Abstract
Background
Pharmacogenetics contributes to inter-individual variability in pharmacokinetics (PK) of efavirenz (EFV), leading to variations in both efficacy and toxicity. The purpose of this study was to assess the effect of genetic factors on EFV pharmacokinetics, treatment outcomes and genotype based EFV dose recommendations for adult HIV-1 infected Ugandans.
Methods
In total, 556 steady-state plasma EFV concentrations from 99 HIV infected patients (64 female) treated with EFV/lamivudine/zidovidine were analyzed. Patient genotypes for CYP2B6 (*6 & *11), CYP3A5 (*3,*6 & *7) and ABCB1 c.4046A>G, baseline biochemistries and CD4 and viral load change from baseline were determined. A one-compartment population PK model with first-order absorption (NONMEM) was used to estimate genotype effects on EFV pharmacokinetics. PK simulations were performed based upon population genotype frequencies. Predicted AUCs were compared between the product label and simulations for doses of 300 mg, 450 mg, and 600 mg.
Results
EFV apparent clearance (CL/F) was 2.2 and 1.74 fold higher in CYP2B6*6 (*1/*1) and CYP2B6*6 (*1/*6) compared CYP2B6*6 (*6/*6) carriers, while a 22% increase in F1 was observed for carriers of ABCB1 c.4046A>G variant allele. Higher mean AUC was attained in CYP2B6 *6/*6 genotypes compared to CYP2B6 *1/*1 (p<0.0001). Simulation based AUCs for 600 mg doses were 1.25 and 2.10 times the product label mean AUC for the Ugandan population in general and CYP2B6*6/*6 genotypes respectively. Simulated exposures for EFV daily doses of 300 mg and 450 mg are comparable to the product label. Viral load fell precipitously on treatment, with only six patients having HIV RNA >40 copies/mL after 84 days of treatment. No trend with exposure was noted for these six patients.
Conclusion
Results of this study suggest that daily doses of 450 mg and 300 mg might meet the EFV treatment needs of HIV-1 infected Ugandans in general and individuals homozygous for CYP2B6*6 mutation, respectively.
Introduction
Efavirenz (EFV) is currently the most widely used non-nucleoside reverse transcriptase inhibitor (NNRTI) for HIV patients, particularly during co-treatment with rifampicin [1]. As a result EFV has been extensively used as part of highly active antiretroviral therapy (HAART). Despite extensive clinical experience with EFV, unpredictable inter-individual variability in efficacy and toxicity remain important limitations associated with its use. EFV exhibits significant inter-individual pharmacokinetic variability as well as a narrow therapeutic window, with plasma concentrations >4 µg/mL being associated with more central nervous system (CNS) toxicity while the rate of virologic failure increases with concentrations <1 µg/mL [2]. Consequently, EFV therapeutic drug monitoring has been recommended [3]. Therapeutic drug monitoring is however not universally achievable, as it is not feasible in resource constrained settings. Among the factors affecting EFV pharmacokinetics are ethnicity, host genetic factors, gender, body weight, drug interactions, binding to plasma proteins, hepatic impairment, disease status and pregnancy [4]–[7].
EFV undergoes oxidative hydroxylation primarily by CYP2B6 to 8-hydroxyEFV as a major metabolite, and to 7-hydroxyEFV as a minor metabolite [8]. CYP2B6 516G>T (*6) has particularly been reported to be associated with a pronounced reduction in enzyme activity and elevated EFV plasma concentrations in studies conducted on different populations [6], [9]–[11]. There is evidence that CYP2B6*6 variants are poor metabolizers and therefore at risk of high EFV plasma concentrations and related consequences such as adverse drug reactions often leading to poor compliance. The EFV alternative metabolic pathways: CYP2A6, CYP3A4/A5 and UGT2B7 appear to influence EFV elimination independent of CYP2B6. Additionally, CYP2B6 c.136A→G) and ABCB1 c.4036 A→G influence both EFV plasma and intracellular concentrations [6], [12].
CYP2B6*6, CYP2B6 (c.136A→G) and ABCB1 c.4036 A→G are expressed differently by various populations. In our previous study we found CYP2B6 516G>T and 785A>G were in complete linkage disequilibrium in Ugandans with an overall expression of the variant allele CYP2B6*6 in at least 50% of the population [6], compared to 3.4% in the western/Caucasian population. The SNP frequencies for CYP2B6 c.136A→G and ABCB1 c.4036 A→G in the same population were 13.6% and 15.8% respectively [6]. Most Sub-Saharan African populations are either heterozygous or homozygous for defective variant alleles of CYP2B6 *6 [6], [13] that might result in different features of EFV kinetics and clinical response than other races. Consequently EFV population based dose stratification may be beneficial. However, dose modification needs to be based upon well-derived exposure measures that take into account clinically relevant genetic factors.
