Abstract
Study Design Preclinical in vitro culture of human degenerated nucleus pulposus (NP) tissue.
Objective Cyclooxygenase 2 inhibitors (e.g., celecoxib) inhibit prostaglandin E2 (PGE2) production, and they have been shown to upregulate regeneration of articular cartilage. In this study, we developed an explant culture system for use with human tissue and tested the potential of celecoxib.
Methods NP explants were cultured with or without 1 μM of celecoxib and were analyzed at days 0 and 7 for biochemical content (water, sulfated glycosaminoglycans, hydroxyproline, and DNA), gene expression (for disk matrix anabolic and catabolic markers), and PGE2 content.
Results Water and biochemical contents as well as gene expression remained close to native values after 1 week of culture. PGE2 levels were not increased in freshly harvested human NP tissue and thus were not reduced in treated tissues. Although no anabolic effects were observed at the dosage and culture duration used, no detrimental effects were observed and some specimens did respond by lowering PGE2.
Conclusions Human degenerated NP explants were successfully cultured in a close to in vivo environment for 1 week. Further research, especially dosage-response studies, is needed to understand the role of PGE2 in low back pain and the potential of celecoxib to treat painful disks.
Keywords: explant culture, intervertebral disk degeneration, inflammation, cyclooxygenase 2 inhibitor, regenerative therapy
Introduction
Low back pain has major financial and socioeconomic implications and is a common cause of disability.1 2 Many different mechanisms can cause low back pain, but it is heavily associated with intervertebral disk degeneration.3 A possible cause of disk degeneration is the shift from an anabolic to a catabolic environment in the disk. The cells in the nucleus pulposus (NP) decrease their production of the main matrix proteins and increase their production of degrading enzymes.4 5 6 7 Consequently, the NP loses proteoglycans and water and over time changes from a gel-like to a fibrous structure.8 9 This leads to a loss in disk height and function, which ultimately can cause pain.10 11
Current treatments for low back pain, such as conservative therapy and spinal fusion, have limited long-term success and do not treat the cause of degeneration. Regeneration of the disk is an alternative approach to treat disk degeneration at an early stage and prevent low back pain from occurring.12 However, these therapies still need to be further improved as they could not fully restore the disk to its original healthy state.13 To screen for possible new therapies, experimental models of disk degeneration are needed.14
Before these therapies can be used, they need to be tested in a preclinical setting. Animal models (in vivo systems) usually involve induced degeneration, which is different from the human disease.14 Besides this, they have low-volume throughput, are cost intensive, and have ethical considerations.14 15 Hence, using in vitro models is appealing. Cell culture, although less expensive and of higher-volume throughput, does not preserve the native tissue environment, which affects the cellular behavior to regenerative stimuli. From all in vitro models, whole-disk explants preserve the native environment best, but their throughput volume is still low and their stability in long-term culture is limited.16 As most of the early detectable changes are in the NP,17 we have developed a long-term explant culture model of NP tissue where osmotic balance was used to keep bovine NP explants in a situation similar to in vivo for 3 weeks.18 The next step is to use human degenerated tissue instead of bovine, so it can be used to test regenerative strategies.
Regenerative strategies aimed to counteract the detrimental matrix changes associated with disk degeneration are promising. A possible cause for increased activity of enzymes degrading the disk matrix, such as ADAMTS (a disintegrin and metalloproteinase with thrombospondin type 1 motifs) and matrix metalloproteinases (MMPs), lies in preinflammatory cytokines. These cytokines, like tumor necrosis factor-α, interleukin-1β and -6, and prostaglandin E2 (PGE2), have been shown indeed to promote catabolic enzyme activity in disk tissue.19 20 As these cytokines are found in moderately degenerated disk tissue,21 22 they can constitute possible targets for early inhibition of degeneration. Elevated levels of PGE2, for example, are involved in pain and irritating nerve roots.23 Such levels have also been shown to increase MMP-13 and ADAMTS-5 and reduce proteoglycans synthesis in osteoarthritic (OA) cartilage and NP cells.24 25 Therefore, inhibiting PGE2 may also be a valid approach for early regenerative therapy, although PGE2 involvement in disk degeneration is not fully known. In OA cartilage, where the equilibrium between synthesis and degradation shifts toward a catabolic environment like in disk degeneration,26 elevated levels of PGE2 compared with healthy tissue were found,27 supporting the idea that PGE2 is involved in degeneration of cartilaginous tissue.
