Abstract
Aim
Glomerular hyperfiltration is commonly observed in diabetics early after the onset of the disease and predicts the progression of nephropathy. Sustained hyperglycemia is also closely associated with kidney hypertrophy and increased electrolyte and glucose reabsorption in the proximal tubule. In this study, we investigated the role of the increased tubular sodium/glucose co-transport for diabetes-induced glomerular hyperfiltration. To eliminate any potential confounding effect of the tubuloglomerular feedback mechanism (TGF), we used adenosine A1-receptor deficient (A1 AR−/−) mice known to lack a functional TGF mechanism, and compared the results to corresponding wild-type animals (A1AR+/+).
Methods
Diabetes was induced by an intravenous bolus injection of alloxan. Glomerular filtration rate (GFR) was determined in conscious mice by a single bolus injection of inulin. The sodium/glucose co-transporters were inhibited by phlorizin 30 minutes prior to GFR measurements.
Results
Normoglycemic animals had a similar GFR independent of genotype (A1AR+/+ 233±11 vs. A1AR−/− 241±25 μL min−1), and induction of diabetes resulted in glomerular hyperfiltration in both groups (A1AR+/+ 380±25 vs. A1AR−/− 336±35 μL min−1 ; both P<0.05). Phlorizin had no effect on GFR in normoglycemic mice, whereas it reduced GFR in both genotypes during diabetes (A1AR+/+ 365±18 to 295±19, A1AR−/− 354±38 to 199±15 μL min−1; both P<0.05). Notably, the reduction was more pronounced in the A1AR−/− (P<0.05).
Conclusion
This study demonstrates that increased tubular sodium/glucose reabsorption is important for diabetes-induced hyperfiltration, and that the TGF mechanism is not involved in these alterations, but rather functions to reduce any deviations from a new set-point.
Keywords: Diabetes Mellitus, Glomerular hyperfiltration, Tubuloglomerular feedback, Adenosine
Introduction
Diabetes is the leading cause of end-stage renal disease worldwide (Parving et al., 2003). Early in the disease, before the development of microalbuminuria and nephropathy, glomerular hyperfiltration is commonly found (Mogensen, 1971a, Ditzel and Schwartz, 1967). Hyperfiltration has clinical importance, since it correlates with an increased risk of developing microalbuminuria and nephropathy later on (Mogensen and Christensen, 1984, Rudberg et al., 1992, Magee et al., 2009). The tubuloglomerular feedback (TGF) mechanism is a negative feedback loop located in the juxtaglomerular apparatus of each nephron. The TGF is activated if the macula densa detects elevated NaCl concentrations, which results in constriction of the afferent arteriole. Thus, the glomerular filtration rate (GFR) matches the transport capacity of the nephron. The signaling from the macula densa to the adjacent afferent arterioles involves adenosine, and knockout mice deficient of adenosine A1-receptors (A1AR−/−) completely lack the TGF response (Brown et al., 2001, Sun et al., 2001).
Glomerular hyperfiltration has been proposed to be secondary to increased proximal reabsorption, resulting in reduced NaCl load to the macula densa, which will inactivate the TGF and consequently dilate the afferent arteriole and increase GFR (Thomson et al., 2001). Several investigators have found increased proximal reabsorption in diabetes, both in patients and in models of experimental diabetes (Hryciw et al., 2004, Vallon et al., 1999). Potential mechanisms include increased activity of the sodium/glucose linked transporters (Bank, 1991), or hypertrophy of the proximal tubule that will increase the reabsorption area (Thomson et al., 2001). However, A1AR−/− mice develop diabetes-induced glomerular hyperfiltration to the same degree as wild-type mice (Sällström et al., 2007, Faulhaber-Walter et al., 2008), indicating the involvement of a TGF-independent mechanism. Reabsorption in the proximal tubule has a direct impact on GFR by altering the proximal intratubular pressure, thus changing the net filtration pressure (Leyssac et al., 1994, Persson et al., 2010). Given the increased proximal reabsorption during diabetes, we hypothesized that glomerular hyperfiltration could be caused by an increased sodium/glucose reabsorption in the proximal tubule independently of the TGF mechanism. Consequently, we investigated the role of sodium/glucose co-transport for diabetes-induced glomerular hyperfiltration by blocking proximal glucose reabsorption with phlorizin in wild-type and A1AR−/− mice.
