Abstract
Infantile hemangioma is a benign vascular tumor that affects 4 to 10% of neonates. A unique feature of hemangiomas is the natural lifecycle, whereby the tumor rapidly grows and then spontaneously regresses to a fibrofatty residuum. We have shown that hemangiomas are derived from multipotential stem cells (hemSCs), which differentiate into endothelial cells during the early proliferating phase and into adipocytes during the later involutive phase. T-box 2 (TBX2) is a transcription factor involved in controlling cell-fate decisions, and is highly expressed during the proliferating phase of hemangioma development. We hypothesize that TBX2 expression would be high in hemSCs derived from human hemangiomas and inhibiting TBX2 would result in changes in hemSC differentiation potential. To test our hypothesis, we analyzed hemSCs for TBX2 mRNA and protein expression. We then used RNA interference and TBX2 overexpression to determine the effect of altering TBX2 levels on hemSC growth and differentiation. Our studies show that TBX2 is highly expressed in hemSCs compared with a panel of normal stem/progenitor cells and mature vascular cells. TBX2 knockdown completely abolished adipogenic differentiation of hemSCs without significantly altering growth. Furthermore, overexpression of TBX2 led to enhanced adipogenic differentiation ability possibly through induction of C/EBPβ. From these findings, we believe that TBX2 is active in hemSCs and that TBX2 maintains an adipogenic differentiation-competent state of hemSCs. These findings may be important in the development of better treatment options for hemangiomas to accelerate involution.
Keywords: infantile hemangioma, adipogenesis, T-box 2, proliferation, differentiation, C/EBP
Introduction
Infantile hemangioma, a benign vascular tumor, is the most common tumor of childhood with up to 10% of neonates being affected.1-3 However, Caucasian, female, and premature infants have a higher risk of developing hemangiomas.4 These tumors are mostly cutaneous, present after birth, and follow a unique life cycle which starts with a rapid proliferation and then spontaneous regression.5 The life cycle consists of three phases. The proliferating phase is characterized by an immature vascular phenotype and lasts for approximately the first year of life. The involuting phase is 3 to 5 years in duration and exhibits a mature vascular phenotype. The involuted phase is reached by age 5 to 8 and is characterized by collapsed vessels surrounded by fibroadipose tissue.5 There are two main hypotheses for the pathogenesis of infantile hemangioma, an intrinsic as well as an extrinsic one.5 We have recently shown that hemangiomas arise from stem cells that give rise to the main cellular constituents of the hemangioma, namely endothelial cells (ECs) and adipocytes.5 The same stem cells have also been shown to differentiate into vascular pericytes.6 These findings support the intrinsic hypothesis for hemangioma pathogenesis.
Hemangiomas are mostly small lesions and pose no serious threat to the wellbeing of the infant. However, some lesions have the potential to interfere with the child’s health depending on size and location.4,5 Currently, treatments for infantile hemangioma consist of corticosteroids, interferon-α, propranolol, and surgery. However, due to the non-specific nature of these treatments, with the exception of surgery, there is the potential for adverse side effects, and thus there still exists a need for more specific therapies.7,8 In order to develop such treatments, specific regulators involved in the hemangioma life cycle must first be identified. In a study by Ritter and colleagues,9 a large-scale analysis of potential regulators of infantile hemangioma was performed, and genes with increased expression levels in the proliferating phase compared with the involuting phase included T-box 2 (TBX2).
TBX2 is part of the T-box transcription factor family, which is important in embryonic development and cell cycle progression control. Specifically, TBX2 is involved in coordinating cell fate in certain tissues and studies have shown its overexpression in several cancers.10 TBX2 seems to be an immortalizing gene in that it is able to repress cell cycle inhibitors such as p19ARF (mouse homolog of human p14 ARF) and p21WAF1/CIP1/SDI1.11
Due to the involvement of TBX2 in coordinating cell fate and its ability to inhibit cell cycle regulation, TBX2 may act as a regulator of infantile hemangioma. We have recently shown that hemangioma-derived stem cells (hemSCs) express platelet-derived growth factor-B (PDGF‑B) which acts to regulate adipogenesis (a process critical for hemangioma involution) in a cell-autogenous manner.12 In an attempt to identify the mechanisms by which PDGF‑B regulates adipogenesis, we performed a mRNA-based screen for putative signaling factors and also identified TBX2 (unpublished data). Therefore, we aim to evaluate the role of TBX2 in hemangioma in this study. We hypothesized TBX2 expression would be high in hemangioma-derived primary hemSCs compared with normal stem/progenitor cell counterparts and disrupting TBX2 would result in changes in hemSC growth and differentiation.