PGE2 belongs to the class of prostanoids, which are local-acting lipid mediators with many regulatory functions throughout the body, and is produced by cyclooxygenase-1 and 2 (COX1 and COX2). Most nonsteroidal anti-inflammatory drugs (NSAIDs) are aimed at shutting down both COX isoforms. Elevated levels of PGE2 in inflammation, however, are produced by COX2, and COX1 mainly produces basal levels of PGE2. With this in mind, COX2-specific NSAIDs, like celecoxib (Cxb), have been developed in recent years and are a focus of research for long-term use as painkillers in OA treatment.26 These studies showed that Cxb stops elevated PGE2 production in OA cartilage through its main action on COX2. Cxb treatments were also able to alleviate the catabolic environment of OA by increasing PG production27 and reducing MMP-13 and ADAMTS-5 in OA explants,24 contrary to COX1-specific or nonspecific inhibitors.28 Besides this main mechanism, there is evidence that Cxb also acts on nuclear factor-kB and JNK MAPK to reduce production of nitrous oxide and MMP-1 and -3.29 Nitrous oxide is involved in inhibiting PG synthesis, inducing chondrocyte apoptosis, and stimulating MMPs.30 So although Cxb is currently used as a painkiller in OA, it might also act as an anabolic agent through the two pathways mentioned above, making it also a good candidate for intervertebral disk regeneration. Tests in explant culture of early degenerated disks can be used to investigate the potential of Cxb to treat early degeneration.
Therefore, in this study we investigated if human early degenerated tissue (Thompson grade 3) could be kept close to their in vivo state, using our NP explant culture model. Second, we used this model to explore the potential of Cxb as a regenerative therapy, by investigating the ability of Cxb treatment to modulate PGE2 production and promote matrix formation in human early degenerated tissue explants.
Materials and Methods
Culture
Human intervertebral disk tissue was obtained within 24 hours postmortem from autopsy subjects. Collection of intervertebral disk specimens was according to the medical ethical regulations of the University Medical Center Utrecht (Utrecht, The Netherlands). Five human disks were harvested from four donors (one woman, three men; age 44 to 77 years; two died of myocardial infarction and two died of carcinoma) under sterile conditions. The disks were opened transversely and the appearance of the NP, annulus fibrosus, end plate, and vertebrae were used to determine the grade of degeneration according to the Thompson classification.31 Only Thompson grade 3 disks were used in this study. The NPs were taken out of each disk and cut into six pieces of ∼ 200 mg. The samples were cultured for 1 week in our polyethylene glycol (PEG) system18: NP explants were placed in a semipermeable membrane (15-kDa molecular weight cutoff: Spectra/Por, Rancho Dominguez, California, United States), closed by custom-made clips, and then cultured in 6 mL of media. Medium was prepared from high glucose (4.5 g/L) Dulbecco's Modified Eagle Medium (DMEM; Gibco Invitrogen, Carlsbad, CA, United States) powder, supplemented with 1% penicillin/streptomycin (Lonza, Basel, Switzerland), 3.7 g/L sodium bicarbonate (Sigma, Zwijndrecht, the Netherlands), 50 mg/L ascorbic acid (Sigma), and 10% fetal bovine serum (Gibco). The medium was filter sterilized and the pH was adjusted to 7.1 to reproduce the pH conditions of a healthy human disk.32 Osmolarity of the culture medium was adjusted based on literature data on the glycosaminoglycan (GAG) content of human samples of different grades33 and on our own experience with bovine samples.18 To this medium, 12.95% (w/v) PEG (20 kDa; Sigma) was added to reach an osmolarity for maintaining grade 3 NP tissues. In this system, samples were cultured either with 0 μM or 1 μM of Cxb (Biovision, San Francisco, California, United States: molecular weight; 381 Da). We selected 1 μM as this concentration was effective in OA explants.27 To control for the effects of dimethyl sulfoxide (DMSO: Merck, Darmstadt, Germany) used to dissolve Cxb, the same amount of DMSO (0.1%) was added to the control group. All cultures were performed in duplicate, in a humidified incubator at 21% O2 and 5% CO2. Media were changed two times a week (i.e., at the beginning of culture and after 3 days). From every donor, six pieces of NP tissue were cultured as follows: two samples used as day 0 control, two samples cultured with 1 μM Cxb, and two samples cultured without Cxb. At days 0 and 7, samples were cut in three pieces and used to determine the biochemical composition, the gene expression levels, and the PGE2 content of the NP tissue.
