Abstract
The senescence delaying effect of cytokinin is well known, however, the details behind how this process occurs remain unclear. Efforts to improve understanding of this phenomenon have led to the identification in Arabidopsis of specific cytokinin signaling components through which senescence signal responses are regulated. These include the cytokinin receptor (AHK3), the type-B response regulator (ARR2) and the recently identified cytokinin response factor (CRF6). At the mechanistic end of this process, it was found that increased cell-wall invertase activity which occurs in response to cytokinin is both necessary and sufficient for the inhibition of senescence. Yet, a direct link between the signaling and mechanistic steps of a cytokinin regulated senescence process has yet to be demonstrated. This may be in part because the relationship between senescence and primary metabolism implied by the key role of cell-wall invertase is the subject of two apparently opposing bodies of evidence. Here we briefly summarize and propose a model in which cytokinin mediated changes in sink/source relationships leads to delayed senescence which is consistent with existing evidence both for and against sugars as a trigger for developmental senescence.
Keywords: senescence, cytokinin, leaf, cell wall invertase, carbon allocation, sink/source relationships
Leaf Senescence: Maximizing Return on Investment
Leaf senescence is a developmental process actively initiated as part of an age-dependent genetic program or in response to environmental stress. Although this may ultimately lead to cellular apoptosis across the organ, it is in no way a simply unintended or unfortunate consequence. In fact, the process of senescence is highly regulated and dependent upon concurrent increases in both synthesis and activity of some proteins as well as degradation or inactivation of others. Precise regulation of senescence is crucial because in preparation for cellular death, the valuable nutrients and energy released by the breakdown of macromolecules during this process are reallocated to the rest of the plant for growth or storage.1,2 This senescence-based recycling of nutrients and energy that are invested in the production of leaves and the photosynthetic machinery within has been described as “altruistic” and evolutionary advantageous process.3
This review focuses on the current understanding of the mechanisms behind the well-known cytokinin inhibition of senescence. The elucidation of the two-component cytokinin signaling (TCS) pathway in Arabidopsis has facilitated the identification of the initial signaling components which mediate senescence-specific responses; specifically the cytokinin receptor (AHK3) and the type-B response regulator (ARR2).4 Recently another transcription factor, cytokinin response factor 6 (CRF6) thought to act as a side branch of the canonical TCS pathway was shown to be involved in senescence delay.5 While at the other end of this process, increased cell wall invertase (CWINV) activity has been shown to be an integral part of the downstream response mechanism through which cytokinin delays leaf senescence.6 Despite this knowledge of upstream signaling and downstream mechanistic parts of a cytokinin-regulated senescence process, these portions have not been connected and a unified pathway remains unresolved (Fig. 1).
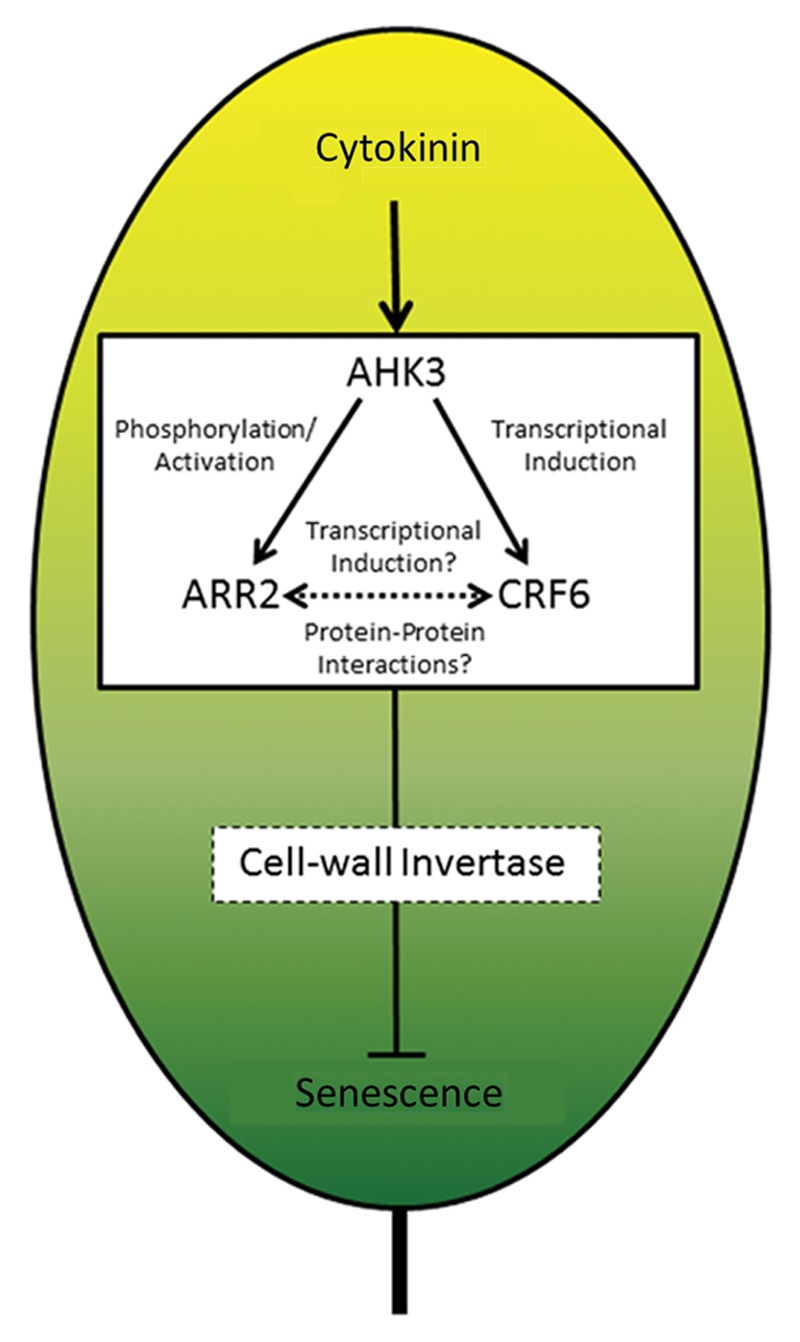
Figure 1. Model of an emerging pathway integrating current knowledge of cytokinin regulation of senescence. In Arabidopsis, cytokinin perception by AHK leads to activation of ARR2 and induced expression CRF6. It is unclear whether ARR2 directly regulates CRF6 expression in this process. ARR2 and CRF6 proteins may also interact physically in the regulation of downstream genes. One such gene may be cell-wall invertase which has been shown in tobacco and tomato to be necessary for senescence inhibition.
Cytokinin: The Foliar Fountain of Youth
Richmond and Lang (1957) first showed that cytokinin treatment leads to greater retention of chlorophyll and protein in excised leaves of cocklebur plants (Xanthium pennsylvanicum).7 This cytokinin effect has since been shown in many other species, even resulting in re-greening of yellowing leaves.8 Experimentation has led to the understanding that cytokinin increases in longevity is specific to leaves and that it can be influenced by other factors such as light and sugars.9,10