Results
hemSCs show robust expression of TBX2
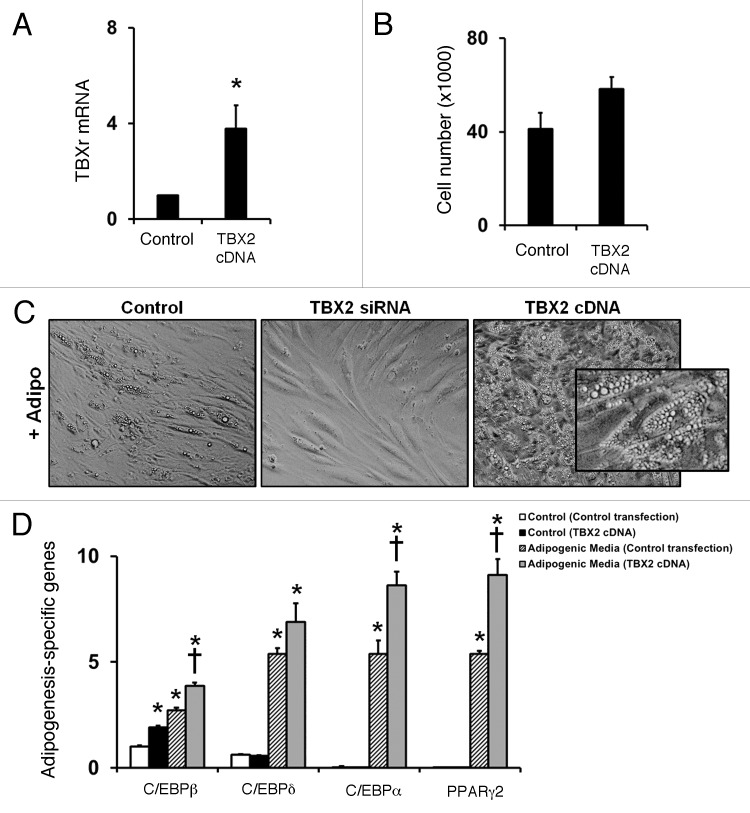
We first examined human hemangioma-derived stem cells (hemSCs) for TBX2 expression and compared the cells to bone marrow-derived mesenchymal progenitor cells (bm-MPCs; used as normal stem/progenitor cells). In addition, we analyzed endothelial progenitor cells (derived from cord blood and adult blood; cbEPCs and abEPCs respectively) and mature human dermal microvascular endothelial cells (HDMECs). Using real time RT-PCR, we found that TBX2 mRNA is highly expressed in hemSCs (Fig. 1A). Expression of TBX2 mRNA was minimal in bm-MPCs and no detectable levels were seen in the cbEPCs, abEPCs, or HDMECs (Fig. 1A). After determining the expression of TBX2 in hemSCs, we wanted to examine the cellular localization of TBX2. Since TBX2 is a transcription factor, an active state should be indicated by nuclear localization. We stained hemSCs for TBX2 and show that TBX2 protein localizes to the nucleus (Fig. 1B).