A pharmacokinetic-pharmacogenetic model was constructed using steady state EFV concentrations in HIV-1 infected patients to: 1) describe genetic effects on EFV steady state pharmacokinetics, 2) estimate the population pharmacokinetic parameters for EFV exposure, and 3) simulated optimal EFV doses for HIV-1 infected Ugandans and CYP2B6*6 and ABCB1 c.4046A>G variants so as to guide dose selection during treatment of these populations.
Subjects and Methods
The current study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from The Uganda National Council for Science and Technology. Written informed consent was obtained from all participants. The dataset contained 556 EFV concentration values collected from 99 HIV/AIDS patients (64 females) over 252 days from the 14th day of initiation of EFV based HAART. All subjects received oral daily dose of 600 mg EFV (Stocrin®; Merck, Sharpe & Dohme, Whitehouse Station, NJ, USA) plus zidovidine/lamivudine (150 mg/300 mg). In addition, subjects received prophylactic trimethoprim/sulfamethoxazole treatment. Mid-dose EFV plasma concentrations samples (11–18 hours after the last dose) were collected on about five different occasions per subject over the study period. CD4 counts and HIV-1 RNA cells/ml measures were performed at baseline, months 3 and 6. Participant genotypes for CYP2B6 (*6 & *11), CYP3A5 (*3, *6 & *7) and ABCB1 (c.4046A>G and c.3435C>T) were also determined.
Bio-Analysis
Efavirenz Pharmacokinetic Analysis
Blood samples were collected into EDTA tubes and prepared for analysis by centrifugation at 3000 rpm for 10 min and stored at −70°C until HPLC analysis was performed. Plasma EFV was determined by reverse phase HPLC with UV-detection as described (12). The HPLC instrument, Agilent series 1100, consisted of a column compartment G1316A, Degasser G132A, Quat pump G1311A, and an auto-sampler ALS, G1329A, and G1315B diode array detector. The column used was Ace3C18, 3 µm 50×30 mm (Advanced Chromatography Technologies, Aberdeen, Scotland). The standard used was EFV (99.9%), supplied by the WHO Collaborating center for chemical reference substances through Apoteket AB Stockholm, Sweden. The retention time for EFV was 2.42 minutes as detected at UV-VIS 1, 210 nm, UV-VIS 2, 220 nm. This method was linear, with a within-day coefficient of variation of 3.2, 3.3 and 5.1% at concentrations of 2.0 mM (n = 17), 8.0 mM (n = 17), and 20 mM (n = 16), respectively, and a between-day coefficient of variation of 4.1% (n = 50).
Genotyping
Genomic DNA was isolated from peripheral blood leukocytes using QIAamp DNA Maxi Kit (QIAGEN GmbH. Hilden. Germany). All participants were genotyped for CYP2B6*6 and *11, CYP3A5*3,*6 and *7 and ABCB1 (3435CT and rs3842). SNP selections, apart from ABCB1 (3435C>T), was based in their role in EFV pharmacokinetics according to our previous report (12). ABCB1 3435C>T was selected on basis of previous conflicting reports on its role in pharmacokinetics and pharmacodynamics of ART (24–28). Allelic discrimination reactions were performed using TaqMan® (Applied Biosystems, CA, USA) genotyping assays: (C___7586657_20 for ABCB1 3435C>T, C___7817765_60, for ABCB1 rs3842T>C, C__29560333_20, for CYP2B6 516G>T [CYP2B6*6 ], for CYP2B6 136A>G [CYP2B6*11], C__26201809_30 for CYP3A5 6986A>G [CYP3A5*3], C__30203950_10 for CYP3A5 14690G>A [CYP3A5*6]) and C__32287188_10 for CYP3A5 g.27131_27132insT [CYP3A5*7] on ABI 7500 FAST (Applied Biosystems, Foster City, CA). The final volume for each reaction was 10 µl, consisting of 2x TaqMan Universal PCR Master Mix (Applied Biosystems), 20 X drug metabolising genotype assay mix and 10 ng genomic DNA. The PCR profile consisted of an initial step at 50°C for 2 min and 50 cycles with 95°C for 10 minutes and 92°C for 15 sec.
Data Analysis
Pharmacokinetic Model Development
A population PK model of EFV was built using nonlinear mixed-effect modeling (NONMEM) (version 7.2.0). The software ggplot2 (Version 9.3.1), PsN 3.4.2, and SAS 9.2 were used for dataset construction, graphical, and statistical analysis. The first-order conditional estimation method (FOCE) was used. A one-compartment model with first-order absorption and elimination (specified to NONMEM by the ADVAN2 and TRANS2 routines) was assumed. Since the data lacked observations within the absorption phase, the absorption rate constant (Ka) could not be estimated but was instead fixed at 0.3 h−1, a value previously reported [14], [15]. Estimated fixed-effect PK parameters included the apparent clearance (CL/F), relative bioavailability (F1) between ABCB1 groups, and the apparent distribution volume (V/F). Model discrimination was based on relative objective function values (OFV), precision of parameter estimates, and goodness-of-fit plots. Interindividual variability (IIV) was included on Cl/F and V/F with exponential error models. Residual error was described with an additive plus proportional error model.