Materials and methods
All chemicals were from Sigma-Aldrich (St. Louis, MO, USA) if not otherwise stated. The study is conform with Good Publishing Practice in Physiology (Persson and Henriksson, 2011).
Animals and induction of diabetes
Adult (3-6 months) male A1AR−/− and corresponding wild-types (A1AR+/+) from the strain developed by Johansson and co-workers were used (Johansson et al., 2001). This strain has been bred into a C57Bl/6J background by the Jackson laboratory (Bar Harbor, ME, USA). Diabetes was induced by a single bolus injection of alloxan (75 mg kg−1 bw) in the tail vein. Animals with a plasma glucose concentration ≥20 mmol/l were considered diabetic. Experiments were performed four to five weeks after the induction of diabetes. Two experimental series were performed using conscious mice.
Series 1 – Role of TGF in the development of diabetes-induced hyperfiltration
In the first series, we investigated whether conscious A1AR−/− could develop a similar degree of glomerular hyperfiltration as their wild-type controls. GFR was determined in normoglycemic animals of both genotypes. Thereafter, diabetes was induced in all animals and GFR once again determined four to five weeks later.
Series 2 – Role of sodium linked glucose transport in diabetes-induced hyperfiltration
In the second series, we investigated whether inhibition of proximal glucose reabsorption could lower GFR in mice lacking the TGF mechanism. Animals of both genotypes were divided into two groups; normoglycemic controls (A1AR+/+ n=13; A1AR−/− n=11) and diabetic animals (A1AR+/+ n=20; A1AR−/− n=5). In the normoglycemic controls, GFR was measured during acute treatment with phlorizin or vehicle. The experimental procedure was repeated four days later, but animals that had previously received phlorizin, were instead administered vehicle, and vice versa, allowing an accurate investigation of the effects of phlorizin on GFR in every subject. The same procedure was performed in mice that had been induced with diabetes four to five weeks earlier.
Determination of GFR in conscious mice
GFR was determined as earlier described (Sällström and Fridén, 2013). The animals were restrained in a Plexiglas chamber. 200 μL dialyzed 3H-methoxy-inulin (~85 kBq; American Radiolabeled Chemicals, St. Louis, MO, USA) was injected into the tail vein of the conscious mice. The injection was facilitated by transilluminating the tail with a fibre optic lamp that visualized the veins. Approximately 5 μL blood was collected from a small cut at the tip of the tail at 1, 3, 7, 10, 15, 35, 55 and 75 minutes after the bolus injection. Throughout the experiment, the animal was kept unrestrained in a normal cage except during blood sampling from the cut tip of the tail. Plasma concentration of inulin was determined by a liquid scintillation counter (Wallac 1409, Wallac Oy, Turku, Finland) and plasma clearance of inulin was calculated using non-compartmental pharmacokinetic data analysis.
Inhibition of glucose reabsorption
Phlorizin (200 mg kg−1) dissolved in propylene glycol or vehicle only was given as a subcutaneous injection 30 minutes before the start of the GFR measurements. Pilot experiments showed that this dose caused glucosuria approximately 15 minutes after the injection.
Measurement of plasma glucose
For normoglycemic mice, the plasma glucose levels in blood from the cut tail were measured using a FreeStyle mini™ device (TheraSense Inc., Alameda, CA, USA) during non-fasting conditions. For diabetic mice, glucose concentration was measured in 3 μL plasma by the Gluco-quant® glucose/HK kit (Roche AB, Stockholm, Sweden) in order to accurately quantify the high plasma glucose concentrations of these animals.
Statistics
All values are expressed as means±SEM. Multiple comparisons between different groups were performed in R version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria) using two-way (series 1) or three-way (series 2) ANOVA and, when appropriate, followed by Fisher’s Least Significant Difference test. For all comparisons, p<0.05 was considered statistically significant.
Results
The body weight was not affected by the glycemic status but was slightly higher (P=0.006) in the A1AR−/− mice (Normoglycemia A1AR+/+ 29±1 and A1AR−/− 36±3 g; Diabetes A1AR+/+ 31±1 and A1AR−/− 36±3 g; 2-way ANOVA interaction genotype and diabetes; P=0.63).
