Abstract
Background
Obesity is the result of chronic positive energy balance. The mechanisms underlying the regulation of energy homeostasis and food intake are not understood. Despite large increases in fat mass (FM), recent evidence indicates that fat-free mass (FFM) rather than FM is positively associated with intake in humans.
Methods
In 184 humans (73F/111M; age 34.5±8.8y; % body fat [PFAT] 31.6±8.1%) we investigated the relationship of FFM index (FFMI kg*m2), FM index (FMI kg*m2;), and 24-hour energy expenditure (EE, n=127) with ad-libitum food intake using a 3d vending machine paradigm. Mean daily calories (CAL), and macronutrient intake (PRO, CHO, FAT) were determined and used to calculate the relative caloric contribution of each (%PRO, %CHO, %FAT) and percent of caloric intake over weight maintaining energy needs (%WMEN).
Results
FFMI was positively associated with CAL (p<0.0001), PRO (p=0.0001), CHO (p=0.0075), and FAT (p<0.0001). This remained significant after adjusting for FMI. Total EE predicted CAL and macronutrient intake (all p<0.0001). FMI was positively associated with CAL (p=0.019), PRO (p=0.025) and FAT (p=0.0008). In models with both FFMI and FMI, FMI was negatively associated with CAL (p=0.019) and PRO (p=0.033). Both FFMI and FMI were negatively associated with %CHO and positively associated with %FAT (all p<0.001). EE and FFMI (adjusted for FMI) were positively (EE p=0.0085; FFMI p=0.0018) and FMI negatively (p=0.0018; adjusted for FFMI) associated with %WMEN.
Conclusion
Food and macronutrient intake is predicted by FFMI and to a lesser degree by FMI. FFM and FM may have opposing effects on energy homeostasis.
Keywords: Body composition, fat-free mass, fat mass, food intake, macronutrient intake
1. Introduction
The obesity epidemic is an ongoing and increasing problem in western societies, with a current prevalence of more than 35% in the United States (1). In 2008, annual health costs attributed to obesity exceeded 140 billion USD (2). Excessive overweight is associated with an increased risk of numerous diseases including cardio- and cerebrovascular disease, diabetes mellitus, certain types of cancer (3;4) and neurodegenerative diseases (5;6). Considering the overall health impact of obesity, further insight into the complex system regulating energy homeostasis and food intake is warranted.
Several mechanisms contributing to the regulation of energy homeostasis have been identified. Adipose tissue itself provides a humoral signal of an individual’s long term energy stores by releasing the adipokin leptin with increasing amounts of body fat tissue (7;8). Other factors contributing to the regulation of appetite and food intake include a variety of gastrointestinal hormones (e.g. ghrelin, PYY, GLP-1, CKK), central peptides (e.g. orexin, NPY) and neural mechanisms (e.g. vagal and hypothalamic signaling), forming the so called gut-brain axis (9).
To achieve energy balance, sensing of energy intake needs to be connected to metabolic rate. Energy expenditure itself is closely related to body size and a large part of its variance (approximately 80%) is explained by fat-free mass (FFM) (10). FFM consists of all non-fat tissues (i.e. skeletal muscle, bones and parenchymal organs) and increases with increased adiposity primarily accounting for the increased energy needs of overweight and obese individuals (11). A recent study by Blundell et al. demonstrated positive associations between FFM, but not fat mass (FM) or BMI with self determined meal size (12). Additionally, Caudwell et al (13) confirmed that RMR is correlated with meal size, energy intake, and hunger ratings in overweight and obese adults.
We investigated the relationship of body composition with ad-libitum food intake in a sample of 184 individuals of mixed ethnic backgrounds with a wide range of adiposity. In a subsample of 127 individuals we further investigated the relationship between 24h energy expenditure (assessed via indirect calorimetry in a metabolic chamber) and energy intake. Food intake was assessed in a clinical research unit environment, using a 3 day vending machine paradigm as an objective and reproducible measure of ad-libitum food intake (14). Outcome measures included mean daily caloric intake (CAL), mean daily macronutrient intake [i.e. protein (PRO), carbohydrates (CHO) and fat (FAT)] and the percentages of caloric intake derived from each individual macronutrient (i.e. %PRO, %CHO, %FAT). Based on each individual’s calculated weight maintenance energy needs we also analyzed how much the study volunteers overate (%WMEN) while having free access to the vending machines.
