Abstract
Ascorbic acid (AsA) is a major antioxidant and plays an important role in plant growth and development. There are two aspects to improve AsA content, including de novo synthesis and recycling from its oxidized form. However, the knowledge of regulatory mechanisms of AsA synthesis pathways and metabolism still remains limited. We have determined that AsA synthesis process was modulated on both transcriptional and post-transcriptional levels in Arabidopsis. GDP-mannose pyrophosphorylase (VTC1) is the initial AsA biosynthetic enzyme in L-galactose pathway, we previously showed that Arabidopsis ERF98 transcriptionally activates gene expression of VTC1 to improve AsA content and respond salt stress; recent discovery of the interaction between photomorphogenic factor COP9 signalosome (CSN) subunit CSN5B and VTC1 indicates that CSN5B promotes VTC1 degradation in the dark, which keeps the change of AsA content from day to night. This mini-review integrates previous reports and recent evidence to better understand the regulatory mechanisms involved in AsA synthesis.
Keywords: ascorbic acid, L-galactose pathway, regulation, VTC1, ERF98, CSN5B
Ascorbic acid (AsA) is a ubiquitous low molecular weight antioxidant in eukaryotes. Plants provide the major source of dietary AsA for humans because they lack the last step AsA synthetic enzyme.1 AsA has a number of physiological roles in plants, preventing plants from reactive oxygen species (ROS) damage, cofactor of many enzymes and electron donor in electron transport chain.2,3 Over past decades, much progress has been made toward understanding the synthesis pathways and metabolism of AsA. There are multiple proposed AsA synthesis pathways, such as L-galactose, uronic acid, L-gulose and myo-inositol pathways and the enzymes participated in these pathways.4 AsA in vivo could be oxidised to monodehydroascorbate (MDHA) and dehydroascorbated (DHA) through ascorbate oxidase (AO)5 and AsA peroxidase (APX),6 then the reduced form is regenerated by the monodehydroascorbate reductase (MDAR) and dehydroascorbate reductase (DHAR).7 Except for the redox state transformation, AsA also undergoes degradation enzymatically or non-enzymatically.8 The fully reduced state of AsA is necessary for the activity, therefore, either improving de novo synthesis or promoting recycling is effective to increase AsA content in vivo.9 Nevertheless, the increase of AsA content through enhancement of reductase activity is confined to substrate levels. Therefore, intending research focused on AsA synthesis regulation will be more viable. Both developmental and environmental cues make effects on AsA content, however, the molecular mechanism of these process and knowledge of related regulators remain largely unclear. Resent reports including our studies have provided evidence that regulators on transcriptional and post-transcriptional levels participate in the control of AsA synthesis.
Contributions of Transcriptional Regulation on AsA Synthesis
In the past years, many reports showed that increased AsA content results in enhanced tolerance to various abiotic stresses,10-12 nevertheless, when the environmental stresses come, the plants adjust themselves to survival through a cascade of responses including activation or inhibition of gene expressions on transcriptional level. So far, there are two reported regulators involved in AsA synthesis in response to stresses. AMR1 was reported as a negative regulator of L-galactose pathway in Arabidopsis. The expression of all enzyme genes in the pathway decreased in AMR1 overexpression transgenic lines and increased in amr1 mutants. Respectively, amr1 mutant showed higher accumulation of AsA levels and more tolerant to oxidative stress.13 Moreover, AMR1 transcripts decreased with increased light intensity, resulting in the inhibition of AMR1 expression, subsequently results in promoted AsA synthesis and higher AsA levels, which will be benefit to plants to eliminate accumulated ROS generated by high light,13 Conversely, a positive regulator ERF98, a member of ethylene response factor (ERF) family, which is induced by ethylene, salt and H2O2, transcriptionally activates AsA synthesis. This regulation enhances tolerance to salt stress through direct activation of AsA synthesis enzyme gene expression in Arabidopsis.14 Moreover, ERF98 transcriptionally regulates the expression of other enzyme genes in the L-galactose pathway. Thus it would be possible that ERF98 has more effects on AsA synthesis than we observed. In addition, AsA pool size is related to photosynthesis mainly due to its antioxidant role in photoprotection.15 Light-induced AsA synthesis is modulated on transcriptional level, the expression of AsA synthetic enzyme genes is light-controlled, high light and continuous light upregulate transcripts of some enzyme genes16,17 and light responsive cis-elements in these genes were found in rice,18 however, the transcription factors involved in this process remains unknown. Further identification of transcription factors will provide us with more information about the mechanism of AsA synthesis on transcriptional level.