Biochemical Content
At days 0 and 7, the samples were blotted dry, weighed, and subsequently stored frozen at −30°C. The samples were then placed in a lyophilizer (Freezone 2.5: Labconco, Kansas City, Missouri, United States) overnight and the dry weight was measured. The water content (%) was calculated from the difference in wet and dry weight, divided by the wet weight. The dried samples were then digested in papain solution (100 mM phosphate buffer, 5 mM L-cystein, 5 mM ethylene diamine tetraacetic acid, and 125 to 140 mg/mL papain; all from Sigma) overnight at 60°C. The digested samples were then used to determine their content of sulfated GAGs (sGAGs), as a measure for PG content; hydroxyproline (HYP), as a measure for collagen content; and DNA. sGAG content was determined with the dimethylmethylene blue assay34 using shark cartilage chondroitin sulfate as reference (Sigma). The fixed charge density was determined from the GAG content per wet weight, similar to the method used by Narmoneva et al.35 HYP content was measured using the chloramine-T assay36 and a trans-4-hydroxyproline (Sigma) reference. DNA content was measured using the Hoechst dye method,37 with a calf thymus DNA reference (Sigma). The amounts of sGAG, HYP, and DNA were expressed per mg dry weight of tissue.
Gene Expression Levels
At days 0 and 7, samples for gene expression analysis were snap frozen in liquid N2 and stored at −80°C. To disrupt the samples, a 420 stainless steel 8-mm bead and 316-L stainless steel custom-made lid were placed in a 2-mL Eppendorf tube and snap frozen in liquid N2. Frozen samples were placed in between bead and lid, and disrupted with a mikro-dismembrator (Sartorius, Goettingen, Germany) for 20 seconds at 1,500 rpm. This was repeated if necessary with the sample snap frozen between each cycle. After disruption, RNA was isolated using TRIzol (Invitrogen) and purified using the Qiagen mini-kit (Qiagen, Venlo, the Netherlands). An additional DNase I treatment (Qiagen) was conducted during purification, according to manufacturer's instructions. The quantity and purity of isolated RNA was measured with a spectrophotometer (ND-1000: Isogen, de Meern, the Netherlands). Absence of genomic DNA contamination in the isolated RNA was checked using real-time polymerase chain reaction (PCR; CFX384: Biorad, Veenendaal, the Netherlands) with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers. The genes of interest and corresponding primer pairs (when available) are summarized in Table 1. The sequences of these primer pairs were either custom designed (PrimerDesign, Southampton, UK) or obtained from literature (Sigma). The VILO kit (Invitrogen) was used to reverse transcribe 70 ng of total RNA. GAPDH was selected as reference gene from three tested genes (RPL13A, GAPDH, and 18S) as it was the most stable gene throughout our experimental conditions. Gene expression was investigated using real-time PCR, and expression difference (ΔCt) was calculated as the difference between the gene of interest and the reference gene GAPDH. Levels of expression are expressed as 2−ΔCt. When gene expression was not detected, the 2−ΔCt value was set to 0 to conduct the statistical analysis.