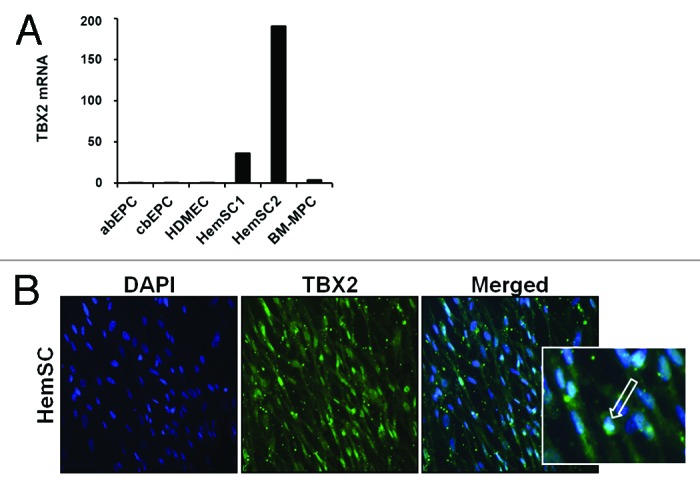
Figure 1. (A) Analysis of TBX2 mRNA levels was performed in adult blood endothelial progenitor cells (abEPC), cord blood endothelial progenitor cells (cbEPC), human dermal microvascular endothelial cells (HDMEC), two different hemangioma stem cells (HemSC1 and HemSC2), and bone marrow mesenchymal progenitor cells (BM-MPC). T-box 2 was highly expressed in HemSCs compared with the other cell types [mRNA levels normalized to β-actin; representative data from 2 independent experiments shown]. (B) Immunofluorescence staining was performed to confirm nuclear localization of TBX2 in hemSCs [TBX2 = green, arrow; DAPI nuclear dye = blue; insert shows higher magnification].
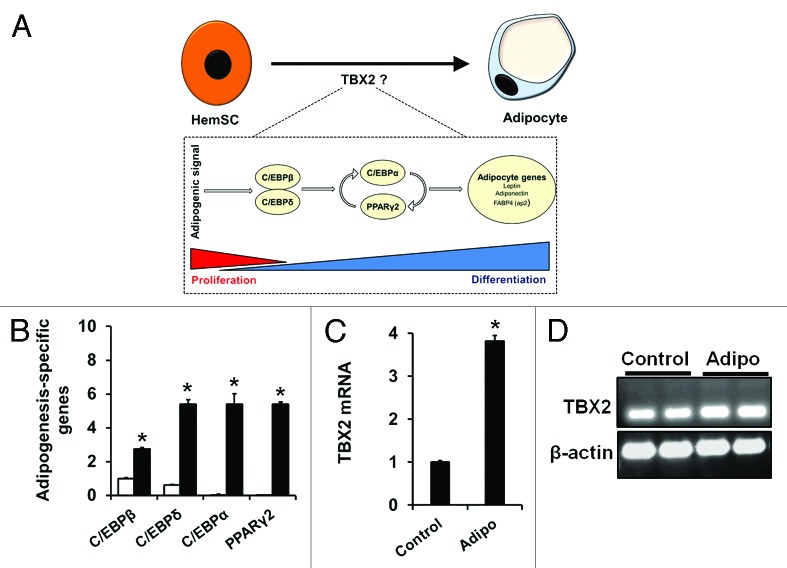
To explore the possibility that TBX2 may be involved in hemSC adipogenesis, we first exposed hemSCs to adipogenic media for 7 d and assessed transcript levels of TBX2. The rationale was to understand the dynamics of TBX2 expression during the process of adipogenesis. We measured the degree of adipogenesis by analyzing specific transcription factors, which are necessary for differentiation12,13 (Fig. 2A). Our results show induction of both early transcription factors (C/EBPβ and δ) as well as late transcription factors associated with adipogenesis (C/EBPα and PPARγ2) when hemSCs were exposed to adipogenic (adipo) media (Fig. 2B). This induction was also associated with significantly higher levels of TBX2 mRNA (Fig. 2C and 2D). This suggests that TBX2 may play a role in hemSC adipogenesis, which may involve regulating cell growth or induction of adipogenesis-specific transcription factors.
Figure 2. (A) Schematic showing the cellular and molecular changes accompanying adipogenic differentiation. Upon receiving adipogenic signals, C/EBPβ and δ are upregulated leading to induction of C/EBPα and PPARγ2. (B) Induction of adipogenesis-specific transcription factors in hemSCs cultured in adipo media (black bars) for 7 d as compared with cells in control media (white bars) [mRNA normalized to β-actin, * p < 0.05 compared with control media]. (C) TBX2 mRNA levels in cells exposed to adipo media [* p < 0.05 compared with control media]. (D) post-PCR products were run on agarose gel to confirm specific amplification.