2. Subjects and Methods
2.1. Subjects
All subjects were healthy as determined by medical history, physical examination, and laboratory testing. Subjects were admitted to our metabolic unit and were fed a standard weight maintaining diet (daily kcal: 20% protein; 50% carbohydrates, 30% fat) for 3 consecutive days prior to testing. Each individual’s weight maintenance energy needs (WMEN) were calculated based on body weight and gender as previously described (15). Body composition (percentage body fat, PFAT) was assessed by dual energy x-ray absorptiomery (DXA; DPX-1; Lunar Corp, Madison, WI); PFAT values were standardized by regressing them back to original underwater weighing data and were used to calculate fat-free mass index (FFMI; FFM kg * height m−2) and fat mass index (FMI; FM kg * height m−2) (16).
In a subsample of 127 individuals, twenty four hour energy expenditure (EE) was measured in a metabolic chamber, as previously described (10). During the measurement, physical activity was recorded by a radar system.
Glucose tolerance was assessed by a 75-g oral glucose tolerance test according to the criteria of the World Health Organization. Only non-diabetic subjects participated in this study. While on the research unit, physical activity was restricted to light activities (e.g. playing pool, television, arts, crafts) during the entire time course of the study.
2.2. Ad libitum energy intake using a computerized vending machine system
Measurement of ad libitum food intake was performed as previously described (14;17). In brief, food preferences were determined based on a Food Preferences Questionnaire (FPQ). Individuals rated each food by using a 9-point Likert scale with 1 = dislike extremely; 5 = neutral; 9 = like extremely; we also included the possibility to rate pleasantness of food items as unknown. During the 3 days of food intake assessment on the metabolic ward, subjects self-selected all food items using a computer-operated vending machine system. Each day vending machines were individually stacked with 40 food items and subjects had 23.5h ad-libitum access to the machines, as previously described. All subjects were asked to eat whenever and whatever they desired and to prepare and consume all foods only in a separate eating area. The weights of the consumed foods were used to calculate daily energy intake (kcal/d) and intake of individual macronutrients. This was done by using the CBORD Professional Diet Analyzer Program (CBORD, Inc., Ithaca, NY) and the Food Processor database (ESHA version 10.0.0, ESHA Research, Salem, OR) modified to reflect the nutrient content of specific food items as indicated by the manufacturer. Calories derived from each individual macronutrient intake were calculated as 4 kcal/gram protein and carbohydrates and 9 kcal/gram fat.
2.3. Statistical Analysis
SAS Software (SAS Institute Inc, version 9.2, Cary, NC) was used for statistical analyses of body composition, energy expenditure and food intake data. General linear models (GLM) were employed to determine associations between body composition variables, BMI, body weight, 24h EE, and measures of food intake. All models were in agreement with underlying statistical assumptions (i.e. normal distribution of multivariate residuals). Average daily energy intake (mean kcal/d; CAL), mean daily intake of macronutrients (mean g/d; protein (PRO), carbohydrates (CHO) and fat (FAT)), their percentage contribution to CAL [%PRO ((PRO*4/CAL)*100), %CHO ((CHO*4/CAL)*100), %FAT ((FAT*9/CAL)*100) and percentages weight maintenance energy needs (%WMEN; [mean daily energy consumed/WMEN] * 100) were used as dependent variables. First, all food intake variables were analyzed entering age, gender and ethnicity as confounding covariates and either FFMI or FMI as independent covariates of interest, thus investigating associations with FFMI and FMI individually. Next, both FFMI and FMI were entered in the same statistical model to investigate independent associations with food intake parameters. Associations of total body weight and BMI with CAL (with and without adjustment for total FFM and FFMI) were analyzed similarly. The relationship between 24h EE and food intake was addressed in two different analyses. In the first analysis, associations of food intake with total 24h EE (adjusted for physical activity only) were determined. In the second analysis we analyzed associations of food intake with unexplained variance of 24h EE by adjusting for age, gender, ethnicity, FM and FFM. Importantly, in this subsample of 127 individuals, no statistically significant differences were observed between the original sample and the sub-sample regarding the subjects main characteristics (i.e. age, gender, ethnicity, FFM, FM, FFMI, FMI, height, total body weight, BMI, measures of food intake).