Table 1. List of primers used for real-time polymerase chain reaction.
| Gene | Accession gene number | Oligonucleotide sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| GAPDH (PD) | – | Not available | – |
| Aggrecan (PD) | NM_001135.3 | 106 | |
| Forward | 5′-TGCTTTGTAGACAGACTTGAGG-3′ | ||
| Reverse | 5′-CCAGCGTAGCATTGTGAGATT-3′ | ||
| Collagen type 2 (PD) | NM_001844.4 | 86 | |
| Forward | 5′-GGGAGAGCCTGGAGATGAC-3′ | ||
| Reverse | 5′-GACCGACGATGCCTCTCTG-3′ | ||
| Collagen type 1 (PD) | NM_000088.3 | 112 | |
| Forward | 5′-TGGCTCTCCTGGTGAACAAG-3′ | ||
| Reverse | 5′-GCCAGGGAGACCGTTGAG-3′ | ||
| Collagen type X | NM_000493.3 | 104 | |
| Forward | 5′-GTGGACCAGGAGTACCTTGC-3′ | ||
| Reverse | 5′-CATAAAAGGCCCACTACCCA-3′ | ||
| COX1 (PD) | NM_000962.2 | 114 | |
| Forward | 5′-CGTGTGTGTGACCTGCTGAA-3′ | ||
| Reverse | 5′-GTACTCCTCGATGACAATCTTGATG-3′ | ||
| COX2 (PD) | NM_000963.2 | 123 | |
| Forward | 5′-CAGGCTTCCATTGACCAGAG-3′ | ||
| Reverse | 5′-TGCAGACATTTCCTTTTCTCCT-3′ | ||
| MMP-13 | NM_002427.3 | 208 | |
| Forward | 5′-GGAGCATGGCGACTTCTAC-3′ | ||
| Reverse | 5′-GAGTGCTCCAGGGTCCTT-3′ | ||
| ADAMTS 4 (PD) | NM_005099.4 | 137 | |
| Forward | 5′-GACCACTTTGACACAGCCATTC-3′ | ||
| Reverse | 5′-AGCCCATCATCCTCCACAATG-3′ | ||
| ADAMTS 5 (PD) | NM_007038.3 | 107 | |
| Forward | 5′-GCAGCACCAACACAACCAG-3′ | ||
| Reverse | 5′-CCAGGGTGTCACATGAATGATG-3′ |
Abbreviations: ADAMTS, a disintegrin and metalloproteinase with thrombospondin type 1 motifs; bp, base pairs; COX, cyclooxygenase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP, matrix metalloproteinases; PD, primer sequences designed by PrimerDesign Ltd. (Southampton, UK).
PGE2 Levels
At days 0 and 7, samples for PGE2 analysis were snap frozen in liquid N2 and stored at −80°C. The samples were disrupted similarly as for RNA isolation. After disruption, lysis buffer (KDalert: Life Technologies, Carlsbad, California, United States) was added and the samples were incubated at 4°C overnight on a roller bank. The PGE2 content per sample was then determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D systems, Minneapolis, Minnesota, United States). For every sample, a commercial BCA assay (Pierce: Thermo Scientific, Rockford, Illinois, United States) was used to determine total protein content, using a bovine serum albumin standard. PGE2 content was normalized to total protein content for every sample.
Statistics
MATLAB (Mathworks Inc., Natick, Massachusetts, United States) was used for statistical analysis. Kruskal-Wallis analysis was performed for all the groups, followed by Bonferroni-corrected post hoc Mann-Whitney U tests. Statistical significance in all cases was assumed for p < 0.05.
Results
Biochemical Content
After 7 days in the PEG system, the water, sGAG, DNA, and HYP contents as well as the fixed charge density in both the treated and untreated explants were not different from day 0 controls (Fig. 1A to E).
Fig. 1.
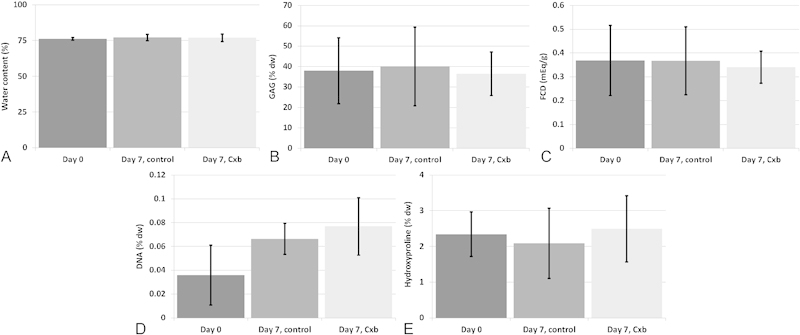
Biochemical content. Water content (A), sulfated glycosaminoglycan (sGAG) content expressed per dry weight (dw) (B), fixed charge density (FCD) (C), DNA content expressed per dry weight (D), and hydroxyproline content expressed per dw (E). All readings taken at days 0 and 7. Values are means ± standard deviation, n = 5 (from four donors).