Much of the evidence supporting the role of cytokinin as an endogenous negative regulator of senescence has come from studies which examined changes in cytokinin content and the expression of cytokinin metabolism genes during senescence. Work in numerous species has indicated a strong correlation between decreased leaf cytokinin content and the onset and progression of senescence.3,11 Cytokinin synthesized in roots is transported into leaves through the transpiration stream; it has been found that the amount of cytokinin in the xylem of Glycine max rapidly decrease at the onset of senescence.12 Similarly, a sorghum cultivar exhibiting delayed leaf senescence had a greater abundance of cytokinin in its xylem sap as compared with a normally senescing cultivar.13 Cytokinin in leaves may also be the product of local synthesis. Transcriptome analyses of Arabidopsis leaves demonstrate that expression of cytokinin biosynthetic genes greatly decreases during senescence, while transcripts of cytokinin degrading enzymes become more abundant.14,15 This suggests that cytokinin may delay leaf senescence not only as a result of exogenous treatment, but as part of an endogenous developmental program. Although an antagonistic role of cytokinin in leaf senescence is strongly supported in these studies, many of them rely on correlations and do not clearly demonstrate a causal relationship.
A new era of investigations in cytokinin physiology began with heterologous expression of the Agrobacterium tumfaciens isopentenyl transferase (IPT) gene which is transferred into plant cells during infection by A. tumfaciens. The IPT enzyme catalyzes the rate-limiting step of cytokinin biosynthesis in plants; ectopic IPT expression in a wide variety of plant species results in dramatic increases in endogenous cytokinin production.16,17 Because constitutive expression using the CaMV 35S promoter severely limited regeneration of plants due to cytokinin inhibition of root organogenesis, tissue-specific or inducible promoters allowing for targeted over-production of cytokinin have been employed to varying levels of success in many species as has been extensively reviewed.9,18
Given the senescence delaying effects of exogenous cytokinin treatment, it was expected that cytokinin overproducing plants would also display increased leaf longevity. However, actual findings were mixed with some plants showing delayed senescence as expected, but many studies found no change or even accelerated leaf senescence.9,19 It has been suggested that these unexpected results are due to a cytokinin imposed shift in sink and source identities of organs.20
An elegant system was designed to resolve these confounding results (as well as concerns regarding the effects of expression-inducing conditions): the IPT gene was expressed in tobacco plants under the promoter of SAG12 (Senescence Associated Gene12), such that the plants had increased cytokinin production limited to leaves which had begun to senesce. This auto-regulatory loop specifically targeted cytokinin increases to senescing cells, yet prevented over-accumulation. The result was a striking delay of leaf senescence.21 The extraordinary leaf longevity exhibited by these plants remains among the most convincing lines of evidence for the negative regulation of leaf senescence by cytokinin. This proSAG:IPT system has since been implemented in a number of important crop species including: lettuce, rice, ryegrass, tomato, alfalfa, cauliflower, wheat, cassava, broccoli and cotton; all of which demonstrated delayed leaf senescence.22-31
A Specific Subset of Cytokinin Signaling Slows Senescence
The two-component cytokinin signal (TCS) pathway is fairly well understood as a result of work done over the past 15 y. It functions as a multi-step phospho-relay involving hybrid histidine kinase receptors (HKs) and downstream transcription factors, such as the type-B response regulators (RRs) and cytokinin response factors (CRFs) that mediate the cytokinin signal.32,33 The first direct link between the TCS pathway and senescence regulation came about with the characterization of an Arabidopsis mutant with a delayed senescence phenotype, ore12, which turned out to be a gain of function allele of the cytokinin receptor AHK3. Further investigation indicated that AHK3 specifically mediates the senescence-delaying response in leaves in a manner partially dependent upon the phosphorylation/activation of the type-B RR ARR2.4 It has since been shown that plants expressing a proteolytic-resistant version of ARR2 exhibit delayed dark-induced leaf senescence.34 A similar phenotype was observed in plants overexpressing