Abstract
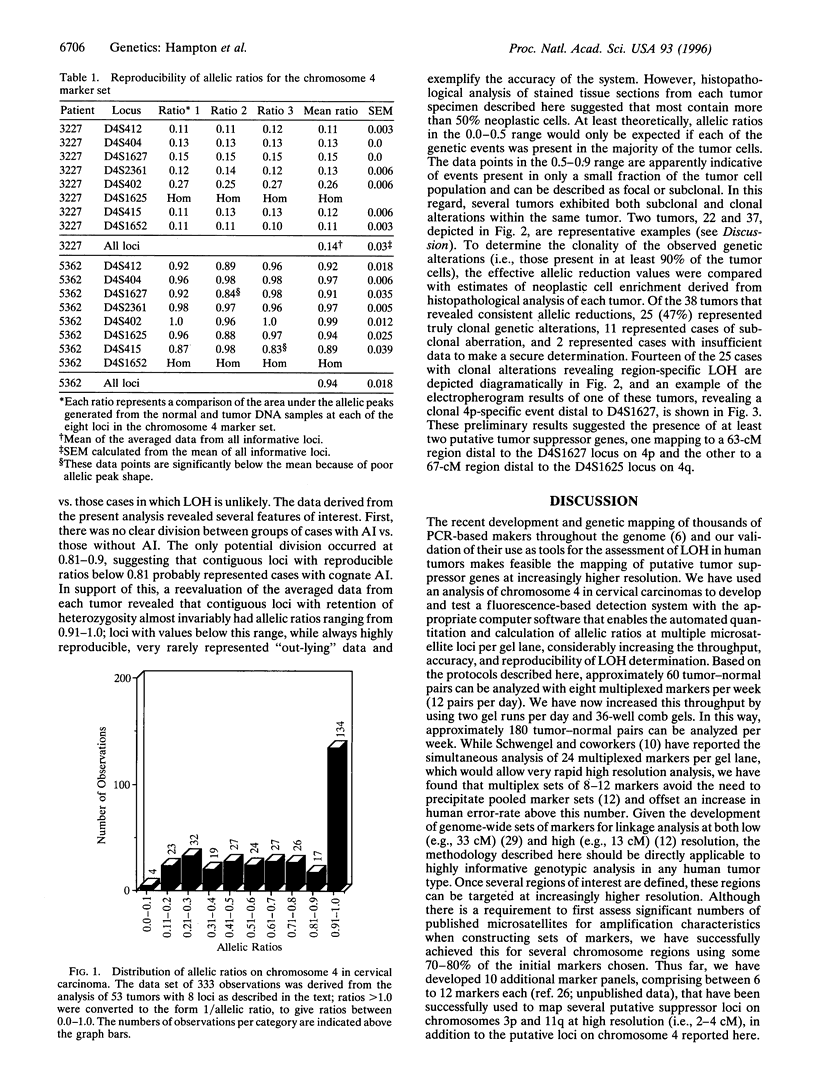
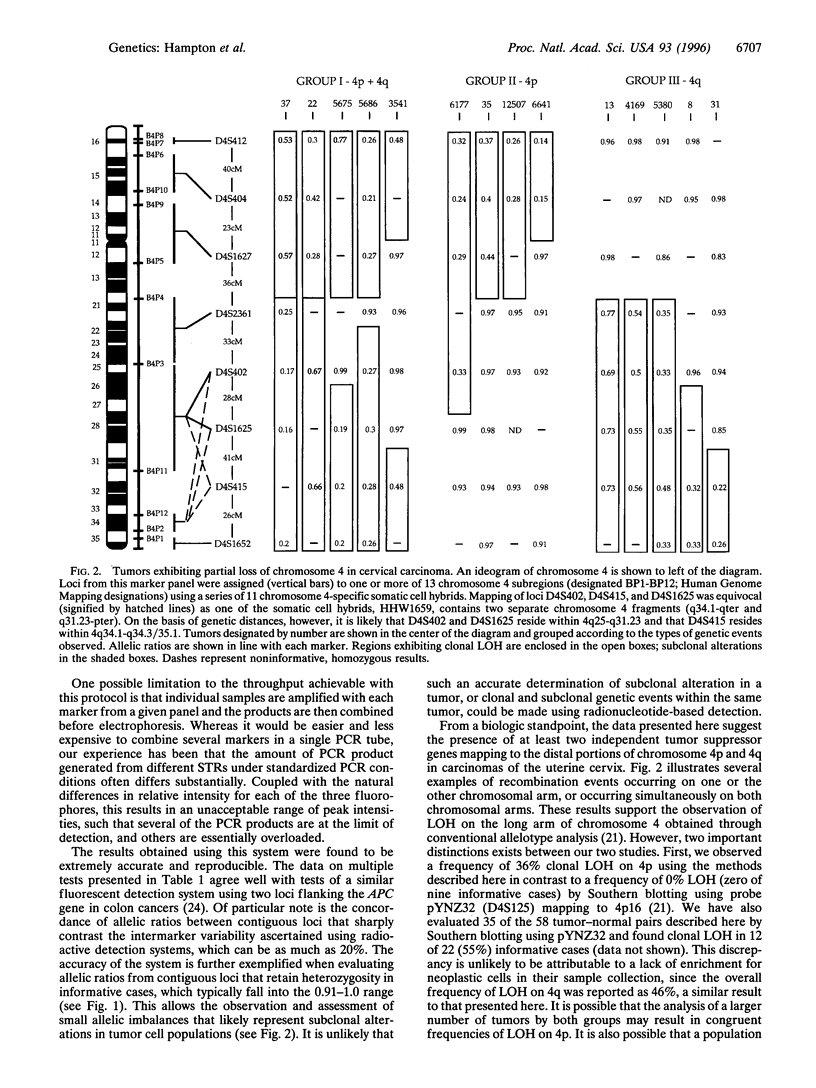
Detection of loss of heterozygosity (LOH) by comparison of normal and tumor genotypes using PCR-based microsatellite loci provides considerable advantages over traditional Southern blotting-based approaches. However, current methodologies are limited by several factors, including the numbers of loci that can be evaluated for LOH in a single experiment, the discrimination of true alleles versus "stutter bands," and the use of radionucleotides in detecting PCR products. Here we describe methods for high throughput simultaneous assessment of LOH at multiple loci in human tumors; these methods rely on the detection of amplified microsatellite loci by fluorescence-based DNA sequencing technology. Data generated by this approach are processed by several computer software programs that enable the automated linear quantitation and calculation of allelic ratios, allowing rapid ascertainment of LOH. As a test of this approach, genotypes at a series of loci on chromosome 4 were determined for 58 carcinomas of the uterine cervix. The results underscore the efficacy, sensitivity, and remarkable reproducibility of this approach to LOH detection and provide subchromosomal localization of two regions of chromosome 4 commonly altered in cervical tumors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B., Baker M. C., Fox M. F. Chromosome changes in 43 carcinomas of the cervix uteri. Cancer Genet Cytogenet. 1990 Feb;44(2):229–241. doi: 10.1016/0165-4608(90)90052-c. [DOI] [PubMed] [Google Scholar]
- Bethwaite P. B., Koreth J., Herrington C. S., McGee J. O. Loss of heterozygosity occurs at the D11S29 locus on chromosome 11q23 in invasive cervical carcinoma. Br J Cancer. 1995 Apr;71(4):814–818. doi: 10.1038/bjc.1995.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawkwell L., Bell S. M., Lewis F. A., Dixon M. F., Taylor G. R., Quirke P. Rapid detection of allele loss in colorectal tumours using microsatellites and fluorescent DNA technology. Br J Cancer. 1993 Jun;67(6):1262–1267. doi: 10.1038/bjc.1993.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung G. T., Huang D. P., Lo K. W., Chan M. K., Wong F. W. Genetic lesion in the carcinogenesis of cervical cancer. Anticancer Res. 1992 Sep-Oct;12(5):1485–1490. [PubMed] [Google Scholar]
- Davies J. L., Kawaguchi Y., Bennett S. T., Copeman J. B., Cordell H. J., Pritchard L. E., Reed P. W., Gough S. C., Jenkins S. C., Palmer S. M. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994 Sep 8;371(6493):130–136. doi: 10.1038/371130a0. [DOI] [PubMed] [Google Scholar]
- Edwards A., Civitello A., Hammond H. A., Caskey C. T. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am J Hum Genet. 1991 Oct;49(4):746–756. [PMC free article] [PubMed] [Google Scholar]