Series 1 – Role of TGF in the development of diabetes-induced hyperfiltration
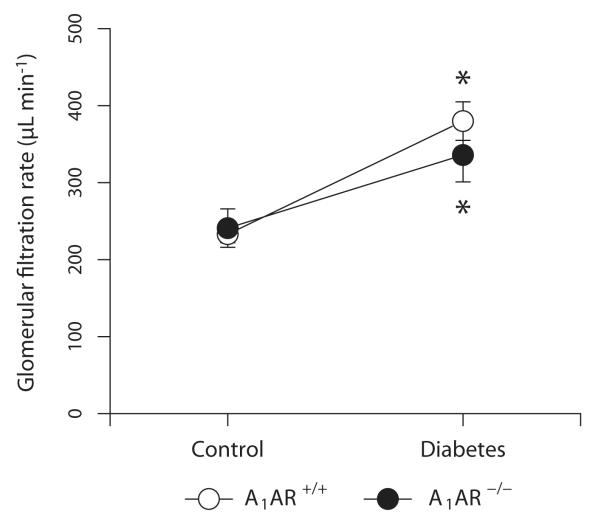
Animals treated with alloxan developed a sustained hyperglycemia that was similar independent of genotype (A1AR+/+ 9±0 and A1AR−/− 10±1 mM versus A1AR+/+ 39±2 and A1AR−/− 41±4 mM; 2-way ANOVA interaction genotype and diabetes; P=0.77). Baseline GFR was similar in both genotypes, and diabetic animals of both genotypes developed a pronounced glomerular hyperfiltration (A1AR+/+ 66±13% and A1AR−/− 45±17%; P=0.36; Fig. 1). There was no statistical interaction in the 2-way ANOVA between genotype and diabetes (P=0.30).
Figure 1.
Glomerular filtration rate in adenosine A1-receptor wild-type (A1AR+/+) and A1-receptor deficient (A1AR−/−) mice with different plasma glucose status (Series 1). * denotes p<0.05 compared to corresponding normoglycemic group.
Series 2 – Role of sodium linked glucose transport in diabetes-induced hyperfiltration
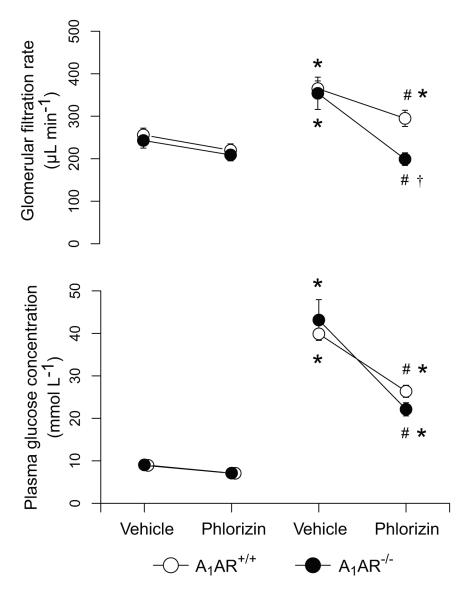
Phlorizin lowered the plasma glucose levels during hyperglycemia in both genotypes (Fig. 2), but the small reduction during normoglycemia did not reach statistical significance (3-way ANOVA interactions: genotype and diabetes P=0.85, genotype and treatment P=0.63, diabetes and treatment P<0.001). Phlorizin had no effect on GFR in normoglycemic animals, whereas it reduced GFR in both diabetic groups (Fig. 2). Notably, the decrease in GFR was more pronounced in A1AR−/− compared to the A1AR+/+. 3-way ANOVA interactions: genotype and diabetes P=0.20, genotype and treatment P=0.61, diabetes and treatment P=0.04.
Figure 2.
Effect of phlorizin on glomerular filtration rate and plasma glucose levels in normoglycemic and diabetic adenosine A1-receptor wild-type (A1AR+/+) and A1-receptor deficient (A1AR−/−) mice (Series 2). * denotes p<0.05 compared to corresponding normoglycemic group, # denotes p<0.05 compared to vehicle within the same genotype and plasma glucose status, and † denotes p<0.05 compared to phlorizin-treated wild-type group.
