Abstract
Study Design Biomechanical study of pedicle screw fixation in osteoporotic bone.
Objective To investigate whether it is better to tap or not tap osteoporotic bone prior to placing a cement-augmented pedicle screw.
Methods Initially, we evaluated load to failure of screws placed in cancellous bone blocks with or without prior tapping as well as after varying the depths of tapping prior to screw insertion. Then we evaluated load to failure of screws placed in bone block models with a straight-ahead screw trajectory as well as with screws having a 23-degree cephalad trajectory (toward the end plate). These techniques were tested with nonaugmented (NA) screws as well as with bioactive cement (BioC) augmentation prior to screw insertion.
Results In the NA group, pretapping decreased fixation strength in a dose-dependent fashion. In the BioC group, the tapped screws had significantly greater loads to failure (p < 0.01). Comparing only the screw orientation, the screws oriented at 23 degrees cephalad had a significantly higher failure force than their respective counterparts at 0 degrees (p < 0.01).
Conclusions Standard pedicle screw fixation is often inadequate in the osteoporotic spine, but this study suggests tapping prior to cement augmentation will substantially improve fixation when compared with not tapping. Angulating screws more cephalad also seems to enhance aging spine fixation.
Keywords: spinal fixation, osteoporosis, cancellous bone, pedicle screw, tapping, screw trajectory
Introduction
As people are living longer due to advances in health care and lifestyles, spine surgeons will be faced with a greater number of surgical problems in the aged population and with that comes an expected increase in perioperative medical complications. Another substantial challenge is advanced osteoporosis, which is found very predictably in this population. With this poor bone mineralization comes an array of complications associated with fixation failure. Until osteoporosis can be better managed in the perioperative period, surgeons will need to aggressively pursue methods to enhance fixation in the aging spine. Pedicle screw fixation is still the most popular fixation method, but to date this fixation proves to be inadequate in many patients with osteoporosis. Previous studies have shown that not tapping prior to placing a pedicle screw will enhance screw fixation in the osteoporotic spine.1 It has also been shown that cement augmentation of screws in the spine will also enhance fixation.2 3 4 5 6 7 8 9 10 11 12 13 Both techniques can be useful in a given situation. However, the effect of tapping versus not tapping when using cement to augment screws has not been evaluated fully.
In addition, surgeons have choices to make regarding the trajectory of the pedicle screws they implant. Although many studies regarded this, very few have addressed implications of changing screw trajectory in the face of osteoporosis. One study by Santoni et al did, however, and espouses a more lateral trajectory in the face of osteoporosis in an attempt to put the screw closer to a cortical buttress.14 To the authors' knowledge, this has not achieved widespread acceptance for unclear reasons.
The purpose of this study is to evaluate the effects of some technical choices a surgeon may make when faced with the need to achieve pedicle screw fixation in the osteoporotic vertebra. One choice faced is whether to pretap prior to placement of pedicle screws into an osteoporotic spinal segment. Another choice the surgeon faces is the preferred screw trajectory in an osteoporotic spinal segment. We chose to investigate the mechanical ramifications of a straight-ahead trajectory versus a cephalad-oriented screw trajectory.