Gene Expression Levels
No significant differences were observed for the expression levels of aggrecan, type I and II collagen, COX1 and COX2, and ADAMTS 4 and 5 for all culture groups compared with day 0 (Fig. 2A to C, E to F, H to I), with expression levels of ADAMTS 4, 5, and COX1 that were not detected in most of the samples. Collagen type X mRNA expression was significantly upregulated in untreated samples compared with day 0 (Fig. 2D). MMP-13 mRNA expression was significantly upregulated in both culture groups compared with day 0 (Fig. 2G), in which MMP-13 was not detected.
Fig. 2.
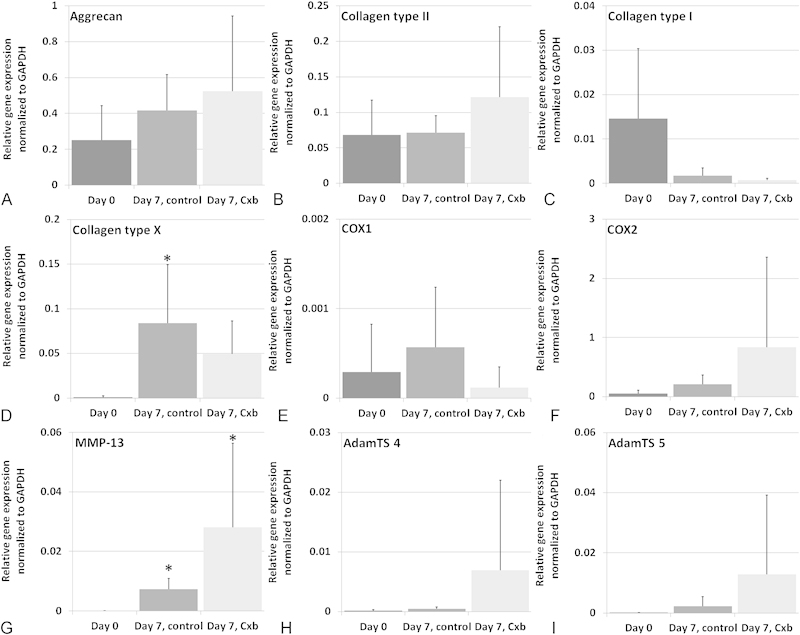
Gene expression levels. Aggrecan (A), collagen type II (B), collagen type I (C), collagen type X (D), cyclooxygenase-1 (COX1) (E), cyclooxygenase-2 (COX2) (F), matrix metalloproteinases (MMP-13) (G), a disintegrin and metalloproteinase (ADAM) metalloproteinases with thrombospondin type 1 motifs 4 (ADAMTS 4) (H), and ADAM metalloproteinases with thrombospondin type 1 motifs 5 (ADAMTS 5) (I). All readings taken at days 0 and 7. Gene expression levels are relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression levels (2−ΔCt). Values are means + standard deviation, n = 5 (from four donors). *Different from day 0; p < 0.05.
PGE2 Content
The PGE2 content measured in the tissue after 7 days of culture, in both the control and the Cxb-treated group, was not different from day 0 (Fig. 3). The nonsignificant increase in the control group is caused by two samples from one donor where a larger PGE2 content was measured, which was not observed in Cxb-treated samples.
Fig. 3.