Percent of protein (%PRO) was not normally distributed. Thus, analyses were repeated with normally distributed log transformed values. However, results were similar in terms of significance so for consistency, we reported results from untransformed data. Associations were graphically illustrated by plotting the dependent variables (i.e. measures of food intake) against the adjusted residuals of the covariate of interest. Correlation coefficients and significance levels were determined by Pearson correlation or Spearman’s ranked correlation whenever appropriate.
3. Results
Characteristics of the study population, including anthropometric and food intake data as well as sex differences, are summarized in Table 1.
Table 1.
Characteristics of study population
| n (female/male)* | Total sample (n=184) | Female (n=73) | Male (n=111) |
|---|---|---|---|
| Ethnicity (A/AA/H/C/NA)* | 1/4/6/52/121 | 0/2/0/18/53 | 1/2/6/34/68 |
| Age (years) | 34.5(±8.8) | 34.4 (±9.3) | 34.5 (±8.6) |
| Weight (kg) | 94.4(±23.1) | 91.1(±23.7) | 96,6 (±22.5) |
| Height (cm) | 169.5(±8.5) | 161.7 (±5.1) | 174.7 (±5.9) |
| PFAT (%) | 31.6(±8.1) | 38.3 (±5.9) | 27.2 (±6.2) |
| FFM (kg) | 63.8(±13.5) | 55.1 (±10.8) | 69.3 (±12.2) |
| FM (kg) | 30.7(±13.1) | 36.0 (±13.7) | 27.3 (±11.4) |
| FFMI (kg*m-2) | 22.1(±3.7) | 21.1 (±3.9) | 22.7 (±3.5) |
| FMI (kg*m-2) | 10.8(±5.0) | 13.8 (±5.3) | 8.9 (±3.6) |
| BMI (kg*m-2) | 32.9(±7.9) | 34.8 (±8.9) | 31.6 (±6.8) |
|
| |||
| Mean Caloric Intake (kcal) | 4369(±1390) | 3639(±1260) | 4849(±1260) |
| Mean Protein Intake (g) | 144.1(±47.6) | 122.5(±41.9) | 158.3(±45.8) |
| Mean Carbohydrate Intake (g) | 545.8(±175.8) | 454.6(±158.7) | 605.8(±160.5) |
| Mean Fat Intake (g) | 190.4(±69.6) | 159.1(±65.6) | 211.0(±64.6) |
| Percentage WMEN (%) | 155.2(±45.3) | 138.2(±47.6) | 166.3(±40.2) |
All results apart from*are presented as mean ± SD;
A Asian; AA African American; H Hispanic; C Caucasian; NA Native American
3.1 Individual associations of FFMI and FMI with food intake
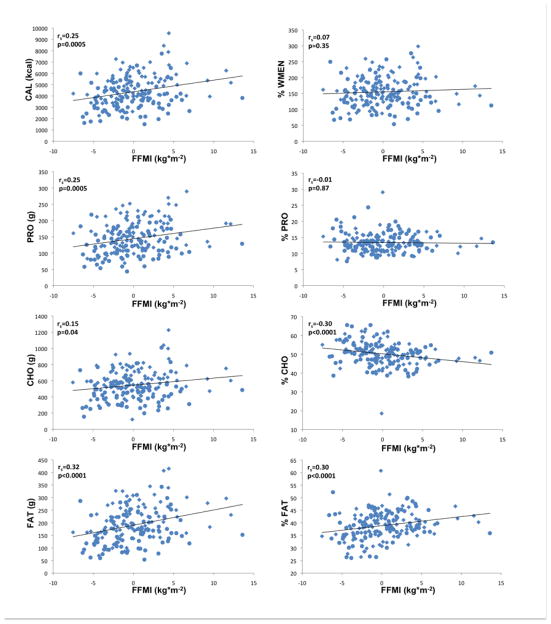
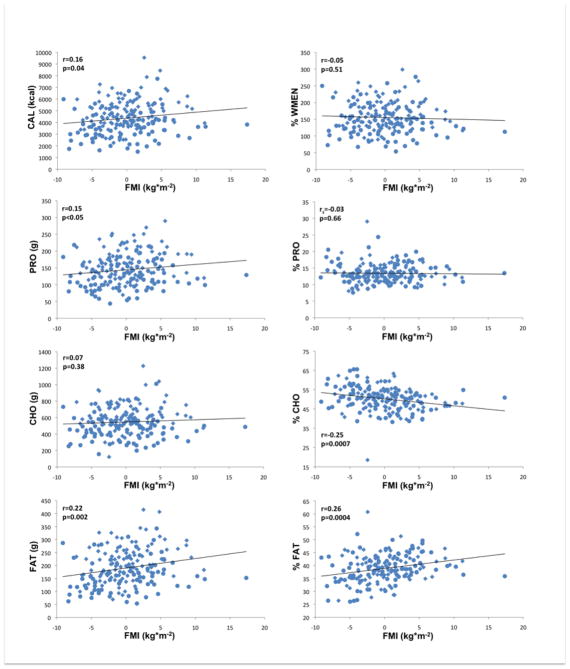
Results obtained by general linear models (GLM) are summarized in Tables 2 and 3 and illustrated in Figures 1 and 2 (i.e. correlations of residualized FFMI and FMI with measures of food intake). Adjusted for age, gender and ethnicity, FFMI was positively associated with CAL, PRO, FAT (all p≤0.0001) and mean CHO (p=0.0075). Adjusted for age, gender and ethnicity, FMI was positively associated (p=0.019) with CAL. FMI was furthermore strongly positively associated with FAT (p=0.0008) and to a lesser degree with PRO (p=0.025) but not with CHO. Both FFMI and FMI were negatively associated with %CHO (FFMI p=0.0007; FMI p=0.0005) and positively associated with %FAT (FFMI p=0.0008; FMI p=0.0003) but neither with %PRO. There were no significant associations of either FFMI or FMI with %WMEN. We also categorized our sample as either Native American or non-native and found no significant effect of this covariate for the relationships between body composition variables, energy expenditure and caloric intake or overconsumption. Interestingly, we did find a significant ethnicgroup*FFMI interaction term in models analyzing caloric intake and overeating (CAL, p=0.0061; %WMEN p=0.012).
Table 2.
Associations of food and macrocronutrient intake with anthropometric measures and energy expenditure
| β | T | P | |
|---|---|---|---|
|
|
|||
| Mean Caloric Intake (kcal) | |||
| FFMI kg*m−2 | 102.8 | 4.17 | <0.0001 |
| FMI kg*m−2 | 50.7 | 2.38 | 0.019 |
| FFMI(adj.) kg*m−2 | 202.4 | 4.16 | <0.0001 |
| FMI(adj.) kg*m−2 | −96.5 | −2.36 | 0.019 |
| 24h EE kcal | 1.62 | 6.93 | <0.0001 |
| 24h EE(adj.) kcal | 2.03 | 4.11 | <0.0001 |
| Mean Protein Intake (g) | |||
| FFMI kg*m−2 | 3.2 | 3.91 | 0.0001 |
| FMI kg*m−2 | 1.6 | 2.26 | 0.025 |
| FFMI(adj.) kg*m−2 | 6.3 | 3.83 | 0.0002 |
| FMI(adj.) kg*m−2 | −3.0 | −2.14 | 0.033 |
| 24h EE kcal | 0.049 | 5.31 | <0.0001 |
| 24h EE(adj.) kcal | 0.07 | 3.70 | 0.0003 |
| Mean Carbohydrate Intake (g) | |||
| FFMI kg*m−2 | 8.7 | 2.70 | 0.0075 |
| FMI kg*m−2 | 2.7 | 0.98 | 0.33 |
| FFMI(adj.) kg*m−2 | 23.8 | 3.75 | 0.0002 |
| FMI(adj.) kg*m−2 | −14.6 | −2.75 | 0.0067 |
| 24h EE kcal | 0.17 | 5.48 | <0.0001 |
| 24h EE(adj.) kcal | 0.25 | 3.81 | 0.0002 |
| Mean Fat Intake (g) | |||
| FFMI kg*m−2 | 6.1 | 4.91 | <0.0001 |
| FMI kg*m−2 | 3.6 | 3.41 | 0.0008 |
| FFMI(adj.) kg*m−2 | 9.2 | 3.74 | 0.0002 |
| FMI(adj.) kg*m−2 | −3.0 | −1.48 | 0.14 |
| 24h EE kcal | 0.08 | 7.12 | <0.0001 |
| 24h EE(adj.) kcal | 0.09 | 3.56 | 0.0005 |
All associations were analyzed using general linear models; β indicates the estimated increase/decrease of each food intake measures (i.e. kcal or g), attributable to one unit increase of anthropometric variables(i.e. 1 kg*m−2) or energy expenditure (i.e. 1 kcal); FFMI fat free mass index; FMI fat mass index; 24h EE 24 hour energy expenditure; All models are described in detail in the methods section;
Table 3.