Materials and Methods
Tapping versus Not Tapping
Synthetic osteoporotic cancellous bone block models were utilized as previously published by Choma et al.3 Osteoporotic bone was simulated by a 0.09-g/mL density open-cell rigid foam block attached with cement to a short fiber-filled epoxy sheet (Sawbones, Pacific Research Laboratories, Vashon, Washington, United States). The epoxy sheet mechanically simulated the dense superior end plate of the vertebra, and the whole structure can be seen in Fig. 1. Section blocks 30 mm wide × 60 mm deep were used for testing. Two groups were tested—a nonaugmented (NA) group and a group augmented with bioactive cement (BioC). This is a proprietary mixture of calcium sulfate and calcium phosphate provided by Wright Medical (Arlington, Tennessee, United States). In each specimen of both groups a centrally located pilot hole, 3.0 mm in diameter by ∼40 mm deep, was drilled into the end of each block using a straight Lenke-type probe, and then the blocks were threaded with a 5.5-mm-diameter tap (Stryker Xia, Kalamazoo, MI, United States) to a depth of 0, 20, 30, or 40 mm depending upon the configuration. Starter holes and screwdrivers were guided along tracks using custom jigs to ensure proper and repeatable orientation of screw placement. BioC, if applicable to the group being tested, was then injected (1.5 to 2.0 mL) for samples using a syringe and Jamshidi needle (CareFusion, San Diego, CA, United States) before a screw was manually inserted until the base of the head rested against the foam. The cement augmentation distribution for various tapping depths was characterized visually using backlit photography (Fig. 2). In the NA group, we tested four samples per specified tap depth, and in the augmented group, we tested five samples for each tap depth. More samples were tested in cement group as there was prestudy concern for more variability with the use of cement. Fixed-head pedicle screws were used in both subgroups (NA and BioC), which were 6.5 mm in diameter by 40 mm long (Stryker Xia). A rod 5.5 mm in diameter by 65 mm long (Stryker Xia) was then inserted into the screw head and secured by tightening the set screw to 12 N/m to complete the test specimen. Each augmented sample cured overnight prior to testing, which was well beyond the manufacturer's recommended minimum time.
Fig. 1.

Synthetic vertebrae with the epoxy end plate.
Fig. 2.
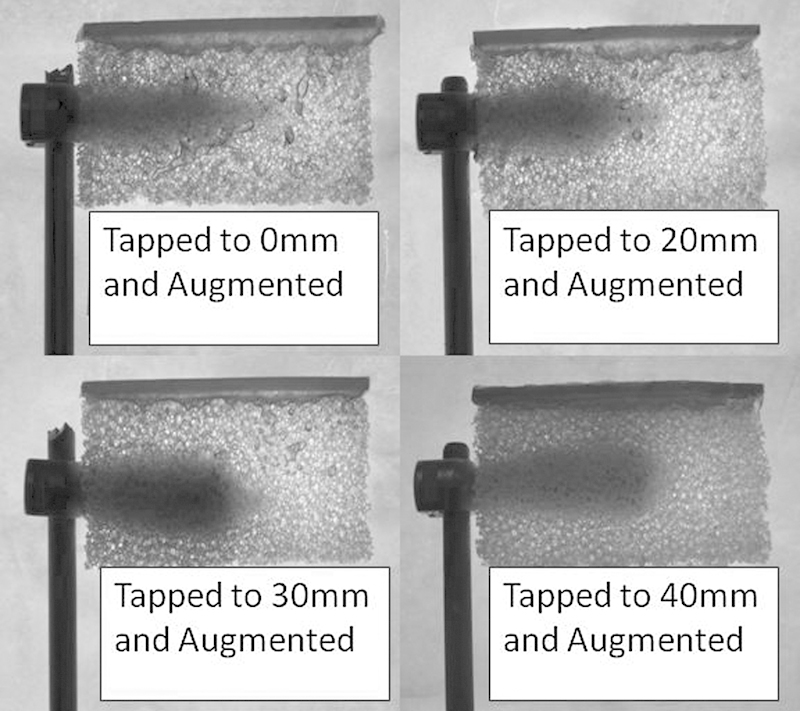
Augmented cases featuring different tap depths.
Screw Orientation
Identical synthetic osteoporotic bone blocks were used to evaluate the effect of varying screw trajectory in the sagittal plane. Two screw trajectories were evaluated in this study: a straight-ahead (0 degrees) screw, parallel to the superior end plate, and a cephalad-oriented screw (23 degrees cephalad), in which the tip approaches the superior end plate. We chose 23 degrees based upon screw geometry so that a consistent entry point was maintained between orientation groups and because it allowed the screw tip to contact the end plate. Four groups were evaluated in this portion of the study: an NA screw control group (NA-0) with the screw placed at 0 degrees relative to the end plate; an NA screw control group (NA-23) with the screw placed at 23 degrees relative to the end plate; a group augmented with calcium phosphate (CP-0) with the screw placed 0 degrees relative to the end plate; and a group augmented with calcium phosphate (CP-23) with the screw placed 23 degrees relative to the end plate. Prior to screw placement, a centrally located pilot hole, 3.0 mm in diameter by 40 mm deep, was drilled into the end of each block using a straight Lenke-type probe and then threaded with a 5.5-mm-diameter tap (Stryker Xia) to a depth of 30 mm. Starter holes and screwdrivers were guided along either 0- or 23-degree degree tracks to ensure proper and repeatable orientation of screw placement. Cement, if applicable, was then injected (1.5 to 2.0 mL) for each sample using a syringe and Jamshidi needle. Next, a pedicle screw was manually inserted until the base of the head rested against the foam. In the NA group, we tested four samples per specified screw trajectory (0 or 23 degrees), and in the augmented group, we tested five samples for each screw trajectory. Each augmented sample cured overnight prior to testing. More samples were tested in cement group as there was prestudy concern for more variability with the use of cement.