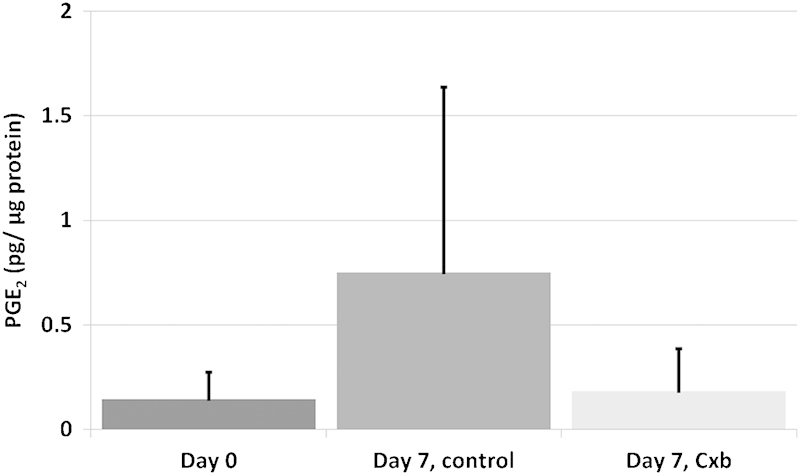
Prostaglandin E2 (PGE2) content expressed per μg of total protein at day 0 and 7. Values are means + standard deviation, n = 5 (from four donors). Cxb, celecoxib.
Discussion
In this study we successfully cultured human early degenerated NP explants in a close to in vivo situation for 1 week, using a previously developed and validated explant model.18 After 7 days in our system, there were no changes in hydroxyproline, sGAG, DNA, and water content in untreated explants compared with day 0 controls. The fixed charge density was also not different from fresh tissue, showing that medium supplemented with a 12.95% (w/v) PEG was sufficient to prevent swelling, and subsequent PG loss, of Thompson grade 3 degenerated NP explants cultured in vitro for 1 week. The cellular behavior was also very close to native tissue, as expression of matrix genes (collagen type I, type II, and aggrecan) was not different from day 0 controls. We did see, however, upregulated MMP-13 expression, which is involved in matrix degradation. We recently showed in a 6-week culture of bovine NP explants that physiologic levels of O2 (5 versus 21% used here) and glucose (1.5 g/L versus 4.5 g/L used here), as well as mechanical pressure to balance osmotic swelling, were beneficial for keeping MMP-13 expression close to native expression.38 So these adjustments should also further improve our in vitro model for human degenerated disk tissue.
We did not see an effect of Cxb on the PGE2 production of human grade 3 degenerated explants (Fig. 3). The dosage used (1 μM), however, was shown to be effective to lower the PGE2 production of human OA cartilage explants to levels produced by healthy explants.27 It is striking that, in the present study, the measured levels of PGE2 in both day 0 and day 7 treated tissues were very similar. This means that, either the treatment with Cxb was not successful as COX2-produced PGE2 was not lowered, or the measured PGE2 levels are a result of COX1 activity. The former is not likely due to Cxb-reported activity in cartilage.27 COX1-produced levels are considered basal levels. In this study, we used a limited number of human tissue from autopsy subjects, which although are of grade III degeneration, are likely nonsymptomatic (i.e., painful). Hence our results could indicate that inflammatory process involving PGE2 in early disk degeneration is not generally pervasive or that this may only be present in painful degenerating disks.
Interesting to note, two samples from one donor in the untreated group contained a higher PGE2 content, which was lower for the treated samples from the same donor, indicating that this Cxb concentration might be effective in reducing PGE2 when elevated. However, to determine if the measured PGE2 levels were abnormally elevated, they need to be compared with literature, which was not possible for several reasons. Not many studies have focused on PGE2 levels in degenerated disk tissue, and if they have, they measured release to the media of surgical tissues.39 40 Measuring release to the media was unfortunately not possible in this study, as PEG, which was used to raise the osmotic pressure, interfered with the ELISA measurement. One study measured PGE2 levels in surgical samples of herniated tissue; because they normalized to tissue weight41 and not to total protein, we cannot compare the values of PGE2. Nevertheless, the dosages of Cxb used in this study did not result in any adverse results compared with controls. Therefore in the future, the levels of PGE2 in tissues of different grades of degeneration, as well as symptomatic and nonsymptomatic disks, may need to be determined, to investigate the value of Cxb treatment for symptomatic disk degeneration.