the CK inducible transcription factor CRF6; and crf6 mutants were found to have reduced sensitivity to the senescence delaying effects of cytokinin.5 While CRFs (cytokinin response factors) have been shown to function as a side branch of the TCS, this is one of the first functional roles in a cytokinin regulated process directly linked to a CRF protein—the first involving senescence.35 Interestingly, the CRF6 protein has been shown to directly interact with type-B ARR proteins.36 Although interaction with ARR2 was not tested, those which were examined (ARR1, ARR10 and ARR12) are closely related and function in a redundant manner.37 This suggests that CRF6 and ARR2 could potentially function in complex to regulate transcriptional response to cytokinin during senescence, however further examination is required (Fig. 1). Notably, AHK3, ARR2 and CRF6 are all expressed in leaf vascular tissues which, as will be addressed in subsequent sections, serve an important role in senescence and may be crucial in regulating this process.38-41
Cell-Wall Invertase: Cytokinin Sweetens the Deal
The downstream mechanism of cytokinin delayed leaf senescence is not fully understood, though it is widely thought to involve the regulation of sink/source relation.20,42,43 The influence of cytokinin upon sink/source relations is exerted in part by regulation of cell-wall invertase (CWINV) activity.
The CWINV enzyme is secreted and ionically bound to cell walls where it catalyzes the cleavage of sucrose into hexose monomers.44 Doing so allows sucrose unloaded from the phloem into the apoplasm of sink organs to be rapidly metabolized and taken up by adjacent cells which possess hexose but not sucrose transport proteins. Because sucrose diffuses passively through the phloem, the rate of its metabolism at the site of unloading is a major determinant of sink strength.45,46
A cytokinin-induced increase in invertase activity was first demonstrated in calli from Cichorium intybus.47 It was later shown that a similar increase specifically of CWINV in the cultured cells of Chenopodium rubrum and leaves of tomato (Solanum lycopersicum) was due to induced gene expression.48,49 Importantly, a coordinated increase was also observed in hexose transporter expression, which is required for uptake of the products of the invertase reaction into cells.48 A link between cytokinin induced CWINV and delayed leaf senescence was first observed in an analysis of tobacco proSAG12::IPT lines, where it was found that the long-lived leaves of these plants had unusually high levels of CWINV activity. It was further demonstrated that plants expressing a proSAG12::CWINV transgene exhibited delayed leaf senescence, as did specific tissue regions in which an inducible CWINV construct was expressed in a localized manner. Moreover when a CWINV inhibitor protein was expressed under a cytokinin inducible promoter, treatment with the hormone no longer resulted in delayed senescence.6 Similar results were obtained in a later study where activity of CWINV in tomato leaves was increased by the silencing of its inhibitor.46 Together these works demonstrate that the induced expression/activity of CWINV which occurs naturally in response to cytokinin is both necessary and sufficient to cause a delay in leaf senescence. This is a highly significant point as it provides both a physiological link between senescence regulation and primary metabolism as well as at least a partial mechanism by which senescence is delayed by cytokinin. Interestingly, it also emphasizes another aspect of senescence regulation which remains poorly understood and in some ways, controversial as discussed below.
Bitter-Sweet Senescence
The implications of CWINV activity in leaves negatively influencing senescence are striking because an accumulation of sugars in leaves, particularly glucose and fructose (the products of the invertase reaction) coincides with the onset of senescence.50,51 It has even been suggested that this accumulation may serve as an underlying trigger of senescence.52,53 Toward this point the overexpression of a yeast invertase in the apoplasm of plants resulted in elevated hexose levels leading to premature senescence.54 However, this finding does not actually conflict with the delayed senescence due to increased CWINV activity reported by Lara et al. (2004) because in that study increased steady-state levels of hexoses were not observed.6 The authors interpreted this as an indication of rapid subsequent metabolism of the glucose and fructose products.