Associations of food and macronutrient intake (expressed as percentages) with body composition variables and energy expenditure
| β | T | P | |
|---|---|---|---|
|
|
|||
| Percentage WMEN (%) | |||
| FFMI kg*m−2 | 0.7 | 0.82 | 0.41 |
| FMI kg*m−2 | −0.6 | −0.82 | 0.41 |
| FFMI(adj.) kg*m−2 | 5.4 | 3.18 | 0.0018 |
| FMI(adj.) kg*m−2 | −4.5 | −3.18 | 0.0018 |
| 24h EE kcal | 0.023 | 2.67 | 0.0085 |
| 24h EE(adj.) kcal | 0.07 | 3.89 | 0.0002 |
| Percentage Protein Intake (%) | |||
| FFMI kg*m−2 | −0.02 | −0.38 | 0.70 |
| FMI kg*m−2 | −0.02 | −0.33 | 0.74 |
| FFMI(adj.) kg*m−2 | −0.02 | −0.19 | 0.85 |
| FMI(adj.) kg*m−2 | −0.00 | −0.00 | 0.99 |
| 24h EE kcal | −0.00 | −0.79 | 0.43 |
| 24h EE(adj.) kcal | 0.00 | 0.07 | 0.94 |
| Percentage Carbohydrate Intake (%) | |||
| FFMI kg*m−2 | −0.42 | −3.45 | 0.0007 |
| FMI kg*m−2 | −0.36 | −3.57 | 0.0005 |
| FFMI(adj.) kg*m−2 | −0.18 | −0.73 | 0.47 |
| FMI(adj.) kg*m−2 | −0.23 | −1.15 | 0.25 |
| 24h EE kcal | −0.003 | −2.21 | 0.029 |
| 24h EE(adj.) kcal | −0.002 | −0.50 | 0.62 |
| Percentage Fat Intake (%) | |||
| FFMI kg*m−2 | 0.36 | 3.43 | 0.0008 |
| FMI kg*m−2 | 0.33 | 3.71 | 0.0003 |
| FFMI(adj.) kg*m−2 | 0.10 | 0.47 | 0.64 |
| FMI(adj.) kg*m−2 | 0.025 | 1.43 | 0.15 |
| 24h EE kcal | 0.003 | 2.65 | 0.009 |
| 24h EE(adj.) kcal | |||
All associations were analyzed using general linear models; β indicates the estimated increase/decrease of percental food intake measures, attributable to one unit increase of body compositin variables (i.e. 1 kg*m−2) or energy expenditure (i.e. 1 kcal); FFMI fat free mass index; FMI fat mass index; 24h EE 24 hour energy expenditure; All models are described in detail in the methods section;
Figure 1.
Associations of fat-free mass index (FFMI kg*m−2), adjusted for age, gender, ethnicity with food and macronutrient intake; CAL, mean daily caloric intake; PRO, mean daily protein intake; CHO, mean daily carbohydrate intake; FAT, mean daily fat intake; %WMEN, percentage weight maintenance energy needs; %PRO, relative caloric contribution of protein; %CHO, relative caloric contribution of carbohydrates; %FAT, relative caloric contribution of fat; rs indicates Spearman’s ranked correlation. Circles indicate female gender, diamonds indicate male gender.
