubMed] [Google Scholar]
- 22.Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, et al. Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ. 2011;34:347–58. doi: 10.1111/j.1365-3040.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang A, Zhang J, Zhang J, Ye N, Zhang H, Tan M, et al. Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant Cell Physiol. 2011;52:181–92. doi: 10.1093/pcp/pcq187. [DOI] [PubMed] [Google Scholar]
- 24.Müssig C, Shin GH, Altmann T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003;133:1261–71. doi: 10.1104/pp.103.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol. 2004;134:1624–31. doi: 10.1104/pp.103.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–5. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Tischner R, Gutiérrez RA, Hoffman M, Xing X, Chen M, et al. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004;136:2512–22. doi: 10.1104/pp.104.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–8. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]