TBX2 knockdown has no significant effect on hemSC growth but completely abolishes adipogenesis
In order to understand the function of TBX2, we knocked down the expression using siRNA. HemSCs were transfected with either TBX2 or control siRNA using electroporation, and subjected to real time RT-PCR to assess knockdown efficiency at 24 h following transfection as well as at the endpoint of all experiments (day 7). TBX2 siRNA transfection led to a significantly reduced level of mRNA expression when compared with the control siRNA group at 24 h (data not shown) as well as at day 7 in normal growth media (approximately 85% reduction; Figure 3A). We first analyzed the effect that decreased levels of TBX2 had on the growth potential of the hemSCs. We determined total live cells and it was found that knocking down TBX2 did not have a significant effect on the growth of hemSCs after 24 h (data not shown) or at day 7 (Fig. 3B).
Figure 3. (A) TBX2 mRNA levels in hemSCs after transfection with control or TBX2 siRNA after 7 d in normal growth media [data normalized to β-actin and shown relative to control siRNA; *p < 0.05 compared with control siRNA]. (B) Effect of TBX2 knockdown on total live cell number at day 7. (C) TBX2 siRNA prevents induction of all adipogenesis-specific transcription factors [mRNA data normalized to β-actin; * p < 0.05 compared with control siRNA transfected cells in control media; † p < 0.05 compared with control siRNA transfected cells in adipo media]. (D) TBX2 mRNA levels in cells exposed to adipo media for 7 d [*p < 0.05 compared with control siRNA]. (E) total live cell numbers following control or TBX2 siRNA transfection and exposure to adipo media for 7 d.
We next cultured hemSCs (transfected with control siRNA or siRNA against TBX2) in adipogenic media to assess the role in the differentiation process. After 7 d, negative Oil Red-O staining showed that TBX2 knockdown prevented adipocyte differentiation in hemSCs (data not shown). Real time RT-PCR based quantitative assessment of adipogenesis showed that TBX2 knockdown completely prevented induction of all adipogenesis-specific transcription factors (Fig. 3C). Since adipogenic differentiation was associated with elevated TBX2 levels (Fig. 2C), we measured whether significant reduction of the transcript levels were maintained in TBX2 siRNA transfected cells following the 7-d differentiation assay. Our results show that TBX2 mRNA levels were significantly reduced in the siRNA transfected cells, although the knockdown was only at 40% (Fig. 3D). Furthermore, there were no differences in cell number when TBX2 siRNA transfected cells were cultured in adipogenic media (Fig. 3E).
TBX2 overexpression leads to induction of C/EBPβ and enhanced adipogenesis
To confirm the role of TBX2 in regulating hemSC adipogenesis, we overexpressed TBX2 and achieved approximately a 4-fold induction in TBX2 levels (Fig. 4A). Surprisingly, increased levels of TBX2 were not associated with changes in cell growth (Fig. 4B). However, when we exposed hemSCs overexpressing TBX2 to adipogenic differentiation media, lipid-laden cells were clearly seen even through regular microscopy (Fig. 4C). This enhanced adipogenic differentiation level was highlighted in the mRNA levels of adipogenesis-specific transcription factors (Fig. 4D). We also found that TBX2 overexpression induces C/EBPβ even in normal growth conditions (Fig. 4D).
Figure 4. (A) TBX2 mRNA levels following TBX2 cDNA overexpression in hemSCs cultured in normal growth media for 7 d after transfection [* p < 0.05 compared with control transfection]. (B) Effect of TBX2 overexpession on cell growth as measured at day 7. (C) Phase contrast image of control transfected cells, cells transfected with TBX2 siRNA, and cells transfected with TBX2 cDNA cultured in adipo media for 7 d [insert shows higher magnification]. (D) TBX2 overexpressing cells were induced to differentiate using adipogenic media for 7 d, at which point they were analyzed for C/EBPβ, C/EBPδ, C/EBPα, and PPARγ2 expression levels to determine degree of adipogenic differentiation. A significant increase in all adipogenesis-specific genes was seen in hemSCs with TBX2 overexpression compared with control transfected cells [*p < 0.05 compared with control transfected cells in control media; †p < 0.05 compared with control transfected cells in adipo media].
