Abstract
Recently we reported that the joint expression of cassava Cu/Zn superoxide dismutase (MeCu/ZnSOD) and catalase (MeCAT1) prolonged the shelf life of cassava storage-roots by the stabilization of reactive oxygen species (ROS) homeostasis after harvest. Since oxidative damage is a major feature of plants exposed to environmental stresses, transgenic cassava showing increased expression of the cytosolic MeCu/ZnSOD and the peroxisomal MeCAT1 should have improved resistance against other abiotic stresses. After cold treatment, the transgenic cassava maintained higher SOD and CAT activities and lower malendialdehyde content than those of wild type plants (WT). Detached leaves of transgenic cassava also showed slower transpirational water loss than those of WT. When plants were not watered for 30 d, transgenic lines exhibited a significant increase in water retention ability, accumulated 13% more proline and 12% less malendialdehyde than WT’s, and showed enhanced activity of SOD and CAT. These results imply that manipulation of the antioxidative mechanism allows the development of staple crops with improved tolerance to abiotic stresses.
Keywords: ROS scavenging, cassava, superoxide dismutase, catalase, abiotic stress resistance
Plants are often subjected to the environmental challenge of abiotic and biotic stresses such as strong light, high and low temperature, drought, salinity and pathogen attacks, which can give rise to excess accumulation of reactive oxygen species (ROS) in their cells.1,2 The ROS homeostasis is maintained by an antioxidative system, which is composed of non-enzymatic (ascorbate, glutathione, α-tocopherol and carotenoid) and ROS-scavenging enzymes, which include superoxide dismutase (EC 1.15.1.1, SOD), catalase (EC 1.11.1.6, CAT), ascorbate peroxidase (EC 1.11.1.11, APX) and glutathione reductase (EC 1.6.4.2, GR).3 A number of studies have demonstrated that improved tolerance to abiotic stress of transgenic plants were successfully achieved by modification of ROS-scavenging systems. In certain cases, however, overexpression of one enzyme may not alter the function of the entire anti-oxidant pathway.4,5 Therefore, it has been suggested and shown that pyramiding of ROS scavenging enzymes (e.g., SOD and CAT or APX) may provide better stress tolerance than overproduction of a single enzyme.6-8 In a recent publication, we co-expressed the cytosolic superoxide dismutase (MeCu/ZnSOD) and peroxisomal catalase (MeCAT1) (Fig. 1) in transgenic cassava in order to explore the intrinsic relationship between ROS scavenging and post-harvest physiological deterioration (PPD) occurrence of storage roots.9 The combined ectopic expression of MeCu/ZnSOD and MeCAT1 leads to an improved synergistic ROS-scavenging capacity of cassava storage roots after harvest, resulting in delayed PPD occurrence. In addition, under exposure to the ROS-generating reagent methyl viologen or to H2O2, the transgenic cassava showed enhanced tolerance to oxidative stresses.9 To test further their performance against other environmental stresses,10,11 the transgenic cassava plants were evaluated after being subjected to cold and drought treatments in current study.
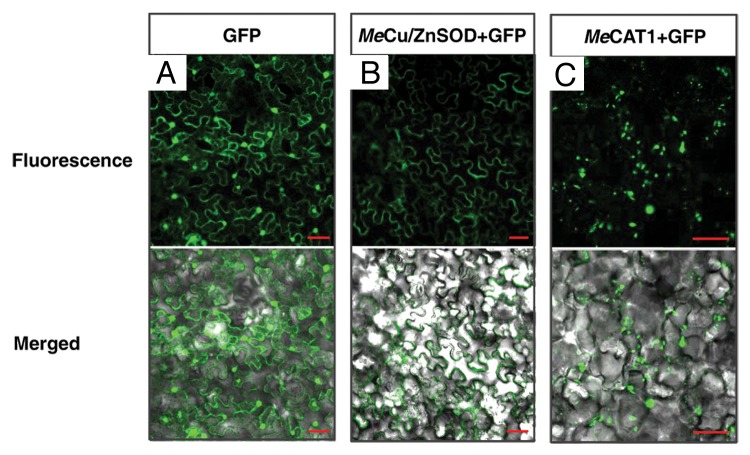
Figure 1. Subcellular localization of MeCu/ZnSOD and MeCAT1 fluorescent fusion proteins in epidermal cells. (A) Subcellular localization of GFP in N. benthamiana epidermal cells as a control; (B) and (C) subcellular localization of MeCu/ZnSOD and MeCAT1 in epidermal cells of N. benthamiana leaves after agroinfiltration with 35S::MeCAT1-GFP and 35S::MeCu/ZnSOD-GFP, respectively. Scale bar = 50 μm.