The debate regarding this point referenced above has come about because in addition to the theory in which sugar accumulation leads to senescence, a strong body of evidence suggests that sugar starvation initiates the onset of senescence.55 For example, the dark-induction of senescence in detached leaves is generally thought to result from starvation due to a lack of photosynthesis; a hypothesis supported by the finding that treatment with sugar can both delay dark-induced senescence (similar to cytokinin) as well as repress the expression of SAGs.56-59
In Arabidopsis, hexokinase1 (HXK1), in addition to its catalytic function, serves as both a sensor of hexose levels and regulator of gene expression.60 Mutants with impaired HXK1 function have reduced sensitivity to glucose and a delayed senescence phenotype, which has been interpreted as a deficiency of the sugar signal causing the delay.61 However, further experimental support of this interpretation may be necessary as it is based on phenotypic analysis of mutants lacking a key regulatory and metabolic enzyme. When interpreting the results from these experiments, it is crucial to consider that the metabolic relevance of sugars is greatly dependent upon their spatial distribution both among and within cells. For instance, sucrose in the phloem of the leaf would be unlikely to have the same metabolic fate as sucrose present in the vacuole of a mesophyll cell, yet these differences cannot be determined in experiments making sugar measurements from a whole leaf extract. Considering these distinctions is important both when measuring sugar levels and in experiments involving sugar-feeding or supplementation.
Sink/Source Identity Theft
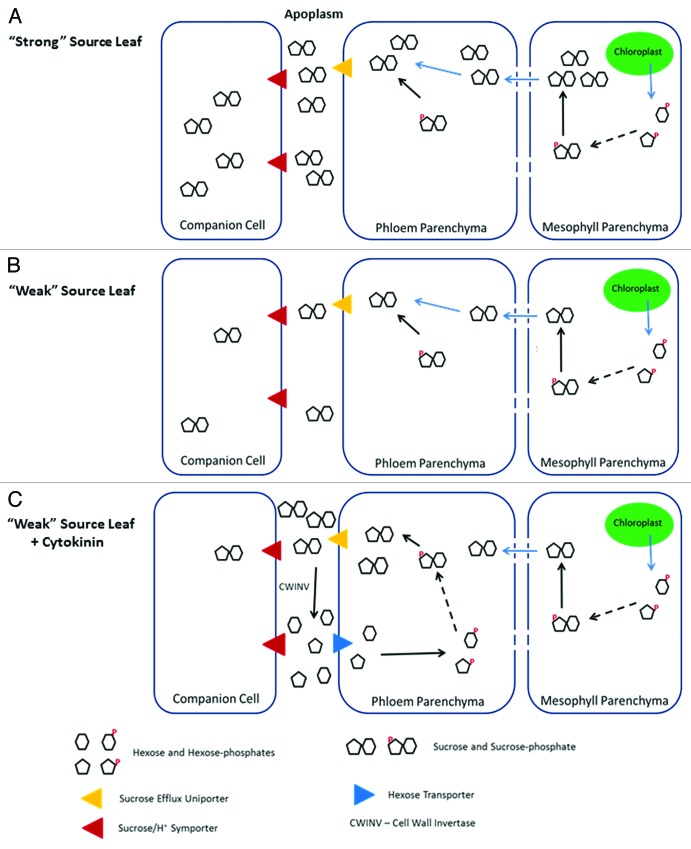
Regardless, both sugar accumulation and starvation appear to serve as triggers for senescence under certain conditions; yet to date neither condition has been irrefutably demonstrated to play such a role. This perhaps suggests that changes in carbon flux rather than steady-state levels of primary metabolites may stimulate the onset of senescence: a mechanism that could also explain the key role of CWINV. The co-induction of CWINV along with a plasma membrane hexose importer would provide a mechanism for increased carbon flux through a so-called “futile cycle.”62,63 Such a “futile cycle” would rely upon processes involved in apoplastic phloem loading (Fig. 2A). In apoplastic phloem loading sucrose produced in mesophyll cell cytoplasm is exported into the apoplastic space prior to uptake into specialized phloem companion cells by sucrose transporters.64 Until recently it was unclear whether sucrose export occurred at the site of synthesis or if the sucrose was symplastically transported to phloem parenchyma and then exported in closer proximity to the companion cells. The identification of the Arabidopsis sucrose efflux proteins involved in this process allowed their localization, specifically to phloem parenchyma adjacent to companion cells to be determined.65 This suggests that the efflux of sucrose directly precedes its uptake into the phloem.
Figure 2. A futile cycle involving CWINV mimics a high rate of carbon export. (A) Loading of sucrose into phloem when photosynthesis rate is high involves pumping large amounts of sucrose into the apoplasm. From the apoplasm it is taken up by companion cells via sucrose symporters. (B) When photosynthesis rate is decreased, less sucrose is available to be pumped into the apoplasm. (C) Cytokinin stimulation of a futile cycle of sucrose export, hydrolyses, uptake and re-synthesis could maintain high rates of sucrose efflux at the expense of long distance transport.