For both parts of this study, the foam block epoxy sheet assembly, which simulated vertebral bone, was securely mounted between the superior and inferior steel plates of the specimen-loading fixture. The specimen's rod was held securely in an angle vise and tilted 45 degrees relative to the test machine's horizontal table in a sagittal plane orientation. A 46.6-cm-long pushrod was attached to the universal joint of the test machine's load cell ram and the axis of the pin in the yoke attaching it to the specimen-loading fixture. The pushrod was used to transmit a force F along the pushrod's axis (vertical initially) to the test specimen at a point 45 mm anterior of the rod's Z axis and 10 mm below the inferior surface of the superior steel end plate. This created equal components of pullout force Fx and transverse force Fz in combination with a flexion bending moment M = Fz ∙ 0.015 N/m at the vertebra center. This configuration simulates a combined loading condition in a kyphotic thoracic spine with no external constraints on the relative motion in the sagittal plane between the screw and vertebra.
Motion of the pedicle screw relative to the simulated vertebra was measured with a 0.01-mm resolution 3D infrared optical tracking system (NDI Optotrak Certus, Waterloo, Ontario, Canada; Fig. 3). Quasi-static loading tests were performed using a servo-hydraulic test machine (Instron 8821s, 100 Royall Street, Canton, Massachusetts, United States). In quasi-static testing, compressive load was applied in displacement control at a rate of 0.40 mm/s until catastrophic failure of the specimen occurred. Failure initiation was defined as the first maximum magnitude of compression force F that resulted in nearly constant or declining load resistance for at least 1 mm of additional ram displacement. Differences in failure initiation force and corresponding screw displacements were statistically compared by using a Student t (two-tailed, unequal variance) test. Significance was taken as p ≤ 0.01.
Fig. 3.
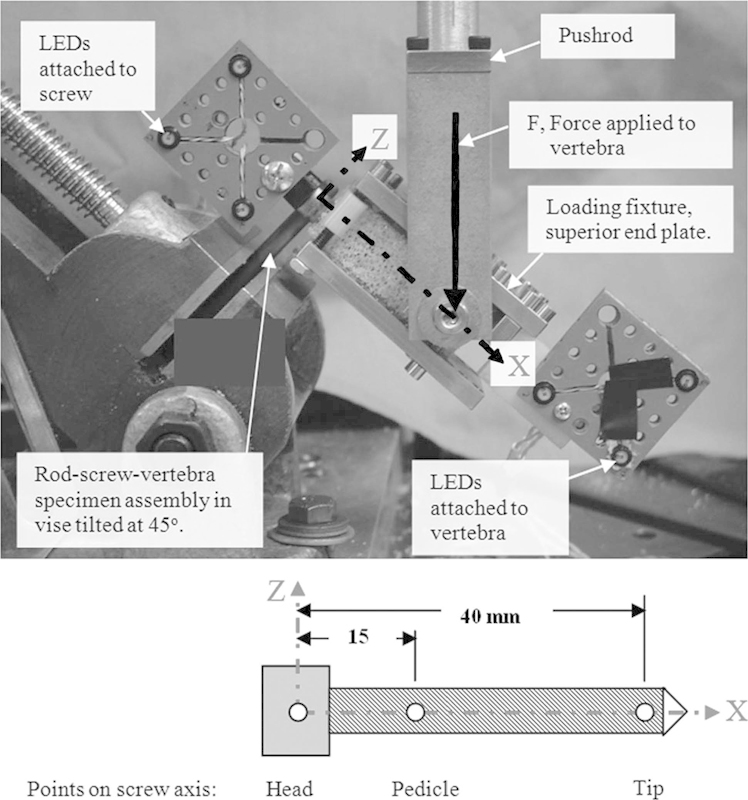
Instron setup with the Optotrak Sensors attached. (Reprinted from Choma TJ, Frevert WF, Carson WL, Waters NP, Pfeiffer FM. Biomechanical analysis of pedicle screws in osteoporotic bone with bioactive cement augmentation using simulated in vivo multicomponent loading. Spine (Phila Pa 1976) 2011;36(6):454–462, with permission from Lippincott Williams & Wilkins.3)
Results
Tapping versus Not Tapping