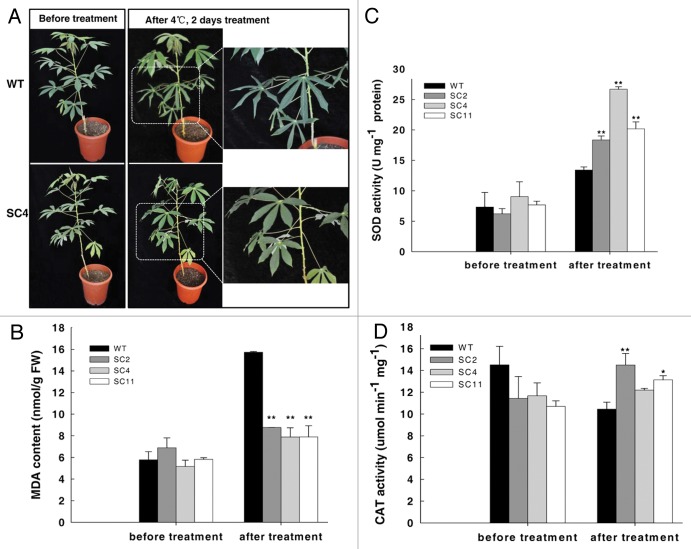
To test whether overexpression of the MeCu/ZnSOD and the MeCAT1 in cassava could enhance cold tolerance, two-month-old transgenic and wild-type (WT) plants were used and transferred into a growth chamber at 4°C for 2 d. As shown in Figure 2A, the WT was severely wilted, especially the bottom leaves, whereas the transgenic lines were hardly affected and no obvious phenotype was observed. Cold treatment causes membrane-lipid peroxidation, leading to an increased malondialdehyde (MDA) content. After the treatment, the MDA content in WT plants increased up to 63%, whereas an average increase of only 29% was found in transgenic lines (Fig. 2B). Both SOD and CAT activities were enhanced after treatment (Fig. 2C and D), especially the SOD activity which showed nearly a 2-fold increase in transgenic plants compared with WT plants. These data confirmed that the altered performance of transgenic cassava under cold stress was due to elevated SOD and CAT activities and was consistent with previous cassava cold transcriptome studies in which the ROS scavenging enzymes, especially SOD, CAT and glutathione-S-transferases (GSTs), were upregulated during a 7°C treatment of greenhouse-grown cassava plants.12 As a tropical crop, cassava is sensitive to cold. Low temperatures and freezing conditions are the major constraints for its geographical distribution and high productivity. The study of transgenic cassava that co-expressed MeCu/ZnSOD and MeCAT1 demonstrated the important role of ROS scavenging under the cold condition
Figure 2. Transgenic cassava shows enhanced resistance to cold stress. (A) Two-mo-old cassava subjected to 4°C for 2 d in a chamber displaying phenotypic changes; (B) MDA contents in cassava leaves exposed to 4°C for 2 d; (C) and (D) SOD and CAT activity changes in cassava leaves exposed to 4°C for 2 d. Values represent the means of three independent experiments ± SD. The double asterisks indicate a statistically significant difference (p < 0.01) by t-test for the data of the stress-treated samples compared with those of the WT samples.