Increased CWINV activity is likely to disrupt apoplastic phloem loading through the hydrolysis of effluxed sucrose (Fig. 2B and C). The abundant hexose monomers resulting from apoplastic sucrose cleavage could then be taken back into cytoplasm of parenchyma cells via the co-induced high-affinity hexose transporters. Hexoses that are then phosphorylated by cytosolic hexokinase (as required for subsequent metabolism) result in a high intracellular concentration of glucose-6-phosphate which in turn enhances the activity of sucrose-phosphate synthase.66 This ultimately results in the regeneration of cytoplasmic sucrose which would again be transported into the apoplasm, generating a “futile cycle” of both the compartmentalization and molecular form of sugars (Fig. 2C). The continuous sucrose efflux affected by this cycle partially mimics conditions in the phloem parenchyma similar to those associated with high rates of phloem loading for carbon export that are characteristic of productive source leaves. This cycle could therefore provide even a poorly productive leaf with an artificially strong source identity despite a reduced amount of sugar actually being exported. Importantly at least one key enzyme in this cycle that is not directly induced or activated by cytokinin (sucrose-phosphate synthase) would be activated as a result of the preceding steps of the cycle (increased glucose-6-phosphate).
It is noteworthy that accelerated senescence has been reported in experiments where whole plants are supplemented with sugar, which may be a consequence of changing sink/source relationships in the opposite direction. For instance, a plant fed glucose through its growth medium would have an abundance of sugars available to its roots, which would result in a decreased amount of sugar unloaded from the phloem of these sink tissues. This would cause a reduced phloem turgor differential between source leaves and sink roots and inhibit bulk flow and diminished sugar export from source leaves. As a result, the phloem parenchyma of such leaves would experience decreased sucrose efflux and could thereby acquire a “weak source” identity and as such become candidates for senescence.
Sink regulation of source tissue identity has been extensively described elsewhere including the increases in sink strength during fruit development and root nodulation that are correlated with elevated photosynthetic rates.67-69 The inverse effect has also been found, where restricting carbon export from leaves (mimicking a lack of a strong sink) reduces photosynthesis in part from the repression of photosynthetic genes.70,71 One known mechanism by which this may occur is through the accumulation of starch within chloroplasts which inhibits efficiency of the thylakoid-bound photosystems.72
It is difficult to interpret experiments where longevity is enhanced in detached leaves supplemented with sugar in terms of sink/source relationships because similar results are not found in intact plants. However, findings appear to suggest that the two seemingly incongruent lines of evidence regarding sugar and senescence actually indicate that it is the loss of a relatively strong source identity which triggers leaf senescence.42,73 Furthermore, that in plants with apoplastic phloem loading it is the rate of sucrose efflux out of phloem parenchyma which provides a sink/source identity. Unfortunately this model may prove difficult to directly test as it is likely to require: (1) the ability to accurately determine changes in sub-cellular (or extracellular) concentrations of sugars; (2) manipulation or misexpression of enzymes involved in indispensable metabolic processes; and (3) a more complete understanding of the role of sugars and primary metabolism in signal transduction.
Concluding Remarks
Elucidation of the mechanism by which cytokinin enhances leaf longevity is likely to have a significant impact on the basic understanding of numerous areas of research including hormone signal transduction, regulatory mechanisms of primary metabolism and the aging processes. Moreover, novel connections among these diverse areas are likely to emerge as the pathway that links the perception of cytokinin to CWINV activity and the resulting changes in carbon allocation is further elucidated.
Acknowledgments
The authors would like to thank Joanna W. Diller, Leslie R. Goertzen, Robert D. Locy and Narendra K. Singh for critical reading of the manuscript and service as PhD advisory committee for P.J.Z. We also thank members of the Rashotte lab for critical reading of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24737
References
- 1.Himelblau E, Amasino RM. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J Plant Physiol. 2001;158:1317–23. doi: 10.1078/0176-1617-00608. [DOI] [Google Scholar]
- 2.Hörtensteiner S, Feller U. Nitrogen metabolism and remobilization during senescence. J Exp Bot. 2002;53:927–37. doi: 10.1093/jexbot/53.370.927. [DOI] [PubMed] [Google Scholar]
- 3.Nooden LD, Leopold AC. (1988) Senescence and aging in plants. Academic Press, London; San Diego. [Google Scholar]