Covariate Analysis
Covariate analysis was performed using a forward-selection (α = 0.05) followed by backward elimination (α = 0.01) method. Albumin, gender and pharmacogenetic covariates including CYP2B6 (*6 and *11) and ABCB1 (c.4046A>G) were tested in the model. Each covariate-parameter relationship was first tested in a univariate manner. Covariates with two and three degrees of freedom were included in the forward selection if they reduced the OFV by at least 5.99 and 7.81 respectively, corresponding to a p-value of <0.05 for a χ2 distribution. The full covariate model was reached when the addition of further covariate-parameter relationships did not decrease the OFV to the specified criteria. The covariate-parameter relationships were re-examined in the backward deletion step in a manner similar to the forward inclusion step but reversed and with a more conservative significance level of α = 0.01. In addition to significantly reducing the OFV, the standard error on the covariate prediction had to be ≤30% of the predicted value.
Estimates of Exposures
For each patient, EFV area under the curve was derived from the estimated individual pharmacokinetic parameter estimates as shown in Equation 1 .
| (1) |
Typical group values of F1 and empiric Bayesian estimates of clearance were used in the computation of AUC. The doses needed to achieve comparable exposure in the different population subgroups were calculated using Equation 2 .
| (2) |
Pharmacokinetic Simulations
PK simulations were performed for 500 datasets of 99 patients each, with the same genotype covariate frequencies as the original dataset. Fixed and random model effects were set equal to the final model. Doses of 300, 450, and 600 mg were simulated for each of the six possible CYP2B6*6 and ABCB1 (c.4046A>G) combinations and their frequencies in the study population. AUC was calculated for each simulated individual and summary statistics are presented.
PK/PD Associations
Efficacy was measured in terms of immunological recovery (change between baseline and last measured CD4 counts or CD4 counts on days 84, 168 and >200) and virologic decay to below detection or <40 copies per milliliter by day 84. Correlations between AUC and change in efficacy were explored graphically.
Results
Overall the pharmacokinetic dataset contained 556 EFV concentration values collected from 99 HIV/AIDS patients (n = 64 females) over days 252 of daily treatment with EFV based HAART. Mean (SD) bodyweight and age were 55.1(8.0) kg, 37.4 (7.6) years, respectively. The baseline mean (SD) serum albumin, alanine aminotransferase, urea and estimated creatinine clearance were 3.87 (0.79) g/dL, 17.79 (10.18) u/L, 2.85 (1.27) mMol/L and 78.9 (24.27) µmMol/L respectively. Other baseline characteristics and dose relevant genotype information on study subjects are summarized in Table 1 . The population allelic frequencies of SNPS without implications for EFV dose modification that included; CYP2B6 *11, CYP3A5 (*3, *6 & *7) and ABCB1 c.3435C>T did not differ from findings of our previous study [6].
Table 1. Baseline characteristics and CYP2B6*6 and ABCB1 (c.4046A>G) genotypes of the study participants (n = 99).
| Measures of disease status | |
| Log10 viral load (±SD) | 4.95±0.71 |
| CD4 cell count/mL(IQR) | 147 (89–207) |
| Genotype | |
| CYP2B6*6 (rs 2279343, 3745274) | |
| *1/*1 | 31.3% (n = 31) |
| *1/*6 | 54.5% (n = 54) |
| *6/*6 | 14.1% (n = 14) |
| ABCB1 -c.4046A>G(rs3842) | |
| A/A | 60.6% (n = 60) |
| A/G | 34.3% (n = 34) |
| G/G | 1% (n = 1) |
| Missing | 4% (n = 4) |
Missing was assigned to individuals whose genotypes were not determined. Data presented in the table are the assignments as analyzed.