- Goold R. D., diSibio G. L., Xu H., Lang D. B., Dadgar J., Magrane G. G., Dugaiczyk A., Smith K. A., Cox D. R., Masters S. B. The development of sequence-tagged sites for human chromosome 4. Hum Mol Genet. 1993 Aug;2(8):1271–1288. doi: 10.1093/hmg/2.8.1271. [DOI] [PubMed] [Google Scholar]
- Gruis N. A., Abeln E. C., Bardoel A. F., Devilee P., Frants R. R., Cornelisse C. J. PCR-based microsatellite polymorphisms in the detection of loss of heterozygosity in fresh and archival tumour tissue. Br J Cancer. 1993 Aug;68(2):308–313. doi: 10.1038/bjc.1993.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hampton G. M., Penny L. A., Baergen R. N., Larson A., Brewer C., Liao S., Busby-Earle R. M., Williams A. W., Steel C. M., Bird C. C. Loss of heterozygosity in cervical carcinoma: subchromosomal localization of a putative tumor-suppressor gene to chromosome 11q22-q24. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6953–6957. doi: 10.1073/pnas.91.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge X. Y., Litt M. A study of the origin of 'shadow bands' seen when typing dinucleotide repeat polymorphisms by the PCR. Hum Mol Genet. 1993 Apr;2(4):411–415. doi: 10.1093/hmg/2.4.411. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Nakamura Y. Deletion mapping of chromosome 3p in female genital tract malignancies using microsatellite polymorphisms. Oncogene. 1992 Aug;7(8):1631–1634. [PubMed] [Google Scholar]
- Jones M. H., Nakamura Y. Deletion mapping of chromosome 3p in female genital tract malignancies using microsatellite polymorphisms. Oncogene. 1992 Aug;7(8):1631–1634. [PubMed] [Google Scholar]
- Jones M. H., Nakamura Y. Detection of loss of heterozygosity at the human TP53 locus using a dinucleotide repeat polymorphism. Genes Chromosomes Cancer. 1992 Jul;5(1):89–90. doi: 10.1002/gcc.2870050113. [DOI] [PubMed] [Google Scholar]
- Karlsen F., Rabbitts P. H., Sundresan V., Hagmar B. PCR-RFLP studies on chromosome 3p in formaldehyde-fixed, paraffin-embedded cervical cancer tissues. Int J Cancer. 1994 Sep 15;58(6):787–792. doi: 10.1002/ijc.2910580606. [DOI] [PubMed] [Google Scholar]
- Kohno T., Takayama H., Hamaguchi M., Takano H., Yamaguchi N., Tsuda H., Hirohashi S., Vissing H., Shimizu M., Oshimura M. Deletion mapping of chromosome 3p in human uterine cervical cancer. Oncogene. 1993 Jul;8(7):1825–1832. [PubMed] [Google Scholar]
- Larson A. A., Kern S., Sommers R. L., Yokota J., Cavenee W. K., Hampton G. M. Analysis of replication error (RER+) phenotypes in cervical carcinoma. Cancer Res. 1996 Mar 15;56(6):1426–1431. [PubMed] [Google Scholar]
- Lasko D., Cavenee W., Nordenskjöld M. Loss of constitutional heterozygosity in human cancer. Annu Rev Genet. 1991;25:281–314. doi: 10.1146/annurev.ge.25.120191.001433. [DOI] [PubMed] [Google Scholar]