Tables 1 and 2 present the force to failure for screws placed into holes tapped to different depths ranging from 0 to 40 mm. In the absence of cement augmentation, tapping the hole prior to screw insertion appears detrimental to the failure force. Each NA sample shows a consistent decrease in force to failure as the tap depth increases. In comparing the untapped versus the tapped screw, the force to failure decreases 52% if the pilot hole is tapped to 40 mm prior to screw insertion (−30.93 ± 2.1 N versus −14.87 ± 0.3 N, respectively). This behavior is consistent with previous studies evaluating the effect of tapping prior to screw placement in the lumbar spine.11 12
Table 1. Average failure load (N) of augmented and nonaugmented specimens versus tap depth.
| Tap depth (mm) | Nonaugmented | Augmented | p value |
|---|---|---|---|
| 0 | −30.93 | −40.34 | <0.01 |
| 20 | −22.30 | −52.00 | <0.01 |
| 30 | −20.95 | −56.82 | <0.01 |
| 40 | −14.87 | −61.95 | <0.01 |
Table 2. Quasi-static failure load (N) nonaugmented group.
| Tap depth (mm) | ||||
|---|---|---|---|---|
| 0 | 20 | 30 | 40 | |
| Trial 1 | −31.5 | −23.1 | −21.5 | −15.1 |
| Trial 2 | −29.2 | −21 | −18.3 | −15 |
| Trial 3 | −33.6 | −22.4 | −23.3 | −14.5 |
| Trial 4 | −29.4 | −22.7 | −20.7 | – |
| Mean | −30.93 | −22.30 | −20.95 | −14.87 |
| Standard deviation | 2.065 | 0.913 | 2.074 | 0.321 |
| Standard error | 1.032 | 0.456 | 1.037 | 0.161 |
Alternatively, the samples with cement augmentation increased the force to failure as the tap depth increased. The average force to failure increased in each case that the tap depth increased (Tables 1 and 3). The average force to failure increased 54% when comparing untapped versus pretapping the entire screw length (−40.35 ± 1.6 N versus −61.95 ± 1.8 N, respectively). A graphical representation of the force to failure for NA and augmented screws relative to varied tap depths is shown in Fig. 4.
Table 3. Quasi-static failure load (N) BioC-augmented group.
| Tap depth (mm) | ||||
|---|---|---|---|---|
| 0 | 20 | 30 | 40 | |
| Trial 1 | −38.1 | −52.1 | −58 | −62.1 |
| Trial 2 | −41.1 | −49.2 | −54.4 | −59.5 |
| Trial 3 | −40.7 | −54.1 | −50.1 | −63.8 |
| Trial 4 | −42.2 | −51.2 | −62.1 | −62.4 |
| Trial 5 | −39.6 | −53.4 | −59.5 | – |
| Mean | −40.34 | −52.00 | −56.82 | −61.95 |
| Standard deviation | 1.560 | 1.927 | 4.676 | 1.794 |
| Standard error | 0.780 | 0.964 | 2.338 | 0.897 |
Abbreviation: BioC, bioactive cement.
Fig. 4.