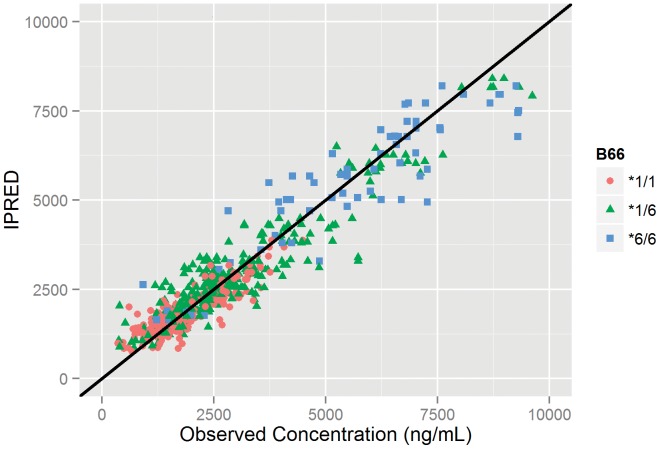
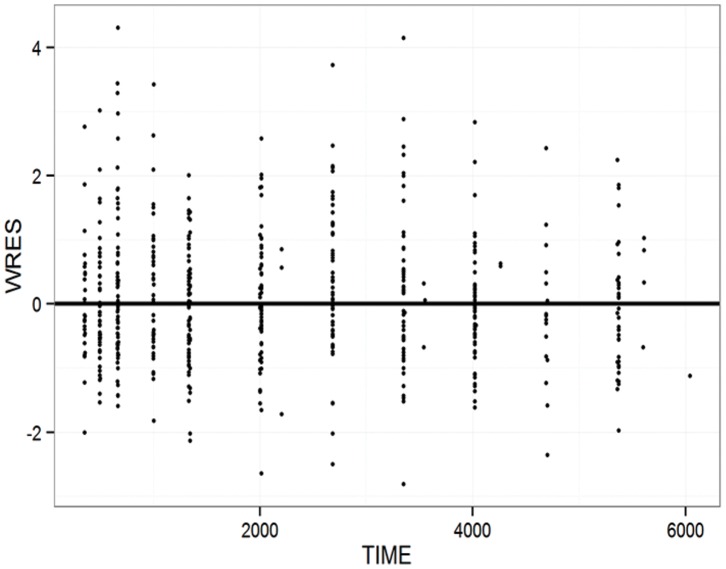
A one-compartment model with first-order absorption described our data well, as can be seen Figure 1 and Figure 2 . All of the highest concentration values, those between 5,000 and 10,000 ng/mL, arose in patients expressing the variant CYP2B6*6 allele. The effects and statistical importance of covariates identified in the study population on pharmacokinetic parameter estimates are depicted in Table 2 . The final model pharmacokinetic parameters are reported in Table 3 . Notably, EFV post-induction CL/F was 2.2 and 1.7 fold higher in CYP2B6*6 (*1/*1) and CYP2B6*6 (*1/*6) compared CYP2B6*6 (*6/*6) carriers, while a 22% increase in F1 was observed for ABCB1 c.4046A>G variants.
Figure 1. Goodness of Fit.
Individual predicted EFV concentrations (IPRED) versus observed concentrations, by CYP2B6*6 genotype (B66).
Figure 2. The individually weighted residuals (WRES) are plotted vs. time.
The dashed line is the zero reference line while the solid line is a smooth nonparametric regression line. The plot demonstrates a good fit of all time point concentration data by the model.
Table 2. Summary of significant factors in the covariate analysis; forward inclusion (α = 0.05) followed by backward elimination (α = 0.01).
| Covariate-Parameter Relationship (functional form) | ΔOFV | p-value |
| Forward Inclusion | ||
| CYP2B6*6– CL (shift for heterozygous and wild-type) | 30.359 | 2.34×10−7 |
| ABCB1 (c.4046A>G – F1 (shift) | 15.883 | 0.0012 |
| Backward Elimination | ||
| ABCB1 (c.4046A>G – F1 (shift) CYP2B6*6– CL (shift for heterozygous and wild-type) | 13.295 25.431 | 0.004 3.004×10−6 |
Table 3. Final model pharmacokinetic parameters.
| Final Pharmacokinetic Model | Parameter | SE(%CV) |
| Ka (hr−1) | 0.300 Fixed | N/A |
| V (L) | 94.5 | 24.4% |
| CL (L/hr) – CYP2B6*6 (*6/*6) – Homozygous Mutant | 4.54 | 11.9% |
| CL (L/hr) – CYP2B6*6 (*1/*6) – Heterozygous Mutant | 7.92 | 33.5% |
| CL (L/hr) – CYP2B6*6 (*1/*1) – Wild-Type | 9.99 | 26.8% |
| F1 - ABRS 1&2 | 1 Fixed | N/A |
| F1 - ABRS 3 | 0.780 | 40.7% |
| F1 - ABRS 4 | 0.513 | 30.4% |
| IIV CL | 0.134 | 19.9% |
| RV – Proportional (%CV) | 17.3 | 26.3% |
| RV – Additive (ng/mL) | 348 | 31.3% |
Ka = Mean population absorption rate constant, V = Mean population Volume of distribution, CL = Mean population clearance, F1 = Bioavailability fraction, IIV CL = inter-individual variability on Clearance in the population, RV = residual variability.