- Levitt R. C., Kiser M. B., Dragwa C., Jedlicka A. E., Xu J., Meyers D. A., Hudson J. R. Fluorescence-based resource for semiautomated genomic analyses using microsatellite markers. Genomics. 1994 Nov 15;24(2):361–365. doi: 10.1006/geno.1994.1628. [DOI] [PubMed] [Google Scholar]
- MacGrogan D., Levy A., Bostwick D., Wagner M., Wells D., Bookstein R. Loss of chromosome arm 8p loci in prostate cancer: mapping by quantitative allelic imbalance. Genes Chromosomes Cancer. 1994 Jul;10(3):151–159. doi: 10.1002/gcc.2870100302. [DOI] [PubMed] [Google Scholar]
- Mitra A. B., Murty V. V., Li R. G., Pratap M., Luthra U. K., Chaganti R. S. Allelotype analysis of cervical carcinoma. Cancer Res. 1994 Aug 15;54(16):4481–4487. [PubMed] [Google Scholar]
- Ning Y., Weber J. L., Killary A. M., Ledbetter D. H., Smith J. R., Pereira-Smith O. M. Genetic analysis of indefinite division in human cells: evidence for a cell senescence-related gene(s) on human chromosome 4. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5635–5639. doi: 10.1073/pnas.88.13.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén J., Adami H. O., Bergström R., Dillner J., Friberg L. G., Gustafsson L., Miller A. B., Parkin D. M., Sparén P., Trichopoulos D. Strategies for global control of cervical cancer. Int J Cancer. 1995 Jan 3;60(1):1–26. doi: 10.1002/ijc.2910600102. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Davies J. L., Copeman J. B., Bennett S. T., Palmer S. M., Pritchard L. E., Gough S. C., Kawaguchi Y., Cordell H. J., Balfour K. M. Chromosome-specific microsatellite sets for fluorescence-based, semi-automated genome mapping. Nat Genet. 1994 Jul;7(3):390–395. doi: 10.1038/ng0794-390. [DOI] [PubMed] [Google Scholar]
- Schwengel D. A., Jedlicka A. E., Nanthakumar E. J., Weber J. L., Levitt R. C. Comparison of fluorescence-based semi-automated genotyping of multiple microsatellite loci with autoradiographic techniques. Genomics. 1994 Jul 1;22(1):46–54. doi: 10.1006/geno.1994.1344. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Srivatsan E. S., Misra B. C., Venugopalan M., Wilczynski S. P. Loss of heterozygosity for alleles on chromosome II in cervical carcinoma. Am J Hum Genet. 1991 Oct;49(4):868–877. [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J. A second generation linkage map of the human genome based on highly informative microsatellite loci. Gene. 1993 Dec 15;135(1-2):275–278. doi: 10.1016/0378-1119(93)90077-g. [DOI] [PubMed] [Google Scholar]
- Ziegle J. S., Su Y., Corcoran K. P., Nie L., Mayrand P. E., Hoff L. B., McBride L. J., Kronick M. N., Diehl S. R. Application of automated DNA sizing technology for genotyping microsatellite loci. Genomics. 1992 Dec;14(4):1026–1031. doi: 10.1016/s0888-7543(05)80126-0. [DOI] [PubMed] [Google Scholar]