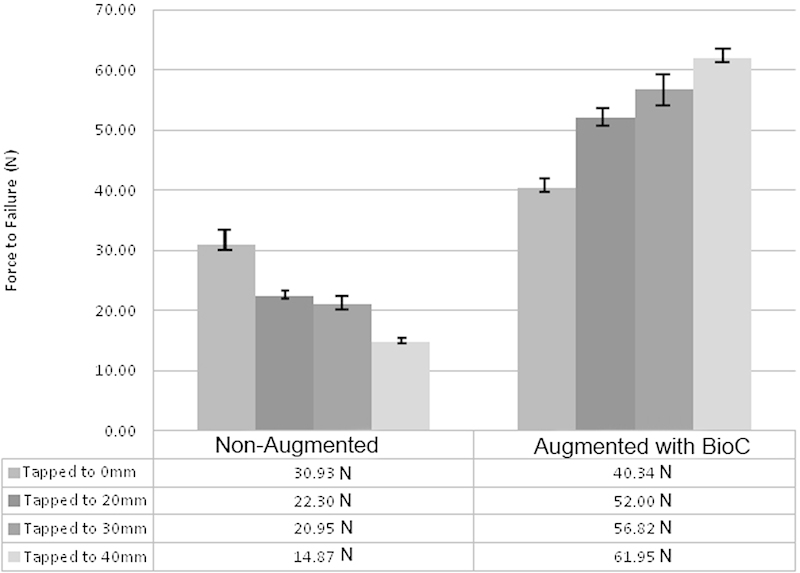
Average force of failure in tapping versus nontapping. Abbreviation: BioC, bioactive cement augmentation.
In comparing each case of the augmented versus NA specimens, the p values are all less than 0.01, taken as a statistically significant difference between the experimental augmented cases and NA control cases. These comparisons were determined with the Student t test (Table 4).
Table 4. Augmented tap depths F max (N) versus NA tap depthsa .
| BioC tap depth (mm) | ||||
|---|---|---|---|---|
| NA tap depth (mm) | 0 | 20 | 30 | 40 |
| 0 | 1.049E-04 | 9.823E-07 | 1.877E-05 | 4.800E-07 |
| 20 | 1.738E-07 | 1.851E-08 | 1.896E-06 | 1.785E-08 |
| 30 | 8.774E-07 | 6.932E-08 | 2.119E-06 | 9.278E-08 |
| 40 | 1.667E-07 | 6.091E-08 | 5.470E-06 | 1.157E-07 |
Abbreviations: BioC, calcium phosphate and calcium sulfate cement augmentation; NA, nonaugmented.
F max (N) p values in Student t test (two-tailed, unequal variance), statistical significance: p < 0.01.
Screw Orientation
Pedicle screws oriented at 23 degrees relative to the end plate had a significantly higher failure force than their respective counterparts at 0 degrees in both the NA and BioC groups (p < 0.01; Tables 5 and 6). In the NA group, simply changing the trajectory of the screw doubled the average force of failure from 20.95 N to 39.82 N. The cases with the largest change in failure strength were the NA screw at 0 degrees versus the calcium phosphate screw at 23 degrees. In this comparison, there was a 3.5-fold increase in the average failure strength from 20.95 N to 73.17 N. Again, this comparison's p value was much less than 0.01, making it statistically significant. In the BioC group, the results were less dramatic but still significant. The average force to failure in the BioC group inserted at 0 degrees was 56.82 ± 4.7 N, and the mean force to failure for the screws inserted at 23 degrees was 73.17 ± 2.1 N (Fig. 5). Orienting the screw up to the vertebral end plate led to significantly higher force to failure values in both cases, with what appears to be an independent effect by augmenting the screw with cement.
Table 5. Orientation of nonaugmented and augmented versus maximal force at failure.
| Maximal force (N) per trial | ||||
|---|---|---|---|---|
| Specimen | NA 0 degrees | BioC 0 degrees | NA 23 degrees | BioC 23 degrees |
| Trial 1 | −21.5 | −58.0 | −41.54 | −72.25 |
| Trial 2 | −18.3 | −54.4 | −38.23 | −75.64 |
| Trial 3 | −23.3 | −50.1 | −40.12 | −74.15 |
| Trial 4 | −20.7 | −62.1 | −39.4 | −70.15 |
| Trial 5 | − | −59.5 | − | −73.65 |
| Mean | −20.95 | −56.82 | −39.82 | −73.17 |
| Standard deviation | 2.074 | 4.676 | 1.385 | 2.077 |
| Standard error | 1.037 | 2.338 | 0.692 | 1.039 |
Abbreviations: BioC, calcium phosphate and calcium sulfate cement augmentation; NA, nonaugmented.