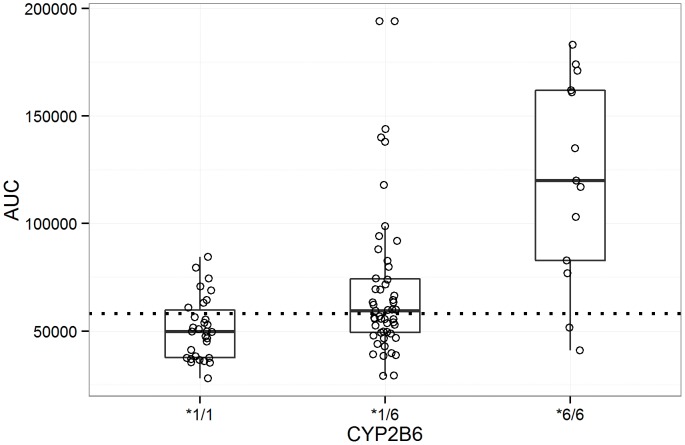
Estimated AUC values stratified by patient genotypes are presented in Table 4 and plotted in Figure 3 . The CYP2B6*6 genotype was found to be a major predicator of exposure to EFV with subjects homozygous for variant CYP2B6 *6 allele exhibiting a mean AUC of 12×104 µg/L·h, more than 2 times mean AUC in the product label. Mean AUC values for both wild type and heterozygous mutants were within the exposure range observed during clinical studies reported in the product label. While plasma EFV exposure differed significantly between CYP2B6*6 (*1/*1)/homozygous wild type ABCB1 (c.4046A>G) and CYP2B6*6 (*6/*6)/mutant ABCB1 (c.4046A>G) (p<0.0001) no difference was observed between CYP2B6*6 (*6/*6)/mutant ABCB1 (c.4046A>G) and CYP2B6*6 (*6/*6)/homozygous wild type ABCB1 (c.4046A>G) p<0.53. Overall population simulation mean AUC was 72523 µg/L·h for the 600 mg dose group, 1.25 fold times the mean AUC in the product label of 58084 µg/L·h.
Table 4. Area under the curve (AUC) NONMEM estimate values for ABCB1 c. 4046A>G and or CYP2B6* 6 genotypes.
| Genotype | n | Area Under the curve(µg/L·h) ×104 | ||
| CYP2B6*6 | ABCB1(c.4046A>G) | Mean (SD) | Range | |
| *1/*6 | 54 | 6.999 (3.509) | 2.917–19.380 | |
| *1/*1 | 31 | 5.160 (1.426) | 2.796–8.456 | |
| *6/*6 | 14 | 12.221 (4.617) | 4.115–18.334 | |
| *1/*6 | mut | 18 | 8.882(4.726) | 3.876–19.378 |
| wt | 35 | 6.147(2.216) | 2.936–14.019 | |
| *1/*1 | mut | 8 | 5.658(1.582) | 3.657–7.948 |
| wt | 21 | 5.114(1.357) | 2.796–8.455 | |
| *6/*6 | mut | 8 | 13.871(3.702) | 7.677–18.334 |
| wt | 4 | 11.070 (5.420) | 5.155–17.367 | |
The estimations are based on the predicted individual apparent clearance and bioavailability values.
mut = heterozygous plus homozygous variant, wt = homozygous variants.
Four patients did not have a reported ABCB1 genotype and one patient was ABCB1 G/G. These five subjects are not included in these summary statistics for ABCB1, but are included in the general CYP2B6*6 summary above.
Figure 3. Distribution of estimated patient AUC values by CYP2B6 genotype.
CYP2B6*1/*1, CYP2B6 *1/*6, and CYP2B6 *6/*6. Dotted line = the mean AUC value in the product label.
By scaling exposures, EFV daily doses of 285 mg would be expected to achieve similar plasma exposure in individuals homozygous to CYP2B6*6 as reported in the EFV product label. Similarly, adjustments to a 487 mg daily dose of EFV would provide the typical Ugandan HIV-1 infected adult with exposure equal to the mean AUC in the drug label. Since these specific dose amounts are not achievable with existing market formulations, simulated exposures for EFV daily doses of 300 mg, 450 mg, and 600 mg are presented in Table 5 to reflect similar doses that are achievable with the currently existing formulations.