Figure 2.
Associations of fat mass index (FMI kg*m−2) adjusted for age, gender and ethnicity with food and macronutrient intake; CAL, mean daily caloric intake; PRO, mean daily protein intake; CHO, mean daily carbohydrate intake; FAT, mean daily fat intake; %WMEN, percentage weight maintenance energy needs; %PRO, relative caloric contribution of protein; %CHO, relative caloric contribution of carbohydrates; %FAT, relative caloric contribution of fat; rs indicates Spearman’s ranked correlation. Circles indicate female gender, diamonds indicate male gender.
3.2 Independent associations of FFMI and FMI with food intake
Results are summarized in Tables 2 and 3 and illustrated in Figure 3 (i.e. correlations of residualized FFMI and FMI with measures of food intake).
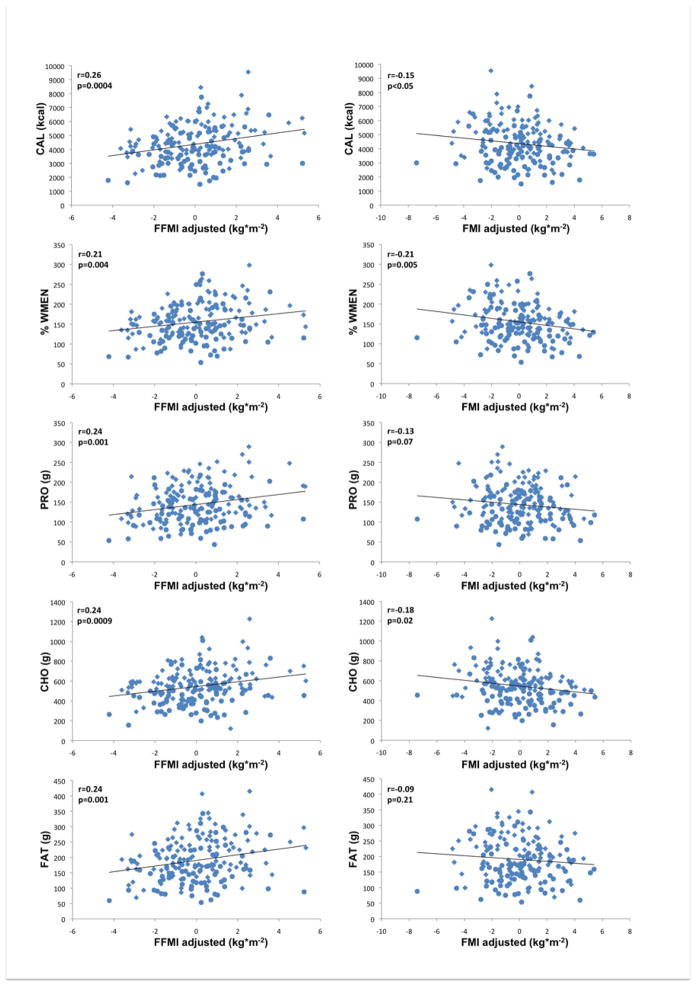
Figure 3.
Left column illustrates associations of fat-free mass index (FFMI kg*m−2,) adjusted for fat mass index, age, gender, and ethnicity with food and macronutrient intake. Right column illustrates associations of fat mass index (FMI kg*m−2) adjusted for fat-free mass index, age, gender, ethnicity with food and macronutrient intake; CAL, mean daily caloric intake; PRO, mean daily protein intake; CHO, mean daily carbohydrate intake; FAT, mean daily fat intake; %WMEN, percentage weight maintenance energy needs; rs indicates Spearman’s ranked correlation coefficient. Circles indicate female gender, diamonds indicate male gender.
After adjustment for age, gender, ethnicity and FMI, highly significant positive associations were observed between FFMI and CAL (p<0.0001) and with PRO, CHO and FAT (all p=0.0002). FMI on the other hand, when accounting for age, gender, ethnicity and FFMI, was negatively associated with CAL (p=0.019), protein intake (p=0.033) and CHO (p=0.0067) but not with FAT. When adjusted for each other, neither FFMI nor FMI were associated with %PRO, %CHO and %FAT. On the other hand, %WMEN was positively associated with FFMI and negatively associated with FMI (both p=0.0018).