Discussion
The major finding of the present study is that inhibition of sodium-linked glucose transport normalizes GFR in hyperfiltering A1AR−/− mice, while normoglycemic animals are unaffected. This suggests that the increased glucose reabsorption found during diabetes, directly contributes to hyperfiltration, without the involvement of the TGF mechanism.
In the present study, we investigated whether the increased proximal reabsorption during diabetes, can contribute directly to hyperfiltration. To eliminate the possible influence of anesthesia, all GFR measurements were performed in conscious mice kept in their usual environment. Wild-type and A1AR−/− mice developed a similar degree of hyperfiltration which agrees with two earlier studies using either alloxan (Sällström et al., 2007) or cross-breeding the animals with a hyperglycemic mouse strain (Faulhaber-Walter et al., 2008). However, in another study, hyperfiltration was absent in A1AR−/− mice where diabetes was induced by streptozotocin (Vallon et al., 2009). Reabsorption in the proximal tubule has a direct impact on GFR by affecting the proximal intratubular pressure, thus also changing the net filtration pressure (Leyssac et al., 1994). Along the same line, carbonic anhydrase inhibition, reduced GFR, which was attributed to both increased proximal intratubular pressure and activated TGF (Persson and Wright, 1982). Furthermore, carbonic anhydrase inhibition also reduced GFR in A1AR−/− mice (Hashimoto et al., 2004).
During diabetes, both absolute and fractional proximal reabsorption is increased (reviewed by (Vallon, 2011)) which is the result of several mechanisms. The SGLT1/2 expression is increased (Vidotti et al., 2008, Freitas et al., 2008, Rahmoune et al., 2005), which allows a higher degree of transport per tubular length. Furthermore, proximal tubules grow longer and have a larger diameter during diabetes (Seyer-Hansen et al., 1980). In patients, these adaptations are illustrated by higher transport maximum for glucose during diabetes (Mogensen, 1971b) and could contribute to the reduced proximal intratubular pressure (Vallon et al., 1999, Jensen et al., 1988). Consequently, the effective filtration pressure is expected to increase, which in turn will increase GFR. Inhibition of SGLT1/2-mediated glucose reabsorption by phlorizin in the present study significantly reduced GFR in diabetic animals of both genotypes, while no reduction was found in normoglycemic mice. Earlier studies have also reported that phlorizin can reduce GFR (Harsing et al., 1957) and diabetic SGLT2 knockout mice do not display hyperfiltration (Vallon et al., 2013). The effect has been stipulated to occur via the TGF mechanism, mediated by an increase in distal NaCl delivery (Vallon et al., 1999). However, the present study indicates that the mechanism appears to be TGF-independent since phlorizin also reduced GFR in the A1AR−/− mice. The reduction could rather be a direct consequence of the inhibited proximal reabsorption, which would increase the proximal intratubular pressure, thus reducing the net filtration pressure. This is supported by earlier findings demonstrating that intratubular perfusion by phlorizin is associated with an increased pressure in Bowman’s space in diabetic rats, while control rats were unaffected (Vallon et al., 1999). The fact that diabetic animals are more sensitive to SGLT1/2 inhibition supports the hypothesis that upregulated proximal reabsorption directly causes hyperfiltration. Notably, phlorizin reduced GFR to a larger extent in diabetic A1AR−/− mice than diabetic wild-types and the reason for this is not clear. Administration of phlorizin can alter TGF function in several possible ways. An increased proximal tubular pressure will reduce GFR and act to lower the NaCl load to the macula densa. Inhibition of proximal reabsorption could also dilute the NaCl concentration at MD. If the tubular sodium chloride concentration sensed by the macula densa is reduced, a functional TGF mechanism will partially preserve GFR via a dilation of the afferent arteriole. The lack of this compensatory component might explain the greater reduction in the A1AR−/− animals. However, phlorizin might also tend to increase the NaCl load to MD since sodium co-transport is reduced and available micropuncture data from rats (Vallon et al., 1999) has indeed demonstrated an increased NaCl concentration in end distal tubules which does not support such speculations.