We also did not observe significant anabolic effects of Cxb, both at the protein or gene level, which was contrary to studies performed with OA tissue.24 27 There were, however, nonsignificant trends of increased aggrecan and collagen type II gene expression after Cxb treatment (Fig. 2A and B), indicating that Cxb might still have an anabolic potential, although not at the concentration and treatment duration used in the present study. In human OA explants though, 1 μM Cxb for 7 days was already effective to increase PG production and retention.27 COX2 clearly plays a very important role in OA, but might not be the key factor in disk degeneration. In untreated samples, collagen type X expression was significantly different from day 0 controls, but not in treated samples. A recent article showed that COX2 is involved in chondrocyte hypertrophy, and that Cxb could reduce collagen type X mRNA expression in a mouse cell line.42
Future work will be to further investigate the role of COX2 and PGE2 in different grades of degenerated disk tissue and to compare symptomatic with nonsymptomatic tissue. If the results show potential for Cxb treatment against disk degeneration, an artificial annulus approach with human degenerated tissue cultured under physiologic oxygen and glucose conditions will be used to validate this potential. However, this explant culture model can also be used to investigate the potential of other therapeutics, possibly in combination with sustained release biomaterials, and to better understand intervertebral disk degeneration.
Conclusion
Human degenerated NP explants were successfully cultured in a close to in vivo environment for 1 week, but did not respond strongly to a continuous stimulus of 1 μM Cxb for 7 days.
Acknowledgment
This research forms part of the Project P2.01 IDiDAS of the research program of the BioMedical Materials institute, cofunded by the Dutch Ministry of Economic Affairs. The financial contribution of the Dutch Arthritis Foundation is gratefully acknowledged.
References
- 1.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Walker B F, Muller R, Grant W D. Low back pain in Australian adults: prevalence and associated disability. J Manipulative Physiol Ther. 2004;27(4):238–244. doi: 10.1016/j.jmpt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Livshits G, Popham M, Malkin I. et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70(10):1740–1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar E J, An H S. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine (Phila Pa 1976) 2002;27(20):2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Anderson D G Tannoury C Molecular pathogenic factors in symptomatic disc degeneration Spine J 20055(6, Suppl):260S–266S. [DOI] [PubMed] [Google Scholar]
- 6.Weiler C, Nerlich A G, Zipperer J, Bachmeier B E, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11(4):308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Maitre C L, Freemont A J, Hoyland J A. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 8.Antoniou J, Steffen T, Nelson F. et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams M A, Stefanakis M, Dolan P. Healing of a painful intervertebral disc should not be confused with reversing disc degeneration: implications for physical therapies for discogenic back pain. Clin Biomech (Bristol, Avon) 2010;25(10):961–971. doi: 10.1016/j.clinbiomech.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Kandel R, Roberts S, Urban J P. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008;17 04:480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urban J P, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuda K An H S Growth factors and the intervertebral disc Spine J 20044(6, Suppl):330S–340S. [DOI] [PubMed] [Google Scholar]
- 13.Masuda K, Lotz J C. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010;16(1):147–158. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alini M, Eisenstein S M, Ito K. et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh K Masuda K An H S Animal models for human disc degeneration Spine J 20055(6, Suppl):267S–279S. [DOI] [PubMed] [Google Scholar]
- 16.Jünger S, Gantenbein-Ritter B, Lezuo P, Alini M, Ferguson S J, Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine (Phila Pa 1976) 2009;34(12):1264–1271. doi: 10.1097/BRS.0b013e3181a0193d. [DOI] [PubMed] [Google Scholar]
- 17.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt K F, Nerlich A G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27(23):2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 18.van Dijk B, Potier E, Ito K. Culturing bovine nucleus pulposus explants by balancing medium osmolarity. Tissue Eng Part C Methods. 2011;17(11):1089–1096. doi: 10.1089/ten.TEC.2011.0215. [DOI] [PubMed] [Google Scholar]