Discussion
The salient finding of our study is that TBX2 is a regulator of adipogenesis in hemSCs. We found that inhibiting TBX2 completely normalizes induction of adipogenic differentiation without altering cell growth. Conversely, overexpression led to basal induction of C/EBPβ and significantly enhanced adipogenesis. It is worthwhile to note the differences in TBX2 expression levels within the two different hemSC specimens that were examined (Fig. 1A). This was expected. Even though hemangiomas exhibit a predictable lifecycle, individual differences lead to slight variations in the timing of the different phases. We have previously shown that hemSCs express varying levels of insulin-like growth factor 2 and the level corresponds to the ability of hemSCs to produce leptin.14 These findings suggest that hemSCs from different phases and different time points would follow a hierarchical stage reflected by their biosynthetic ability and varying expression of different cell fate determining factors.
We had expected that because TBX2 is a cell cycle regulator and an immortalizing factor in certain other tumors, it would positively regulate growth and proliferation.10,11 When we knocked down TBX2, we did note a slight decrease in cell growth, although this decrease is not statistically significant. It is possible that had the assay been continued over a longer time, it will have produced a larger disparity in cell numbers between the control and TBX2 siRNA groups. Another explanation could be the presence of various growth promoting factors in culture media. This is difficult to test, as serum-depleted (or reduced) media would lead to confounding variables and most certainly change cell growth and differentiation itself. We can, however, conclude that the primary role of TBX2 in hemangioma is related to cell differentiation. Supporting evidence also comes from our overexpression studies, which show non-significant effect on cell growth but highly enhanced adipogenic differentiation potential.
Unlike most transcription factors regulating stem cell phenotype whose knockdown leads to enhanced differentiation, TBX2 seems to regulate the differentiation competent state. We expected that knocking down the expression of TBX2 would allow the cells to complete adipogenesis at a faster rate than under control conditions; however, our results indicate the opposite is true. The fact that overexpression of TBX2 in hemSCs resulted in an increase in adipogenic markers supports the idea that TBX2 maintains the differentiation competent state and is a positive regulator of adipogenic differentiation in hemSCs. Interestingly, when TBX2 was overexpressed in hemSCs our results show a basal induction of C/EBPβ. There may be an indirect link between TBX2 and adipogenesis that may not be restricted to hemSCs. Recent studies have shown that TBX2 interacts with retinoblastoma protein.15 It has also been reported that in differentiating cells retinoblastoma protein and C/EBPs share a physical interaction but only in actively differentiating cells.16 The C/EBP form identified in this report was C/EBPβ, same form that was induced in our TBX2 overexpressing cells. Together these studies show a possible link between TBX2 and C/EBPβ and provide insight into how TBX2 may positively regulate adipogenic differentiation in hemSCs. As hemSCs are capable of differentiating into other cell lineages, such as endothelial cells, neuroglial cells, osteocytes, and chondrocytes,17It would therefore be interesting to evaluate the effect that TBX2 knockdown would have on the differentiation ability of hemSCs into these other cell lineages to determine if the effects of TBX2 are adipogenesis-specific.
Overall, our findings indicate that TBX2 is involved in the adipogenic potential of hemangioma stem cells. Most importantly, we have identified a potential mechanism through which infantile hemangioma may be targeted. Finding a way to manipulate the link between TBX2 and C/EBPβ could provide a way to increase the involution rate of the tumor thereby decreasing the potential size reached and therefore decreasing risk of complications caused by the tumor.