Table 5. Simulation based AUC for 300.
| Dose = 300 mg daily | Dose = 450 mg daily | Dose = 600 mg daily | ||||||
| Area Under the curve (µg/L·h) x104 | ||||||||
| number of obsa | Mean (±SD) | 95% CI | Mean (±SD) | 95% CI | Mean (±SD) | 95% CI | ||
| All participants | 47000 | 3.63 (1.93) | 1.57–7.58 | 5.44 (2.90) | 2.35–11.06 | 7.25 (3.86) | 3.14–14.75 | |
| ABCB1(c.4046A>G) wt | CYP2B6*6 | |||||||
| *1/*1 | 17500 | 2.5 (0.94) | 1.28–5.41 | 3.75 (1.41) | 1.92–3.50 | 5.0 (1.88) | 2.56–8.49 | |
| *1/*6 | 2000 | 3.16 (1.17) | 1.63–5.34 | 4.74 (1.75) | 2.45–8.01 | 6.32 (2.34) | 3.27–10 68 | |
| *6/*6 | 10500 | 5.57 (2.14) | 2.85–9.53 | 8.36 (3.21) | 4.27–14.29 | 11.14 (4.28) | 5.7–19.05 | |
| ABCB1(c.4046A>G) Mut | ||||||||
| *1/*1 | 4000 | 3.20 (1.21) | 1.65–5.41 | 4.80 (1.81) | 2.48–8.11 | 6.41 (2.41) | 3.31–10.81 | |
| *1/*6 | 9000 | 4.05 (1.54) | 2.10–7.05 | 6.07 (2.32) | 3.14–10.58 | 8.10 (3. 09) | 4.19–14.10 | |
| *6/*6 | 4000 | 7.12 (2.72) | 3.63–12.20 | 10.67 (4.09) | 5.45–18.30 | 14.23 (5.45) | 7.26–24.40 | |
The frequency of each group in these simulations reflects the proportional frequency of the groups in the observed patient dataset.
Pharmacodynamic Evaluations
Baseline mean (SD) log10 HIV RNA copies per ml and CD4 counts were 4.972 (0.61) and 147.8 (81.0) respectively. Mean (SD) change from baseline CD4 counts at days 84, 168 and after 200 days of EFV based ART was 93.7 (87.2), 154.3 (83.0) and 206.1 (104.5) respectively, while CD4 change by last time of measurement was 177.9 (101.2). Six participants (6.1%) had HIV RNA >40 copies mL−1 after 84 days of ART. AUC values for these 6 patients, as well as their baseline and day 84 HIV RNA values are presented in Table 6 .
Table 6. HIV viral loads at baseline and day 84 of treatment and AUC of study participants who failed to attain viral suppression to below detection by day 84.
| AUC | HIV RNA >40 copies mL−1 | |
| (µg/L·h) | Baseline | Day 84 |
| 27962 | 12170 | 6660 |
| 29359 | 356662 | 108 |
| 36573 | 534688 | 667 |
| 55512 | 900246 | 134 |
| 102750 | 123164 | 134 |
| 183340 | 18030 | 666 |
Data are arranged in order of increasing AUC.
Neither change in CD4 counts nor achievement of HIV RNA <40 copies mL−1 demonstrated a correlation with EFV exposure in this study. In part this may be because the pharmacodynamics response is so strong for most patients at all observed exposure levels on this combination HAART therapy.
Discussion
Despite the existing evidence of extensive inter-ethnic EFV pharmacokinetic variability that is dependent on host-genetic-factors, its dosing in the sub-Saharan African region is to date based largely on data derived from studies conducted among Caucasians. We predicted EFV optimal dose for adult HIV-1 infected Ugandans based upon population genetic make-up and recommend a dose reduction from 600 mg to 450 mg daily dose. Findings of our study also reveal need for EFV dose reduction by 50% when treating patients homozygous for the variant CYP2B6*6 allele.
Consistent with existing reports on EFV pharmacogenetics, the current study demonstrates that variability in EFV pharmacokinetics is largely dependent upon CYP2B6*6 genotype [7], [9], [16]. Reduced EFV metabolism in individuals either homozygous or heterozygous for CYP2B6*6 ultimately results in increased plasma exposure to the drug with higher likelihood of EFV CNS related symptoms [16]. The black race has been associated with higher plasma EFV exposure [4], [17], consistent with our observation of the 1.25 fold higher AUC among HIV-infected Ugandans than the mean AUC in the product label. Higher frequency of EFV concentration dependent CNS adverse events that in turn influence treatment discontinuation rates [18] have previously been reported among Africans. Presumably the supra-therapeutic EFV exposure might play a role in adherence issues and consequent treatment outcomes of EFV based ART.