Table 6. p Values of BioC-augmented and nonaugmented screw pullout strengths at either 0 or 23 degreesa .
| Nonaugmented 0 degrees | Nonaugmented 23 degrees | |
|---|---|---|
| Augmented 0 degrees | 2.119E-06 | 2.225E-04 |
| Augmented 23 degrees | 2.496E-09 | 9.765E-05 |
Student t test (two-tailed, unequal variance, statistical significance: p < 0.01).
Fig. 5.
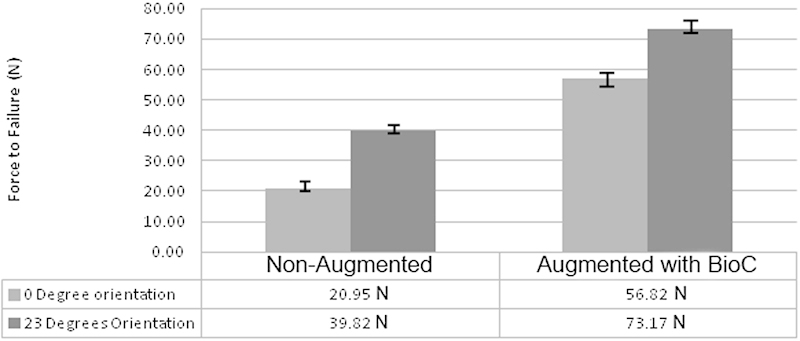
Average force of failure in screw orientation.
Discussion
Pedicle screw fixation in the osteoporotic spine is expected to be a major challenge facing spinal surgeons for the foreseeable future. The data presented here may help with fixation decision making. Fairly clear evidence—including the results of this study—supports the biomechanical superiority of not tapping prior to placing standard NA pedicle screws in an osteoporotic spine. In our study, tapping prior to screw placement led to a 52% decrease in force to failure of the screw fixation. Tapping removes bone that could potentially be compressed by the placement of a screw and therefore not tapping and leaving this bone in place will likely lead to better fixation. The choice of tapping versus not tapping when placing cement-augmented screws certainly seems less intuitive, which we felt justified further study.
Our study used an osteoporotic model with multicomponent loading that was previously established by Choma et al.3 The multicomponent loading is thought to more closely approximate how screws might fail in vivo, and therefore this combined pullout, toggle, and translational force was used in our study instead of a one-dimensional screw pullout model. In our study, we evaluated tapping versus not tapping prior to cement augmentation of a screw in a cancellous bone block model. Tapping to 40 mm prior to augmentation and screw placement led to a 54% increase in force to failure when compared with a nontapped cement-augmented screw. The results of our study suggest that tapping prior to placement of cement augmentation substantially improves loads to failure of screw fixation in an osteoporotic model. It was also clear in our data that tapping longer instead of shorter prior to cement augmentation led to substantially greater stability. We hypothesize that the tapping creates a larger cavity in which the bioactive cement can more uniformly distribute around the screw and the screw–bone interface. Tapping longer likely allows more of this process to occur.
Limitations exist whenever a synthetic bone model is used and this should be recognized, but this previously established model provides a uniform reproducible simulation of osteoporotic bone.3 Synthetic bone blocks helped to minimize the effect of geometric and material property variability associated with cadaveric vertebra. They also simulated a “worst-case” scenario of minimal cortical contact of the screw in the pedicle, which is inherent in a cadaveric model due to the pedicle's oval cross-sectional shape.
There should be some caution translating this research to an in vivo application as tapping prior to placing augmentation and screws may have consequences that have not been well studied. One potential disadvantage of tapping first, prior to augmentation and screw placement, is that the tap creates a larger hole and could lead to a pedicle breach and therefore a conduit for cement extravasation. This breach may not have been present if the tract was not tapped prior to cement placement. The obvious advantage to tapping first, beyond the enhanced fixation as suggested in our research, is that screws are more likely to follow their intended pathway. The tapping also more closely approximates the diameter of the screw and therefore should better allow the surgeon to palpate a breach or a potentially misdirected screw trajectory.