Materials and Methods
Hemangioma stem cell and other cell specimens
Proliferating hemangioma-derived CD133+ cells (hemSCs) were kindly provided by Dr. Joyce Bischoff (Children’s Hospital Boston). We have previously shown full characterization of hemSCs and culture conditions.17 Bone marrow-mesenchymal progenitor cells (bm-MPCs; isolated from bone marrow mononuclear preparations; 2M-125B, Lonza Inc.) were used as a control for hemSCs. Three endothelial cell types were also included: human dermal microvascular endothelial cells (HDMECs; CC-2516, Lonza Inc.), cord blood endothelial progenitor cells (cbEPCs) and adult blood endothelial progenitor cells (abEPCs), both isolated from mononuclear fraction (2C-150A, Lonza Inc.; and B2150-01-50ML, US Biological) as shown previously.13 All cells were cultured in Endothelial Basal Media-2 (EBM2; Lonza Inc.) supplemented with 20% Fetal Bovine Serum (FBS; CA-95042-112; Lonza Inc.), EBM2 SingleQuots (Lonza Inc.) and 1x antibiotic antimycotic media (CA45000-616; Mediatech Inc.) on fibronectin-coated (FN; 1 µg/cm2, FC010-10, Millipore) plates. All studies were conducted following approval by the Research Ethics Board at Western University, London, Ontario, Canada.
Real time RT-PCR analysis
Extraction of RNA, cDNA synthesis and real time RT-PCR analysis was performed by following previous laboratory protocols.12-14 TBX2 primers were obtained from Qiagen (QT00091266). All other primer sequences and sources are reported in our previous studies.12-14 mRNA levels were normalized to β-actin and the data was analyzed using relative quantity (ΔCT) with CFX Manager Software (Bio-Rad).
Immunofluorescence staining
HemSCs were plated at a density of 10 000 cells/cm2 on FN-coated chambered slides in complete EMB2/20% FBS media. Cells were fixed with 100% methanol and incubated with anti-TBX2 antibody (1:100 dilution; sc-17880, Santa Cruz Biotech) for 1 h at room temperature. Fluorescein-conjugated anti-goat secondary antibody (1:200 dilution; Vector Laboratories) was used for detection. Counterstaining with DAPI nuclear dye (Vector Laboratories) was performed and Fluoromount K 024 (Diagnostic BioSystems) was used for mounting the slides. Images were taken using the Olympus BX-51 microscope (Olympus Canada In.) equipped with a Spot Pursuit digital camera (SPOT Imaging Solutions).
Transfection of hemangioma stem cells
HemSCs were grown in complete EBM2 media to subconfluence. The day prior to the transfections, the media was replaced with antibiotic/antimycotic-free media. Twenty-four hours later, the cells were trypsinized and then transfected with either TBX2 siRNA (sc-38469) or control siRNA (sc-37007) using electroporation (Neon® Transfection System, Life Technologies). For siRNA transfections, we used the following protocol: 1400v, 20 ms, 2 pulses. Knockdown efficiency was determined at 24 h post-transfection or at day 7 using real time RT-PCR. For TBX2 overexpression, we transfected hemSCs with transfection ready TBX2 full-length cDNA in pCMV6-XL5 (SC125962, Origene) using 1600v, 20 ms, 3 pulses.
Cell activity assays
Post-transfection, the growth of hemSCs was assayed by measuring total live cell number with Scepter 2.0 Automated Cell Counter (Millipore). To assay adipogenic differentiation, hemSCs (with or without transfection) were plated at a density of 40,000 cells/cm2 in Dulbecco’s Modification of Eagle’s Medium (DMEM; Mediatech Inc.) supplemented with 10% FBS (control media) or with StemPro® Adipogenesis Differentiation media (adipogenic media; A10070–01, Life Technologies Inc.). After 7 d, cells were subjected to staining with Oil Red-O (O0625–25G; Sigma-Aldrich) to identify adipocytes and RNA isolation and subsequent real time RT-PCR for the purpose of quantifying adipogenesis-specific markers (C/EBPβ, C/EBPδ, C/EBPα, and PPARγ2) and thus adipogenic differentiation.
Statistical analysis
The data were expressed as means ± SEM. Differences were determined using ANOVA with post hoc Bonferroni’s correction. P values < 0.05 were considered statistically significant.
Acknowledgments
The authors would like to acknowledge support from the Canadian Institutes of Health Research (ZAK; MOP 97783). ZAK is a recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada (Great-West Life and London Life New Investigator Award).
Author’s Contributions
SMT performed most of the experiments, interpreted the data, and drafted the manuscript. ZAK conceived the project, interpreted the data, edited and finalized the manuscript.