- 4.Kim HJ, Ryu H, Hong SH, Woo HR, Lim PO, Lee IC, et al. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:814–9. doi: 10.1073/pnas.0505150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwack PJ, Robinson BR, Risley MG, Rashotte AM. Cytokinin response factor 6 negatively regulates leaf senescence and is induced in response to cytokinin and numerous abiotic stresses. Plant Cell Physiol. 2013 doi: 10.1093/pcp/pct049. [DOI] [PubMed] [Google Scholar]
- 6.Balibrea Lara ME, Gonzalez Garcia MC, Fatima T, Ehness R, Lee TK, Proels R, et al. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell. 2004;16:1276–87. doi: 10.1105/tpc.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richmond AE, Lang A. Effect of Kinetin on Protein Content and Survival of Detached Xanthium Leaves. Science. 1957;125:650–1. doi: 10.1126/science.125.3249.650-a. [DOI] [Google Scholar]
- 8.Dyer TA, Osborne DJ. Leaf Nucleic Acids. 2. Metabolism During Senescence and Effect of Kinetin. J Exp Bot. 1971;22:552. doi: 10.1093/jxb/22.3.552. [DOI] [Google Scholar]
- 9.Gan SS, Amasino RM. Cytokinins in plant senescence: From spray and pray to clone and play. Bioessays. 1996;18:557–65. doi: 10.1002/bies.950180707. [DOI] [Google Scholar]
- 10.Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP. Regulation of leaf senescence by cytokinin, sugars, and light - Effects on NADH-dependent hydroxypyruvate reductase. Plant Physiol. 1998;116:329–35. doi: 10.1104/pp.116.1.329. [DOI] [Google Scholar]
- 11.Singh S, Letham DS, Palni LMS. Cytokinin Biochemistry in Relation to Leaf Senescence. 7. Endogenous Cytokinin Levels and Exogenous Applications of Cytokinins in Relation to Sequential Leaf Senescence of Tobacco. Physiol Plant. 1992;86:388–97. doi: 10.1111/j.1399-3054.1992.tb01334.x. [DOI] [Google Scholar]
- 12.Noodén LD, Singh S, Letham DS. Correlation of xylem sap cytokinin levels with monocarpic senescence in soybean. Plant Physiol. 1990;93:33–9. doi: 10.1104/pp.93.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambler JR, Morgan PW, Jordan WR. Amounts of Zeatin and Zeatin Riboside in Xylem Sap of Senescent and Nonsenescent Sorghum. Crop Sci. 1992;32:411–9. doi: 10.2135/cropsci1992.0011183X003200020027x. [DOI] [Google Scholar]
- 14.Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–85. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 15.Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell. 2011;23:873–94. doi: 10.1105/tpc.111.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiyoshi DE, Klee H, Amasino RM, Nester EW, Gordon MP. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci USA. 1984;81:5994–8. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smigocki AC, Owens LD. Cytokinin gene fused with a strong promoter enhances shoot organogenesis and zeatin levels in transformed plant cells. Proc Natl Acad Sci USA. 1988;85:5131–5. doi: 10.1073/pnas.85.14.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson S, Kudoyarova GR, Veselov DS, Arkhipova TN, Davies WJ. Plant hormone interactions: innovative targets for crop breeding and management. J Exp Bot. 2012;63:3499–509. doi: 10.1093/jxb/ers148. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol. 1992;153:386–95. doi: 10.1016/0012-1606(92)90123-X. [DOI] [PubMed] [Google Scholar]
- 20.Roitsch T, Ehness R. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 2000;32:359–67. doi: 10.1023/A:1010781500705. [DOI] [Google Scholar]
- 21.Gan SS, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–8. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- 22.McCabe MS, Garratt LC, Schepers F, Jordi WJ, Stoopen GM, Davelaar E, et al. Effects of P(SAG12)-IPT gene expression on development and senescence in transgenic lettuce. Plant Physiol. 2001;127:505–16. doi: 10.1104/pp.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YJ, Cao ML, Xu CG, Chen H, Wei J, Zhang QF. Cultivating rice with delaying led-senescence by P-SAG12-IPT gene transformation. Acta Bot Sin. 2002;44:1333–8. [Google Scholar]
- 24.Li Q, Robson PRH, Bettany AJE, Donnison IS, Thomas H, Scott IM. Modification of senescence in ryegrass transformed with IPT under the control of a monocot senescence-enhanced promoter. Plant Cell Rep. 2004;22:816–21. doi: 10.1007/s00299-004-0762-6. [DOI] [PubMed] [Google Scholar]
- 25.Swartzberg D, Dai N, Gan S, Amasino R, Granot D. Effects of cytokinin production under two SAG promoters on senescence and development of tomato plants. Plant Biol (Stuttg) 2006;8:579–86. doi: 10.1055/s-2006-924240. [DOI] [PubMed] [Google Scholar]
- 26.Calderini O, Bovone T, Scotti C, Pupilli F, Piano E, Arcioni S. Delay of leaf senescence in Medicago sativa transformed with the ipt gene controlled by the senescence-specific promoter SAG12. Plant Cell Rep. 2007;26:611–5. doi: 10.1007/s00299-006-0262-y. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen KH, Jordi W, Van Dun K, Schepers F, Davelaar E, Stoopen G, et al. Delayed senescence in cauliflower transformed with an autoregulated isopentenyl transferase gene. Int J Plant Sci. 2008;169:339–47. doi: 10.1086/526466. [DOI] [Google Scholar]