The other component of this study was the evaluation of screw trajectory and the effect of sagittal screw angulation with or without screw augmentation. In the NA screws, changing the angulation from 0 (straight ahead) to 23 degrees cephalad doubled the load to failure in our model. The hypothesis for such a dramatic difference is that this angulation of screws allows the screw tip to better approximate the simulated superior end plate. In vivo this cephalad angulation of the screw could better approximate the screw tip to the relatively denser bone found adjacent to the superior end plate. This might lead to fewer early instrumentation failures in the osteoporotic spine and hopefully translate to improved clinical results. Further clinical study is clearly needed. One certainly needs to be aware of anatomic constraints of placing screws with more cephalad orientation. Violating the end plate or pedicle with this cephalad trajectory could certainly have very undesirable consequences.
The results between the augmented screws with different trajectories also showed a substantial improvement in the group with greater cephalad angulation, but the difference between the two trajectories was less dramatic (29% increase in load to failure). Cement augmentation alone provides substantial stability, and the marginal increase in stability achieved with a cephalad oriented and augmented screw is likely not worth further investigation given the anatomic constraints and risks of angulating screws cephalad.
Unfortunately, the number of patients with osteoporosis and patients with low bone mineral density continues to rise as our population is living longer. Standard pedicle screw fixation in this population is often inadequate and can lead to devastating complications.15 16 17 18 Because of this, we need to investigate and exploit every potential advantage for stable fixation in the aging spine. Based on an established osteoporotic bone block model with multicomponent loading, it appears that there is a substantial improvement in stability when tapping and tapping long prior to bioactive cement augmentation of pedicle screws. In our study, pretapping the entire screw length prior to placement of augmented screws led to a 54% increase in load to failure when compared with a nontapped but still augmented screw. This is a relatively straightforward adjustment in surgical technique when placing augmented screws into the osteoporotic spine and has the potential to further limit early fixation failure.
Based on the same osteoporotic model, we conclude that it appears cephalad angulated pedicle screw placement provides substantially greater stability when compared with a standard straight-ahead trajectory with both augmented and NA screw constructs. The screws that are oriented cephalad would more closely approximate the dense bone of the superior end plate and therefore lead to greater fixation. Further clinical studies should investigate if this cephalad screw trajectory is a viable option.
Acknowledgments
The authors wish to thank Stryker Spine (screws and instruments) and Wright Medical (cement) for in-kind support of this study.
Footnotes
Disclosures Dr. Kuhns serves as a paid consultant to Stryker Spine and also received money for an instructional course with Stryker Spine. Michael Reiter and Ferris Pfeiffer have no disclosures. Dr. Choma serves as a paid consultant to Stryker Spine and receives stock/stock options from Gentis, Inc.