Proteolysis Modulation in AsA Synthesis on Post-Transcriptional Level
Ubiquitin-proteasome degradation has been involved in the plant development, phytohormone signaling and responses to abiotic stresses.19 Proteolysis is also involved in the regulation of AsA synthesis. AMR1 might function as a component of an SCF ubiquitin ligase complex due to its typical F-box motif, to degrade its target protein that positively regulates AsA synthesis.13 Though the molecular mechanism of AMR1 functions in AsA synthesis is still unclear, this finding indicates that proteolysis is possibly involved in the regulation of AsA synthesis.
It is well known that plants have circadian rhythms to respond environmental changes, photosynthesis is typically rhythmic and results in a series of periodical physiological processes, such as AsA synthesis. AsA content in plant is rhythmic over a 24 h period, AsA levels increase toward the end of the light period and decrease along with the dark period.20 As human taking vitamins, plants make AsA synthesis themselves as antioxidant in the light, while AsA levels decrease when the light goes out. Light induces the expression of AsA biosynthesis genes at transcriptional level, but it is unclear how the decrease of AsA levels in the dark is modulated. It is significant for the plant to use its limited metabolic resources most efficiently and protein degradation limiting the responses is a good way to keep metabolic cost. Similarly, proteolysis has a role in inhibition of AsA synthesis in the dark.21 Our most resent breakthrough finds that VTC1 degradation results in decreased AsA levels in dark, photomorphogenic complex CSN inhibits AsA synthesis in darkness via direct interaction between its subunit CSN5B and VTC1.22 CSN functions in the ubiquitin-proteasome pathway through regulating the activity of E3 ligase.23 Interestingly, we examined VTC1 could be ubiquitinationed and CSN5B promotes VTC1 degradation via 26S proteasome pathway in the dark, loss function of CSN5B results in increased AsA content and enhanced tolerance to salt and oxidative stresses. Therefore, CSN plays a negative role in regulation AsA content on post-transcriptional level in darkness. Nevertheless, there are still some supplemental questions, the CSN functions exhibit in nucleus and VTC1 is cytosol-localized, the translocation of VTC1 is still unknown.24 For the target protein degraded through 26S proteasome pathway generally needs a special E3 ligase assistance and other components, the identification of these factors involved in VTC1 degradation is under investigation in our laboratory.
Concluding Remarks and Perspective
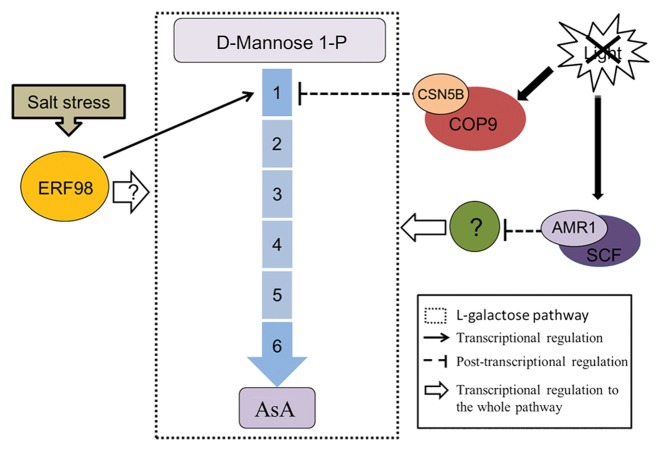
L-galactose pathway has been recognized as the main AsA synthesis pathway in plants,25 studies focused on the modulation of this pathway will contribute significant instruction to metabolism engineering. Based on our and previous studies, we propose a summary model to overview the current progresses on regulation of AsA L-galactose synthesis pathway in plants (Fig. 1). On the transcriptional level, AMR1 negatively regulates transcript levels of L-galactose pathway genes, while light deactivates the expression of AMR1. In addition, the expression of ERF98 is induced under salt stress and then activates VTC1 expression to improve AsA content, resulting in increased tolerance to salt stress. On the post-transcriptional level, CSN5B promotes VTC1 degradation under darkness in order to decrease AsA content, which is helpful to keep the balance of redox state in vivo. It is definitely changed that AsA content varies environmental conditions, these discoveries show that plants adjust the AsA synthesis to respond salt stress and light/dark cycles. More functions of these regulators and additional factors involved in the modulation of AsA synthesis will be identified in coming years.
Figure 1. Overview of the reported regulators on L-galactose pathway of AsA synthesis. The broken line box shows L-galactose pathway. Enzymes: (1) GDP-Man pyrophosphorylase (VTC1);(2) GDP-Man-3′,5′-epimerase (GME);(3) GDP-L-Gal phosphorylase (VTC2/VTC5); (4) L-Gal 1-phosphate phosphatase (GPP);(5) L-Gal dehydrogenase (GalDH); (6) L-GalL dehydrogenase (GalLDH). Salt stress triggers ERF98 expression and subsequently VTC1 higher expression on transcriptional level, finally improving AsA content to respond stress, but the modulation of ERF98 on other enzymes needs further research. Light inhibits AMR1 expression, which further deactivates the expression of the synthesis enzyme genes mediated by an unknown protein. When the light goes out, CSN5B carry out function to inhibit AsA synthesis on post-transcriptional level through interaction with VTC1 directly to promote its degradation.