Earlier studies have found that inhibition of proximal reabsorption can increase the renal interstitial pressure (Ott et al., 1971), probably due to swelling of the tubules and the capsule’s low compliance. This indeed has the potential to increase vascular resistance and reduce GFR and it cannot be excluded that this contributes to the reduced GFR seen after phlorizin administration. There are also reports implying a direct vascular cause for diabetes-induced glomerular hyperfiltration. Some studies have found increased glomerular capillary pressure (PGC) in diabetic rats (Hostetter et al., 1981, Zatz et al., 1986), whereas the pressure was reported to be unaltered in other studies (Vallon et al., 1999, Jensen et al., 1981). The reason for this discrepancy is presently unclear, but different rat strains and different methods for assessment of PGC might have influenced the results. Neuronal nitric oxide synthase (nNOS) has been shown to be upregulated in diabetic rats (Komers et al., 2000), which may act to increase PGC and contribute to the hyperfiltration. Furthermore, acute inhibition of nNOS reduced the glomerular hyperfiltration (Komers et al., 2000), whereas long-term treatment delayed the development of renal damage (Komers et al., 2004).
Clinically, diabetes-induced hyperfiltration is associated with an increased risk of developing microalbuminuria and nephropathy (Mogensen and Christensen, 1984, Rudberg et al., 1992, Magee et al., 2009). Several pharmaceutical companies are currently developing selective SGLT2 inhibitors as a new solution to the reduction of plasma glucose concentration in type 2 diabetes (Sheridan, 2012). Given the GFR-lowering effects of phlorizin described in the present paper, long term administration of SGLT2 inhibitors might also be effective as a renoprotective agent in type 1 diabetes, which warrants further studies.
In conclusion, tubular glucose reabsorption plays a pivotal role in maintaining elevated GFR during diabetes. This finding suggests that the observed increased reabsorption during diabetes, directly increases GFR by lowering the proximal intratubular pressure. Furthermore, our findings support earlier data demonstrating that the causative mechanism for diabetes-induced glomerular hyperfiltration is independent of the TGF mechanism. Given that diabetes-induced hyperfiltration contributes to the development of nephropathy, tubular glucose transport is an interesting target for future drugs preventing the development of diabetes-induced kidney damage.
Acknowledgements
This study was financially supported by The Swedish Research Council (K2009-64X-03522-38-2, 2008-2302), The Wallenberg Foundation, H.K.H. Kronprinsessan Lovisas Förening för Barnasjukvård, The Swedish Heart-Lung Foundation (20100183), The Ingabritt and Arne Lundberg Foundation and NIH/NIDDK DK077858 to FP.
Footnotes
Conflict of interest There are no conflicts of interest.
References
- Bank N. Mechanisms of diabetic hyperfiltration. Kidney Int. 1991;40:792–807. doi: 10.1038/ki.1991.277. [DOI] [PubMed] [Google Scholar]
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–7. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- Ditzel J, Schwartz M. Abnormally increased glomerular filtration rate in short-term insulin-treated diabetic subjects. Diabetes. 1967;16:264–7. doi: 10.2337/diab.16.4.264. [DOI] [PubMed] [Google Scholar]
- Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 Adenosine Receptors Augments Diabetic Hyperfiltration and Glomerular Injury. J Am Soc Nephrol. 2008;19:722–30. doi: 10.1681/ASN.2007060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas HS, Anhe GF, Melo KF, Okamoto MM, Oliveira-Souza M, Bordin S, Machado UF. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology. 2008;149:717–24. doi: 10.1210/en.2007-1088. [DOI] [PubMed] [Google Scholar]
- Harsing L, Fonyodi S, Kabat M, Kover G. Effect of phlorizin and of mercurial diuretics on renal haemodynamics. Acta Physiol Hung. 1957;12:363–71. [PubMed] [Google Scholar]
- Hashimoto S, Huang YG, Castrop H, Hansen PB, Mizel D, Briggs J, Schnermann J. Effect of carbonic anhydrase inhibition on GFR and renal hemodynamics in adenosine-1 receptor-deficient mice. Pflugers Arch. 2004;448:621–8. doi: 10.1007/s00424-004-1330-1. [DOI] [PubMed] [Google Scholar]