- 28.Sykorová B, Kuresová G, Daskalova S, Trcková M, Hoyerová K, Raimanová I, et al. Senescence-induced ectopic expression of the A. tumefaciens ipt gene in wheat delays leaf senescence, increases cytokinin content, nitrate influx, and nitrate reductase activity, but does not affect grain yield. J Exp Bot. 2008;59:377–87. doi: 10.1093/jxb/erm319. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, Wang WQ, Zhang GL, Kaminek M, Dobrev P, Xu J, et al. Senescence-inducible expression of isopentenyl transferase extends leaf life, increases drought stress resistance and alters cytokinin metabolism in cassava. J Integr Plant Biol. 2010;52:653–69. doi: 10.1111/j.1744-7909.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu MS, Li HC, Chang YM, Wu MT, Chen LFO. Proteomic analysis of stress-related proteins in transgenic broccoli harboring a gene for cytokinin production during postharvest senescence. Plant Sci. 2011;181:288–99. doi: 10.1016/j.plantsci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Liu YD, Yin ZJ, Yu JW, Li J, Wei HL, Han XL, et al. Improved salt tolerance and delayed leaf senescence in transgenic cotton expressing the Agrobacterium IPT gene. Biol Plant. 2012;56:237–46. doi: 10.1007/s10535-012-0082-6. [DOI] [Google Scholar]
- 32.Gupta S, Rashotte AM. Down-stream components of cytokinin signaling and the role of cytokinin throughout the plant. Plant Cell Rep. 2012;31:801–12. doi: 10.1007/s00299-012-1233-0. [DOI] [PubMed] [Google Scholar]
- 33.Shi X, Rashotte AM. Advances in upstream players of cytokinin phosphorelay: receptors and histidine phosphotransfer proteins. Plant Cell Rep. 2012;31:789–99. doi: 10.1007/s00299-012-1229-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim K, Ryu H, Cho YH, Scacchi E, Sabatini S, Hwang I. Cytokinin-facilitated proteolysis of ARABIDOPSIS RESPONSE REGULATOR 2 attenuates signaling output in two-component circuitry. Plant J. 2012;69:934–45. doi: 10.1111/j.1365-313X.2011.04843.x. [DOI] [PubMed] [Google Scholar]
- 35.Rashotte AM, Mason MG, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ. A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proc Natl Acad Sci USA. 2006;103:11081–5. doi: 10.1073/pnas.0602038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cutcliffe JW, Hellmann E, Heyl A, Rashotte AM. CRFs form protein-protein interactions with each other and with members of the cytokinin signalling pathway in Arabidopsis via the CRF domain. J Exp Bot. 2011;62:4995–5002. doi: 10.1093/jxb/err199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishida K, Yamashino T, Yokoyama A, Mizuno T. Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant Cell Physiol. 2008;49:47–57. doi: 10.1093/pcp/pcm165. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA. 2004;101:8821–6. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tajima Y, Imamura A, Kiba T, Amano Y, Yamashino T, Mizuno T. Comparative studies on the type-B response regulators revealing their distinctive properties in the His-to-Asp phosphorelay signal transduction of Arabidopsis thaliana. Plant Cell Physiol. 2004;45:28–39. doi: 10.1093/pcp/pcg154. [DOI] [PubMed] [Google Scholar]
- 40.Mason MG, Li J, Mathews DE, Kieber JJ, Schaller GE. Type-B response regulators display overlapping expression patterns in Arabidopsis. Plant Physiol. 2004;135:927–37. doi: 10.1104/pp.103.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwack PJ, Shi X, Robinson BR, Gupta S, Compton MA, Gerken DM, et al. Vascular expression and C-terminal sequence divergence of cytokinin response factors in flowering plants. Plant Cell Physiol. 2012;53:1683–95. doi: 10.1093/pcp/pcs110. [DOI] [PubMed] [Google Scholar]
- 42.Hensel LL, Grbić V, Baumgarten DA, Bleecker AB. Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in arabidopsis. Plant Cell. 1993;5:553–64. doi: 10.1105/tpc.5.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas H. Senescence, ageing and death of the whole plant. New Phytol. 2013;197:696–711. doi: 10.1111/nph.12047. [DOI] [PubMed] [Google Scholar]
- 44.Eschrich W. Free space invertase, its possible role in phloem unloading. Ber Dtsch Bot Ges. 1980;93:363–78. [Google Scholar]
- 45.Roitsch T, Tanner W. Cell wall invertase: Bridging the gap. Bot Acta. 1996;109:90–3. [Google Scholar]
- 46.Jin Y, Ni DA, Ruan YL. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell. 2009;21:2072–89. doi: 10.1105/tpc.108.063719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefebvre R, Vasseur J, Backoula E, Couillerot JP. Role of Sugar Metabolism in Tissue Organogenesis of Chciorium-Intybus Cultivated Invitro. Canadian Journal of Botany-Revue Canadienne De Botanique. 1992;70:1897–902. doi: 10.1139/b92-235. [DOI] [Google Scholar]
- 48.Ehness R, Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11:539–48. doi: 10.1046/j.1365-313X.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- 49.Godt DE, Roitsch T. Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol. 1997;115:273–82. doi: 10.1104/pp.115.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jongebloed U, Szederkényi J, Hartig K, Schobert C, Komor E. Sequence of morphological and physiological events during natural ageing and senescence of a castor bean leaf: sieve tube occlusion and carbohydrate back-up precede chlorophyll degradation. Physiol Plant. 2004;120:338–46. doi: 10.1111/j.0031-9317.2004.0245.x. [DOI] [PubMed] [Google Scholar]