- 19.Le Maitre C L, Freemont A J, Hoyland J A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Séguin C A, Pilliar R M, Madri J A, Kandel R A. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 2008;33(4):356–365. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 21.Burke J G, G Watson R W Conhyea D et al. Human nucleus pulposis can respond to a pro-inflammatory stimulus Spine (Phila Pa 1976) 200328242685–2693. [DOI] [PubMed] [Google Scholar]
- 22.Weiler C Nerlich A G Bachmeier B E Boos N Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls Spine (Phila Pa 1976) 200530144–53., discussion 54 [DOI] [PubMed] [Google Scholar]
- 23.Muramoto T, Atsuta Y, Iwahara T, Sato M, Takemitsu Y. The action of prostaglandin E2 and triamcinolone acetonide on the firing activity of lumbar nerve roots. Int Orthop. 1997;21(3):172–175. doi: 10.1007/s002640050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attur M, Al-Mussawir H E, Patel J. et al. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J Immunol. 2008;181(7):5082–5088. doi: 10.4049/jimmunol.181.7.5082. [DOI] [PubMed] [Google Scholar]
- 25.Vo N V, Sowa G A, Kang J D, Seidel C, Studer R K. Prostaglandin E2 and prostaglandin F2α differentially modulate matrix metabolism of human nucleus pulposus cells. J Orthop Res. 2010;28(10):1259–1266. doi: 10.1002/jor.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zweers M C, de Boer T N, van Roon J, Bijlsma J W, Lafeber F P, Mastbergen S C. Celecoxib: considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res Ther. 2011;13(5):239. doi: 10.1186/ar3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastbergen S C, Bijlsma J W, Lafeber F P. Selective COX-2 inhibition is favorable to human early and late-stage osteoarthritic cartilage: a human in vitro study. Osteoarthritis Cartilage. 2005;13(6):519–526. doi: 10.1016/j.joca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Mastbergen S C, Jansen N W, Bijlsma J W, Lafeber F P. Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study. Arthritis Res Ther. 2006;8(1):R2. doi: 10.1186/ar1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsutsumi R, Ito H, Hiramitsu T. et al. Celecoxib inhibits production of MMP and NO via down-regulation of NF-kappaB and JNK in a PGE2 independent manner in human articular chondrocytes. Rheumatol Int. 2008;28(8):727–736. doi: 10.1007/s00296-007-0511-6. [DOI] [PubMed] [Google Scholar]
- 30.Henrotin Y E, Bruckner P, Pujol J P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11(10):747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 31.Thompson J P, Pearce R H, Schechter M T, Adams M E, Tsang I K, Bishop P B. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990;15(5):411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40(1):23–42. doi: 10.3109/17453676908989482. [DOI] [PubMed] [Google Scholar]
- 33.Antoniou J, Demers C N, Beaudoin G. et al. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn Reson Imaging. 2004;22(7):963–972. doi: 10.1016/j.mri.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Farndale R W, Sayers C A, Barrett A J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 35.Narmoneva D A, Wang J Y, Setton L A. Nonuniform swelling-induced residual strains in articular cartilage. J Biomech. 1999;32(4):401–408. doi: 10.1016/s0021-9290(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 36.Huszar G, Maiocco J, Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980;105(2):424–429. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
- 37.Cesarone C F, Bolognesi C, Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- 38.van Dijk B G, Potier E, Ito K. Long-term culture of bovine nucleus pulposus explants in a native environment. Spine J. 2013;13(4):454–463. doi: 10.1016/j.spinee.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Kang J D, Georgescu H I, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson W F III, Evans C H. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1996;21(3):271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 40.Burke J G, Watson R W, McCormack D, Dowling F E, Walsh M G, Fitzpatrick J M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell J L O'Donnell A L Prostaglandin E2 content in herniated lumbar disc disease Spine (Phila Pa 1976) 199621141653–1655., discussion 1655–1656 [DOI] [PubMed] [Google Scholar]
- 42.Welting T J Caron M M Emans P J et al. Inhibition of cyclooxygenase-2 impacts chondrocyte hypertrophic differentiation during endochondral ossification Eur Cell Mater 201122420–436., discussion 436–437 [DOI] [PubMed] [Google Scholar]