References
- 1.Pfeiffer F M, Abernathie D L, Smith D E. A comparison of pullout strength for pedicle screws of different designs: a study using tapped and untapped pilot holes. Spine (Phila Pa 1976) 2006;31(23):E867–E870. doi: 10.1097/01.brs.0000244658.35865.59. [DOI] [PubMed] [Google Scholar]
- 2.Bai B, Kummer F J, Spivak J. Augmentation of anterior vertebral body screw fixation by an injectable, biodegradable calcium phosphate bone substitute. Spine (Phila Pa 1976) 2001;26(24):2679–2683. doi: 10.1097/00007632-200112150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Choma T J, Frevert W F, Carson W L, Waters N P, Pfeiffer F M. Biomechanical analysis of pedicle screws in osteoporotic bone with bioactive cement augmentation using simulated in vivo multicomponent loading. Spine (Phila Pa 1976) 2011;36(6):454–462. doi: 10.1097/BRS.0b013e3181d449ec. [DOI] [PubMed] [Google Scholar]
- 4.Evans S L, Hunt C M, Ahuja S. Bone cement or bone substitute augmentation of pedicle screws improves pullout strength in posterior spinal fixation. J Mater Sci Mater Med. 2002;13(12):1143–1145. doi: 10.1023/a:1021133819646. [DOI] [PubMed] [Google Scholar]
- 5.Hashemi A, Bednar D, Ziada S. Pullout strength of pedicle screws augmented with particulate calcium phosphate: an experimental study. Spine J. 2009;9(5):404–410. doi: 10.1016/j.spinee.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Lei W, Wu Z X. et al. Augmentation of pedicle screw stability with calcium sulfate cement in osteoporotic sheep: biomechanical and screw-bone interfacial evaluation. J Spinal Disord Tech. 2011;24(4):235–241. doi: 10.1097/BSD.0b013e3181ecf88a. [DOI] [PubMed] [Google Scholar]
- 7.Masaki T, Sasao Y, Miura T. et al. An experimental study on initial fixation strength in transpedicular screwing augmented with calcium phosphate cement. Spine (Phila Pa 1976) 2009;34(20):E724–E728. doi: 10.1097/BRS.0b013e3181adc0e9. [DOI] [PubMed] [Google Scholar]
- 8.Renner S M, Lim T H, Kim W J, Katolik L, An H S, Andersson G B. Augmentation of pedicle screw fixation strength using an injectable calcium phosphate cement as a function of injection timing and method. Spine (Phila Pa 1976) 2004;29(11):E212–E216. doi: 10.1097/00007632-200406010-00020. [DOI] [PubMed] [Google Scholar]
- 9.Rohmiller M T, Schwalm D, Glattes R C, Elalayli T G, Spengler D M. Evaluation of calcium sulfate paste for augmentation of lumbar pedicle screw pullout strength. Spine J. 2002;2(4):255–260. doi: 10.1016/s1529-9430(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 10.Tan J S, Kwon B K, Dvorak M F, Fisher C G, Oxland T R. Pedicle screw motion in the osteoporotic spine after augmentation with laminar hooks, sublaminar wires, or calcium phosphate cement: a comparative analysis. Spine (Phila Pa 1976) 2004;29(16):1723–1730. doi: 10.1097/01.brs.0000134569.63542.49. [DOI] [PubMed] [Google Scholar]
- 11.Wittenberg R H, Lee K S, Shea M, White A A III, Hayes W C. Effect of screw diameter, insertion technique, and bone cement augmentation of pedicular screw fixation strength. Clin Orthop Relat Res. 1993;(296):278–287. [PubMed] [Google Scholar]
- 12.Carmouche J J, Molinari R W, Gerlinger T, Devine J, Patience T. Effects of pilot hole preparation technique on pedicle screw fixation in different regions of the osteoporotic thoracic and lumbar spine. J Neurosurg Spine. 2005;3(5):364–370. doi: 10.3171/spi.2005.3.5.0364. [DOI] [PubMed] [Google Scholar]
- 13.Wuisman P I, Van Dijk M, Staal H, Van Royen B J. Augmentation of (pedicle) screws with calcium apatite cement in patients with severe progressive osteoporotic spinal deformities: an innovative technique. Eur Spine J. 2000;9(6):528–533. doi: 10.1007/s005860000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santoni B G, Hynes R A, McGilvray K C. et al. Cortical bone trajectory for lumbar pedicle screws. Spine J. 2009;9(5):366–373. doi: 10.1016/j.spinee.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Cauley J A, Hochberg M C, Lui L Y. et al. Long-term risk of incident vertebral fractures. JAMA. 2007;298(23):2761–2767. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 16.Cooper C O'Neill T Silman A; European Vertebral Osteoporosis Study Group. The epidemiology of vertebral fractures Bone 19931401S89–S97. [DOI] [PubMed] [Google Scholar]
- 17.Melton L J III Epidemiology of spinal osteoporosis Spine (Phila Pa 1976) 199722(24, Suppl):2S–11S. [DOI] [PubMed] [Google Scholar]
- 18.Melton L J III, Kan S H, Frye M A, Wahner H W, O'Fallon W M, Riggs B L. Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129(5):1000–1011. doi: 10.1093/oxfordjournals.aje.a115204. [DOI] [PubMed] [Google Scholar]