- Hostetter TH, Troy JL, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981;19:410–5. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- Hryciw DH, Lee EM, Pollock CA, Poronnik P. Molecular changes in proximal tubule function in diabetes mellitus. Clin Exp Pharmacol Physiol. 2004;31:372–9. doi: 10.1111/j.1440-1681.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- Jensen PK, Christiansen JS, Steven K, Parving HH. Renal function in streptozotocin-diabetic rats. Diabetologia. 1981;21:409–14. [PubMed] [Google Scholar]
- Jensen PK, Kristensen KS, Rasch R, Persson AEG. Decreased sensitivity of the tubuloglomerular feedback mechanism in experimental diabetic rats. In: PERSSON AEG, BOBERG U, editors. 11th Eric K. Fernström Symposium; Örenäs Castle, Lund, Sweden. Elsevier; 1988. [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–12. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komers R, Lindsley JN, Oyama TT, Allison KM, Anderson S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am J Physiol Renal Physiol. 2000;279:F573–83. doi: 10.1152/ajprenal.2000.279.3.F573. [DOI] [PubMed] [Google Scholar]
- Komers R, Lindsley JN, Oyama TT, Anderson S. Effects of long-term inhibition of neuronal nitric oxide synthase (NOS1) in uninephrectomized diabetic rats. Nitric Oxide. 2004;11:147–55. doi: 10.1016/j.niox.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Leyssac PP, Karlsen FM, Holstein-Rathlou NH, Skott O. On determinants of glomerular filtration rate after inhibition of proximal tubular reabsorption. Am J Physiol. 1994;266:R1544–50. doi: 10.1152/ajpregu.1994.266.5.R1544. [DOI] [PubMed] [Google Scholar]
- Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–7. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- Mogensen CE. Kidney function and glomerular permeability to macromolecules in early juvenile diabetes. Scand J Clin Lab Invest. 1971a;28:79–90. doi: 10.3109/00365517109090666. [DOI] [PubMed] [Google Scholar]
- Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest. 1971b;28:101–9. doi: 10.3109/00365517109090668. [DOI] [PubMed] [Google Scholar]
- Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- Ott CE, Navar LG, Guyton AC. Pressures in static and dynamic states from capsules implanted in the kidney. Am J Physiol. 1971;221:394–400. doi: 10.1152/ajplegacy.1971.221.2.394. [DOI] [PubMed] [Google Scholar]
- Parving HH, Mauer M, Ritz E. Diabetic nephropathy. In: BRENNER BM, LEVINE SA, editors. The Kidney. 7th ed Saunders; Philadelphia: 2003. [Google Scholar]
- Persson AE, Wright FS. Evidence for feedback mediated reduction of glomerular filtration rate during infusion of acetazolamide. Acta Physiol Scand. 1982;114:1–7. doi: 10.1111/j.1748-1716.1982.tb06945.x. [DOI] [PubMed] [Google Scholar]
- Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 2010;200:3–10. doi: 10.1111/j.1748-1716.2010.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson PB, Henriksson J. Good Publishing Practice in Physiology. Acta physiol. 2011;203:403–407. [Google Scholar]
- Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–34. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy - an 8-year prospective study. Kidney Int. 1992;41:822–8. doi: 10.1038/ki.1992.126. [DOI] [PubMed] [Google Scholar]
- Seyer-Hansen K, Hansen J, Gundersen HJG. Renal hypertrophy in experimental diabetes. Diabetologia. 1980;18:501–505. doi: 10.1007/BF00261707. [DOI] [PubMed] [Google Scholar]
- Sheridan C. SGLT2 inhibitors race to enter type-2 diabetes market. Nat Biotechnol. 2012;30:899–900. doi: 10.1038/nbt1012-899. [DOI] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–8. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sällström J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 2007;190:253–9. doi: 10.1111/j.1748-1716.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- Sällström J, Fridén M. Simultaneous determination of renal plasma flow and glomerular filtration rate in conscious mice using dual bolus injection. J Pharmacol Toxicol Methods. 2013 doi: 10.1016/j.vascn.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–24. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1009–22. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–76. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156–67. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol. 2009;111:p30–8. doi: 10.1159/000208211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidotti DB, Arnoni CP, Maquigussa E, Boim MA. Effect of long-term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol. 2008;28:107–14. doi: 10.1159/000109967. [DOI] [PubMed] [Google Scholar]
- Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77:1925–30. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]