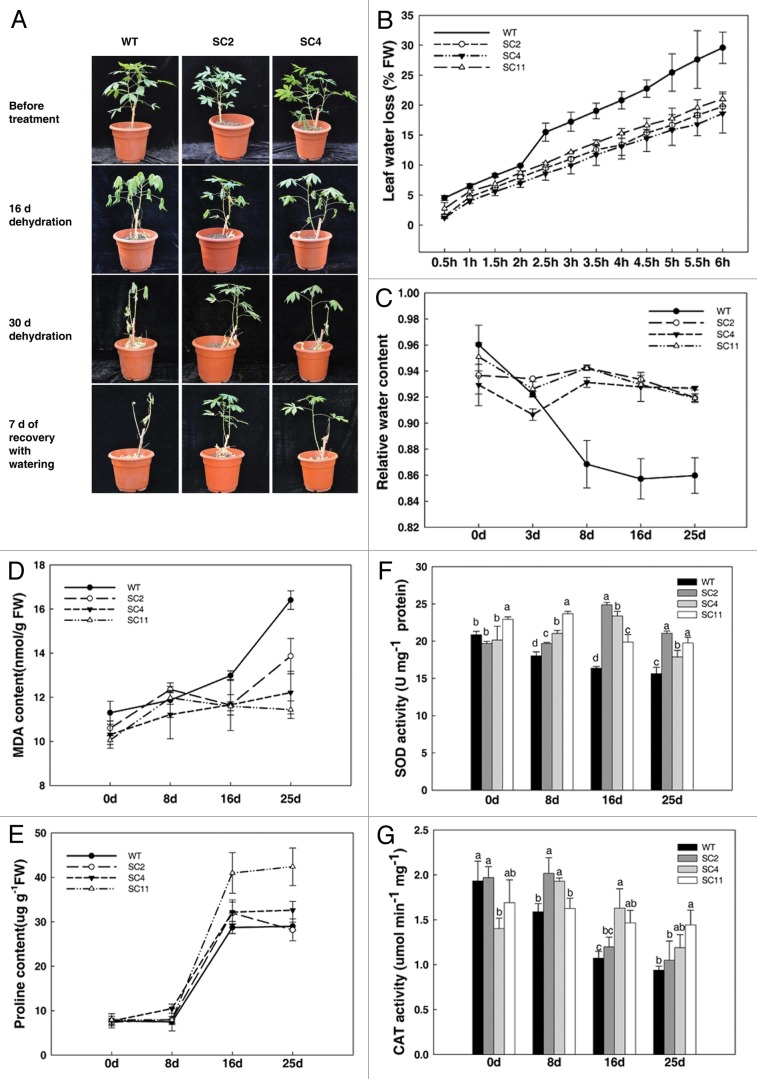
Drought tolerance experiment was performed by depriving 2-mo-old WT and transgenic cassava plants of irrigation for 30 d. Under water deficiency, the wild-type control displayed collapsed leaves, especially the bottom leaves, while the transgenic lines were less affected (Fig. 3A). Seven days after resuming watering the transgenic lines survived but the WT tended to not to recover. Analysis of transpirational water loss by detached leaves confirmed that transgenic plants lost water more slowly than WT (Fig. 3B). Furthermore, upon treatment, the relative water content (RWC) in leaves of WT and transgenic cassava under normal watered conditions were similar. However, transgenic plants maintained a higher RWC of 92% compared with WT’s 84% at 25 d (Fig. 3C). Drought stress also caused obviously physiological changes in cassava. Two stress responsive metabolites MDA and proline, the most frequently used indicators for plant stress, were monitored. The MDA content in drought-stressed plants was 12% lower in the leaves of transgenic plants compared with those of WT in 25 d (Fig. 3D); while the concentration of proline increased rapidly both in WT and transgenic lines, but was 13% more in transgenic plants (Fig. 3E). Drought stress markedly increased the activities of the antioxidant enzymes. During the treatment, the SOD and CAT activities of WT decreased over the time course, however, their activities in transgenic cassava was always higher than those of WT during the time course (Fig. 3F and G).
Figure 3. Transgenic cassava shows enhanced resistance to drought stress. (A) Two-mo-old cassava subjected to drought treatment for 30 d showing phenotypic changes; (B) comparison of rates of transpirational water loss from detached leaves of WT and transgenic cassava. Water loss is expressed as a proportion of the initial FW; leaf RWC (C), MDA content (D), proline (E), SOD activity (F) and CAT activity (G) in WT and transgenic cassava under normal and drought conditions. Values represent the means of three independent experiments ± SD. Values labeled with different letters a, b, c are significantly different by Duncan’s multiple comparison tests at p < 0.05.