- 51.Wingler A, Purdy S, MacLean JA, Pourtau N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot. 2005;57:391–9. doi: 10.1093/jxb/eri279. [DOI] [PubMed] [Google Scholar]
- 52.Ono K, Watanabe A. Levels of endogenous sugars, transcripts of rbcS and rbcL, and of RuBisCO protein in senescing sunflower leaves. Plant Cell Physiol. 1997;38:1032–8. doi: 10.1093/oxfordjournals.pcp.a029268. [DOI] [Google Scholar]
- 53.Parrott D, Yang L, Shama L, Fischer AM. Senescence is accelerated, and several proteases are induced by carbon “feast” conditions in barley (Hordeum vulgare L.) leaves. Planta. 2005;222:989–1000. doi: 10.1007/s00425-005-0042-x. [DOI] [PubMed] [Google Scholar]
- 54.Stitt M, Vonschaewen A, Willmitzer L. Sink Regulation of Photosynthetic Metabolism in Transgenic Tobacco Plants Expressing Yeast Invertase in Their Cell-Wall Involves a Decrease of the Calvin-Cycle Enzymes and an Increase of Glycolytic-Enzymes. Planta. 1991;183:40–50. doi: 10.1007/BF00197565. [DOI] [PubMed] [Google Scholar]
- 55.van Doorn WG. Is the onset of senescence in leaf cells of intact plants due to low or high sugar levels? J Exp Bot. 2008;59:1963–72. doi: 10.1093/jxb/ern076. [DOI] [PubMed] [Google Scholar]
- 56.Thimann KV, Tetley RM, Krivak BM. Metabolism of Oat Leaves during Senescence: V. Senescence in Light. Plant Physiol. 1977;59:448–54. doi: 10.1104/pp.59.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh SA, Lee SY, Chung IK, Lee CH, Nam HG. A senescence-associated gene of Arabidopsis thaliana is distinctively regulated during natural and artificially induced leaf senescence. Plant Mol Biol. 1996;30:739–54. doi: 10.1007/BF00019008. [DOI] [PubMed] [Google Scholar]
- 58.Chung BC, Lee SY, Oh SA, Rhew TH, Nam HG, Lee CH. The promoter activity of sen1, a senescence-associated gene of Arabidopsis, is repressed by sugars. J Plant Physiol. 1997;151:339–45. doi: 10.1016/S0176-1617(97)80262-3. [DOI] [Google Scholar]
- 59.Fujiki Y, Yoshikawa Y, Sato T, Inada N, Ito M, Nishida I, et al. Dark-inducible genes from Arabidopsis thaliana are associated with leaf senescence and repressed by sugars. Physiol Plant. 2001;111:345–52. doi: 10.1034/j.1399-3054.2001.1110312.x. [DOI] [PubMed] [Google Scholar]
- 60.Xiao WY, Sheen J, Jang JC. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol. 2000;44:451–61. doi: 10.1023/A:1026501430422. [DOI] [PubMed] [Google Scholar]
- 61.Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, et al. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science. 2003;300:332–6. doi: 10.1126/science.1080585. [DOI] [PubMed] [Google Scholar]
- 62.Foyer CH. The Basis for Source-Sink Interaction in Leaves. Plant Physiol Biochem. 1987;25:649–57. [Google Scholar]
- 63.Geigenberger P, Stitt M. A Futile Cycle of Sucrose Synthesis and Degradation Is Involved in Regulating Partitioning between Sucrose, Starch and Respiration in Cotyledons of Germinating Ricinus-Communis L Seedlings When Phloem Transport Is Inhibited. Planta. 1991;185:81–90. doi: 10.1007/BF00194518. [DOI] [PubMed] [Google Scholar]
- 64.Turgeon R, Wolf S. (2009) Phloem Transport: Cellular Pathways and Molecular Trafficking. In: Annual Review of Plant Biology: 207-221. [DOI] [PubMed] [Google Scholar]
- 65.Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335:207–11. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- 66.Huber SC. Biochemical Mechanism for Regulation of Sucrose Accumulation in Leaves during Photosynthesis. Plant Physiol. 1989;91:656–62. doi: 10.1104/pp.91.2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall AJ, Brady CJ. Assimilate Source-Sink Relationships in Capsicum-Annuum-L. 2. Effects of Fruiting and Defloration on Photosynthetic Capacity and Senescence of Leaves. Aust J Plant Physiol. 1977;4:771–83. doi: 10.1071/PP9770771. [DOI] [Google Scholar]
- 68.Kaschuk G, Hungria M, Leffelaar PA, Giller KE, Kuyper TW. Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biol (Stuttg) 2010;12:60–9. doi: 10.1111/j.1438-8677.2009.00211.x. [DOI] [PubMed] [Google Scholar]
- 69.Ainsworth EA, Bush DR. Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011;155:64–9. doi: 10.1104/pp.110.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–38. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paul MJ, Pellny TK. Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot. 2003;54:539–47. doi: 10.1093/jxb/erg052. [DOI] [PubMed] [Google Scholar]
- 72.Stitt M, Lunn J, Usadel B. Arabidopsis and primary photosynthetic metabolism - more than the icing on the cake. Plant J. 2010;61:1067–91. doi: 10.1111/j.1365-313X.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- 73.Ono K, Nishi Y, Watanabe A, Terashima I. Possible mechanisms of adaptive leaf senescence. Plant Biol. 2001;3:234–43. doi: 10.1055/s-2001-15201. [DOI] [Google Scholar]