The current standard 600 mg/daily EFV dose is effective; but the higher EFV plasma exposure may pause a risk for toxicity and non-adherence particularly in CYP2B6 slow metabolizers without increased virologic and immunologic response. Recent prospective studies from African HIV patients indicated association of higher EFV plasma exposure with CNS adverse events [16], [19] and liver enzyme abnormalities [19], [20]. The recommended dose reduction to 450 mg daily according to this study that is plausibly explained by the higher allelic frequency of CYP2B6*6 in the study population also draw its justification from the higher frequency of EFV CNS symptoms among Africans. Our dose adjustment recommendation is also supported by a clinical case report of CYP2B6*6 heterozygous patient who successfully attained viral suppression and sustained it for more than 18 months on EFV dose of 400 mg daily as well as other studies including the recently concluded one by Puls et al [21]–[23]. Successful HIV viral suppression has been demonstrated at EFV doses of 400 mg and 200 mg daily among patients that exhibited supra-therapeutic plasma concentrations following 600 mg daily EFV dose.
The current study had a long follow up period of up to 252 days and pharmacokinetic data were collected at assumed steady state conditions. Six patients did not achieve viral suppression to below detection by day 84 of treatment. However, the lack of a correlation between their day 84 viral loads and either AUC or baseline viral load in the current study suggests other possible causes. Although there is need for studies designed to address this specific question, we postulate that some of the factors responsible for failure to achieve viral suppression to below detection in this particular study might include erratic adherence or intrinsic viral resistance. Considering that 4 (66.7%) of the individuals who failed to achieve viral suppression to below detection by day 84 had their AUC either within range or above the product label AUC, pre-treatment HIV viral resistance that was reported at a rate of 12.3% in Kampala Uganda [24] might have played a role.
Our findings may have extensive applications for most of the Sub-Saharan African region, and are supported by the high frequency of the defective CYP2B6*6 variant alleles among Africans as well as previous reports [21], [22], [25]. Among individuals homozygous for CYP2B6*6, simulated exposures for EFV daily doses of 300 mg (55712.3±21420.9 µg/L·h) were comparable to the product label (58084±23044 µg/L·h). This finding is supported by previous findings according to Mello et al and Gatanaga et al. who demonstrated sustained HIV viral suppression on an EFV dose of 200 mg daily [22], [26].
Even as sub-therapeutic concentrations of EFV are associated with treatment failure [2], [27], supra-therapeutic EFV plasma concentrations may lead to poor adherence, which is in turn a major predictor of treatment outcomes [28]. These EFV dose reduction recommendations are therefore important for sustained efficacy of EFV in HIV management but may also lead to improved quality of life among HIV patients receiving EFV based ART.
In summary based on the current population pharmacokinetic analysis and simulation study, we propose CYP2B6 genotype based EFV dosage adjustment for HIV/AIDS patients in Uganda and the entire sub-Saharan Africa. While our recommended daily doses of 450 mg and 300 mg might meet the EFV treatment needs of HIV-1 infected Ugandans in general and individuals homozygous for variant CYP2B6*6 allele respectively, there is need for caution in events where known drug-drug interactions suspected. We recommend a large multinational clinical trial across the sub-Saharan Africa to validate the EFV dose recommendations by the current study.
Acknowledgments
The authors acknowledge the contribution of all study participants, the laboratory staff at Department of Pharmacology and Therapeutics, Makerere University College of Health Sciences and Karolinska Institutet Hospital Huddinge.
Funding Statement
This study was funded in-part through a CIHR Canadian HIV Trials Network (Clinical Trials Network) international HIV postdoctoral grant and through a SIDA/SAREC, grant No. SWE 2007–270 to Makerere University-Karolinska Institutet research collaboration. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.(2009) WHO/HTM/TB/2009.420, Treatment of tuberculosis: guidelines - 4th ed., http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf.2009, WHO Library Cataloguing-in-Publication Data.