Plants perceive various abiotic stresses through cell membrane receptors, most likely transmembrane proteins, and signal transduction may stimulate cellular metabolism changes, such as ROS signaling, hormone signal transduction and the calcium signaling cascade. ROS are now considered as key regulatory signaling molecules, but they also cause cellular damage when produced in excess.13 Severe abiotic stress disrupts the metabolic balance of plant cells, resulting in a perturbed equilibrium between the production and the scavenging of ROS in the cytoplasm, chloroplasts and mitochondria in plants. Therefore, it is essential to have an efficient antioxidant network in order to scavenge ROS effectively and to maintain low levels of intracellular ROS pools.14 The different cellular compartment localization of the MeCu/ZnSOD and MeCAT1 enhanced scavenge ability of ROS both in the cytoplasm and peroxidsome, which is strongly consistent with the report by Faiz M et al.7 The increased SOD and CAT enzyme activities were able rapidly to scavenge ROS at the site of generation, as well as preventing the production of hydroxyl radicals, thereby enhancing stress tolerance. Thus, our study confirmed that improved cold and drought tolerance is probably attributed to enhanced expression of ROS-scavenging genes (jointly by SOD and CAT genes) resulting in increased ROS homeostasis under the abiotic stress. These results imply that pyramiding of ROS scavenging enzymes through genetic engineering and thereby controlling the levels of cellular ROS is an effective approach to obtaining abiotic stress tolerant plants.
Acknowledgments
This work was supported by grants from the National Basic Research Program (2010CB126605), the National Natural Science Foundation of China (31271775), the National High Technology Research and Development Program of China (2012AA101204), the Earmarked Fund for China Agriculture Research System (CARS-12) and Shanghai Municipal Afforestation and City Appearance and Environmental Sanitation Administration (G102410, F132427) to J.X., X.D., J.Y. and P.Z. The study was partially funded by a grant from the Bill and Melinda Gates Foundation (BioCassava Plus program) to J.R.B. and P.Z.
Glossary
Abbreviations:
- ROS
reactive oxygen species
- WT
wild type plants
- PPD
post-harvest physiological deterioration
- RWC
relative water content
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24525
References
- 1.Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–79. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 2.Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–7. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- 3.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 4.Pitcher LH, Brennan E, Hurley A, Dunsmuir P, Tepperman JM, Zilinskas BA. Overproduction of petunia chloroplastic copper/zinc superoxide dismutase does not confer ozone tolerance in transgenic tobacco. Plant Physiol. 1991;97:452–5. doi: 10.1104/pp.97.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payton P, Allen RD, Trolinder N, Holaday AS. Overexpression of chloroplast-targeted Mn superoxide dismutase in cotton (Gossypium hirsutum L., cv. Coker 312) does not alter the reduction of photosynthesis after short exposures to low temperature and high light intensity. Photosynth Res. 1997;52:233–44. doi: 10.1023/A:1005873105596. [DOI] [Google Scholar]
- 6.Lee SH, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, et al. Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol. 2007;164:1626–38. doi: 10.1016/j.jplph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, et al. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599–613. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- 8.Tseng MJ, Liu CW, Yiu JC. Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol Biochem. 2007;45:822–33. doi: 10.1016/j.plaphy.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Duan XG, Yang J, Beeching JR, Zhang P. Enhanced ROS scavenging by over-production of superoxide dismutase and catalase delays post-harvest physiological deterioration of cassava storage roots. Plant Physiol. 2013;161:1517–28. doi: 10.1104/pp.112.212803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittler R, Zilinskas BA. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J. 1994;5:397–405. doi: 10.1111/j.1365-313X.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez JA, Jimenez A, Mullineaux PM, Sevilla F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000;23:853–62. doi: 10.1046/j.1365-3040.2000.00602.x. [DOI] [Google Scholar]
- 12.An D, Yang J, Zhang P. Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genomics. 2012;13:64. doi: 10.1186/1471-2164-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–9. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]