Glossary
Abbreviations:
- abEPC
adult blood endothelial progenitor cell
- Adipo
adipogenic differentiation media
- bm-MPC
bone marrow-mesenchymal progenitor cell
- cbEPC
cord blood endothelial progenitor cell
- C/EBP
CCAAT/enhancer-binding protein
- DAPI
4',6-diamidino-2-phenylindole
- FN
fibronectin
- HemSC
hemangioma stem cell
- HDMEC
human dermal microvascular endothelial cell
- PDGF
platelet-derived growth factor
- PPAR
peroxisome proliferator-activated receptor
- RT-PCR
reverse transcriptase-polymerase chain reaction
- siRNA
small interfering RNA
Citation: M. Todorovich S, A. Khan Z. Elevated T-box 2 in infantile hemangioma stem cells maintains an adipogenic differentiation-competent state. Dermato-Endocrinology 2013; 5: - ;
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/26739
References
- 1.Holmdahl K. Cutaneous hemangiomas in premature and mature infants. Acta Paediatr. 1955;44:370–9. doi: 10.1111/j.1651-2227.1955.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruckner AL, Frieden IJ. Hemangiomas of infancy. J Am Acad Dermatol. 2003;48:477–93, quiz 494-6. doi: 10.1067/mjd.2003.200. [DOI] [PubMed] [Google Scholar]
- 3.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–22. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Boscolo E, Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. 2009;12:197–207. doi: 10.1007/s10456-009-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleiman A, Keats EC, Chan NG, Khan ZA. Evolution of hemangioma endothelium. Exp Mol Pathol. 2012;93:264–72. doi: 10.1016/j.yexmp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Boscolo E, Stewart CL, Greenberger S, Wu JK, Durham JT, Herman IM, Mulliken JB, Kitajewski J, Bischoff J. JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:2181–92. doi: 10.1161/ATVBAHA.111.232934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon LM, MacDonald DM, Mulliken JB. Complications of systemic corticosteroid therapy for problematic hemangioma. Plast Reconstr Surg. 1999;104:1616–23. doi: 10.1097/00006534-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Greinwald JH, Jr., Burke DK, Bonthius DJ, Bauman NM, Smith RJ. An update on the treatment of hemangiomas in children with interferon alfa-2a. Arch Otolaryngol Head Neck Surg. 1999;125:21–7. doi: 10.1001/archotol.125.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Ritter MR, Dorrell MI, Edmonds J, Friedlander SF, Friedlander M. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci U S A. 2002;99:7455–60. doi: 10.1073/pnas.102185799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahams A, Parker MI, Prince S. The T-box transcription factor Tbx2: its role in development and possible implication in cancer. IUBMB Life. 2010;62:92–102. doi: 10.1002/iub.275. [DOI] [PubMed] [Google Scholar]
- 11.Prince S, Carreira S, Vance KW, Abrahams A, Goding CR. Tbx2 directly represses the expression of the p21(WAF1) cyclin-dependent kinase inhibitor. Cancer Res. 2004;64:1669–74. doi: 10.1158/0008-5472.CAN-03-3286. [DOI] [PubMed] [Google Scholar]
- 12.Roach EE, Chakrabarti R, Park NI, Keats EC, Yip J, Chan NG, Khan ZA. Intrinsic regulation of hemangioma involution by platelet-derived growth factor. Cell Death Dis. 2012;3:e328. doi: 10.1038/cddis.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keats E, Khan ZA. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS One. 2012;7:e38752. doi: 10.1371/journal.pone.0038752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleiman A, Keats EC, Chan NG, Khan ZA. Elevated IGF2 prevents leptin induction and terminal adipocyte differentiation in hemangioma stem cells. Exp Mol Pathol. 2013;94:126–36. doi: 10.1016/j.yexmp.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Vance KW, Shaw HM, Rodriguez M, Ott S, Goding CR. The retinoblastoma protein modulates Tbx2 functional specificity. Mol Biol Cell. 2010;21:2770–9. doi: 10.1091/mbc.E09-12-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen PL, Riley DJ, Chen Y, Lee WH. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- 17.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–9. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]