- 2. Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, et al. (2001) Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15: 71–75. [DOI] [PubMed] [Google Scholar]
- 3. Solas C, Gagnieu MC (2011) [Evidence-based therapeutic drug monitoring for efavirenz]. Therapie 66: 197–205. [DOI] [PubMed] [Google Scholar]
- 4. Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, et al. (2006) Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol 61: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stohr W, Back D, Dunn D, Sabin C, Winston A, et al. (2008) Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther 13: 675–685. [PubMed] [Google Scholar]
- 6. Mukonzo JK, Roshammar D, Waako P, Andersson M, Fukasawa T, et al. (2009) A novel polymorphism in ABCB1 gene, CYP2B6*6 and sex predict single-dose efavirenz population pharmacokinetics in Ugandans. Br J Clin Pharmacol 68: 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ngaimisi E, Habtewold A, Minzi O, Makonnen E, Mugusi S, et al. (2013) Importance of Ethnicity, CYP2B6 and ABCB1 Genotype for Efavirenz Pharmacokinetics and Treatment Outcomes: A Parallel-Group Prospective Cohort Study in Two Sub-Saharan Africa Populations. PLoS One 8: e67946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, et al. (2003) The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 306: 287–300. [DOI] [PubMed] [Google Scholar]
- 9. Arab-Alameddine M, Di Iulio J, Buclin T, Rotger M, Lubomirov R, et al. (2009) Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin Pharmacol Ther 85: 485–494. [DOI] [PubMed] [Google Scholar]
- 10. Ribaudo HJ, Liu H, Schwab M, Schaeffeler E, Eichelbaum M, et al. (2010) Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J Infect Dis 202: 717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habtewold A, Amogne W, Makonnen E, Yimer G, Riedel KD, et al. (2011) Long-term effect of efavirenz autoinduction on plasma/peripheral blood mononuclear cell drug exposure and CD4 count is influenced by UGT2B7 and CYP2B6 genotypes among HIV patients. J Antimicrob Chemother 66: 2350–2361. [DOI] [PubMed] [Google Scholar]
- 12. Elens L, Vandercam B, Yombi JC, Lison D, Wallemacq P, et al. (2010) Influence of host genetic factors on efavirenz plasma and intracellular pharmacokinetics in HIV-1-infected patients. Pharmacogenomics 11: 1223–1234. [DOI] [PubMed] [Google Scholar]
- 13. Nyakutira C, Roshammar D, Chigutsa E, Chonzi P, Ashton M, et al. (2008) High prevalence of the CYP2B6 516G–>T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol 64: 357–365. [DOI] [PubMed] [Google Scholar]
- 14. Wade JR, Kelman AW, Howie CA, Whiting B (1993) Effect of misspecification of the absorption process on subsequent parameter estimation in population analysis. J Pharmacokinet Biopharm 21: 209–222. [DOI] [PubMed] [Google Scholar]
- 15. Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, et al. (2003) Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther 73: 20–30. [DOI] [PubMed] [Google Scholar]
- 16. Mukonzo JK, Okwera A, Nakasujja N, Luzze H, Sebuwufu D, et al. (2013) Influence of efavirenz pharmacokinetics and pharmacogenetics on neuropsychological disorders in Ugandan HIV-positive patients with or without tuberculosis: a prospective cohort study. BMC Infect Dis 13: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rotger M, Csajka C, Telenti A (2006) Genetic, ethnic, and gender differences in the pharmacokinetics of antiretroviral agents. Curr HIV/AIDS Rep 3: 118–125. [DOI] [PubMed] [Google Scholar]
- 18.Leutscher PD, Stecher C, Storgaard M, Larsen CS (2013) Discontinuation of efavirenz therapy in HIV patients due to neuropsychiatric adverse effects. Scand J Infect Dis. [DOI] [PubMed]
- 19. Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, et al. (2004) Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 18: 2391–2400. [PubMed] [Google Scholar]
- 20. Yimer G, Amogne W, Habtewold A, Makonnen E, Ueda N, et al. (2012) High plasma efavirenz level and CYP2B6*6 are associated with efavirenz-based HAART-induced liver injury in the treatment of naive HIV patients from Ethiopia: a prospective cohort study. Pharmacogenomics J 12: 499–506. [DOI] [PubMed] [Google Scholar]
- 21. Torno MS, Witt MD, Saitoh A, Fletcher CV (2008) Successful use of reduced-dose efavirenz in a patient with human immunodeficiency virus infection: case report and review of the literature. Pharmacotherapy 28: 782–787. [DOI] [PubMed] [Google Scholar]
- 22. Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, et al. (2007) Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis 45: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 23.Puls R (2013) A daily dose of 400-mg efavirenz (EFV) is non-inferior to the standard 600-mg dose: week 48 data from the ENCORE1 study, a randomised, double-blind, placebo controlled, non-inferiority trial. IAS 2013. KUALA LUMPUR: North American Correspondent, MedPage Today.
- 24. Hamers RL, Wallis CL, Kityo C, Siwale M, Mandaliya K, et al. (2011) HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 11: 750–759. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez A, Cabrera S, Santos D, Valverde MP, Fuertes A, et al. (2011) Population pharmacokinetic/pharmacogenetic model for optimization of efavirenz therapy in Caucasian HIV-infected patients. Antimicrob Agents Chemother 55: 5314–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fayet Mello A, Buclin T, Decosterd LA, Delhumeau C, di Iulio J, et al. (2011) Successful efavirenz dose reduction guided by therapeutic drug monitoring. Antivir Ther 16: 189–197. [DOI] [PubMed] [Google Scholar]
- 27. Ahoua L, Guenther G, Pinoges L, Anguzu P, Chaix ML, et al. (2009) Risk factors for virological failure and subtherapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern Uganda. BMC Infect Dis 9: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ford N, Darder M, Spelman T, Maclean E, Mills E, et al. (2010) Early adherence to antiretroviral medication as a predictor of long-term HIV virological suppression: five-year follow up of an observational cohort. PLoS One 5: e10460. [DOI] [PMC free article] [PubMed] [Google Scholar]