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB114204 and 2013CB127003) and the National Science Foundation of China (90917018 and 31171171).
Glossary
Abbreviations:
- AsA
ascorbic acid
- VTC1
GDP-mannose pyrophosphorylase
- ROS
reactive oxygen species
- MDHA
monodehydroascorbate
- DHA
dehydroascorbate
- AO
ascorbate oxidase
- APX
AsA peroxidase
- MDAR
monodehydroascorbate reductase
- DHAR
dehydroascorbate reductase
- ERF
ethylene response factor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24536
References
- 1.Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- 2.Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol. 2000;3:229–35. [PubMed] [Google Scholar]
- 3.Tóth SZ, Puthur JT, Nagy V, Garab G. Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol. 2009;149:1568–78. doi: 10.1104/pp.108.132621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valpuesta V, Botella MA. Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci. 2004;9:573–7. doi: 10.1016/j.tplants.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Pignocchi C, Foyer CH. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr Opin Plant Biol. 2003;6:379–89. doi: 10.1016/S1369-5266(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T, Shigeoka S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci Biotechnol Biochem. 2008;72:1143–54. doi: 10.1271/bbb.80062. [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, et al. Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta. 2010;231:609–21. doi: 10.1007/s00425-009-1075-3. [DOI] [PubMed] [Google Scholar]
- 8.Green MA, Fry SC. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature. 2005;433:83–7. doi: 10.1038/nature03172. [DOI] [PubMed] [Google Scholar]
- 9.Conklin PL. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004;27:959–970. doi: 10.1111/j.1365-3040.2004.01203.x. [DOI] [Google Scholar]
- 10.Hemavathi, Upadhyaya CP, Akula N, Young KE, Chun SC, Kim DH, et al. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett. 2010;32:321–30. doi: 10.1007/s10529-009-0140-0. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, He W, Guo J, Chang X, Su P, Zhang L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot. 2005;56:3041–9. doi: 10.1093/jxb/eri301. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW. Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol Plant. 2010;139:421–34. doi: 10.1111/j.1399-3054.2010.01369.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Lorence A, Gruszewski HA, Chevone BI, Nessler CL. AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009;150:942–50. doi: 10.1104/pp.109.138453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Wang J, Zhang R, Huang R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012;71:273–87. doi: 10.1111/j.1365-313X.2012.04996.x. [DOI] [PubMed] [Google Scholar]
- 15.Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci. 2000;355:1455–64. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamaoki M. Light-controlled expression of a gene encoding L-galactono-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci. 2003;164:1111–7. doi: 10.1016/S0168-9452(03)00122-5. [DOI] [Google Scholar]
- 17.Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, et al. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot. 2007;58:2661–71. doi: 10.1093/jxb/erm124. [DOI] [PubMed] [Google Scholar]
- 18.Fukunaga K, Fujikawa Y, Esaka M. Light regulation of ascorbic acid biosynthesis in rice via light responsive cis-elements in genes encoding ascorbic acid biosynthetic enzymes. Biosci Biotechnol Biochem. 2010;74:888–91. doi: 10.1271/bbb.90929. [DOI] [PubMed] [Google Scholar]
- 19.Serino G, Xie Q. The ever expanding role of ubiquitin and SUMO in plant biology. J Integr Plant Biol. 2013;55:5–6. doi: 10.1111/jipb.12020. [DOI] [PubMed] [Google Scholar]
- 20.Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–89. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 21.Mach J. COP9 signalosome-regulated proteolysis: turning off ascorbic acid synthesis when the lights go out. Plant Cell. 2013;25:359. doi: 10.1105/tpc.113.250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Yu Y, Zhang Z, Quan R, Zhang H, Ma L, et al. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013;25:625–36. doi: 10.1105/tpc.112.106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei N, Serino G, Deng XW. The COP9 signalosome: more than a protease. Trends Biochem Sci. 2008;33:592–600. doi: 10.1016/j.tibs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Xiong L. F1000Prime Recommendation of Wang J et al., Plant Cell 2013. F1000Prime. 2013 doi: 10.3410/f.717980339.793471561. [DOI] [Google Scholar]
- 25.Laing WA, Wright MA, Cooney J, Bulley SM. The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA. 2007;104:9534–9. doi: 10.1073/pnas.0701625104. [DOI] [PMC free article] [PubMed] [Google Scholar]