3.3 Associations of 24 hour energy expenditure with food intake
Results are summarized in Table 2 and illustrated in Figure 4. As expected, total FFM and FFMI correlated strongly with 24h energy expenditure (EE; FFM: r=0.89; FFMI r=0.78; both p<0.0001).
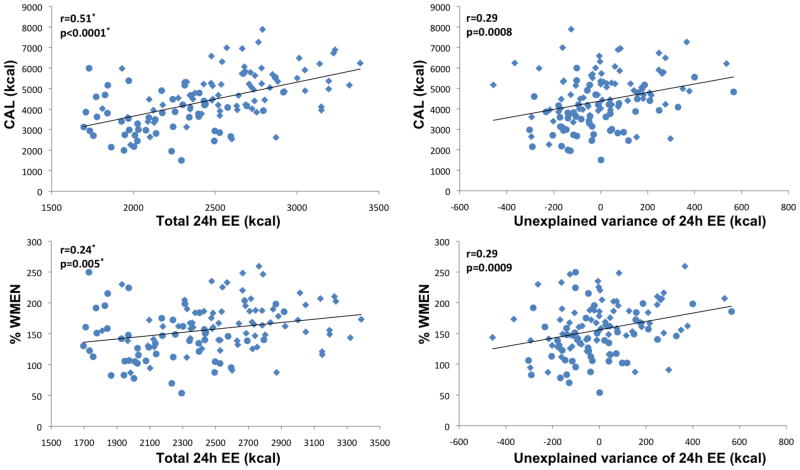
Figure 4.
Left column illustrates associations of total 24h energy expenditure (24h EE) with mean daily caloric intake (CAL) and overconsumption (%WMEN, percentage weight maintenance energy needs). Right column illustrates associations of 24h EE adjusted for age, gender, ethnicity, fat-free mass and fat mass (i.e. unexplained variance of 24h EE) with CAL and %WMEN. Circles indicate female gender, diamonds indicate male gender. * p values and correlation coefficients were calculated accounting for physical activity.
EE (adjusted for physical activity only) was strongly positively associated with CAL and intake of individual macronutrients (all p<0.0001). EE was also negatively associated with %CHO (p=0.029) and positively associated with %FAT (p=0.009) and %WMEN (p=0.0085) but not %PRO. After adjustment for age, gender, ethnicity, FFM and FM, EE associations with CAL (p<0.0001), PRO (p=0.0003), CHO (p=0.0002), FAT (p=0.0005) and %WMEN (p=0.0002), but not %CHO and %FAT, remained significant.
3.4 Associations of total body weight and BMI with caloric intake
Both total body weight and BMI were significantly and positively associated with CAL (p=0.0003 and p=0.0012). However, when adjusted for FFM (i.e. total body weight adjusted for total FFM and BMI adjusted for FFMI) neither total body weight nor BMI were negatively associated with CAL (data not shown), while FFM and FFMI remained significantly and positively associated with caloric intake (FFM adjusted for total body weight: p=0.0051; FFMI adjusted for BMI p=0.0007).
4. Discussion
We investigated the relationship between measures of body composition and ad-libitum food intake in 184 healthy individuals. FFMI was positively associated with mean daily caloric intake (CAL) and mean daily intake of protein (PRO), carbohydrates (CHO), and fat (FAT). Additionally, we found significant positive associations of FMI with CAL, PRO and FAT. These positive associations seemed to be entirely explained by FFMI, as in models with both FFMI and FMI, FMI was negatively associated with CAL and CHO. In contrast, all positive associations of FFMI with CAL and intake of individual macronutrients remained highly significant after adjustment for FMI. Both FFMI and FMI were associated with increased energy intake based on fat (%FAT) and reduced energy intake based on carbohydrates (%CHO). FFMI adjusted for FMI was also positively associated with how much individuals overate relative to their weight maintenance energy needs (% WMEN), while FMI was negatively associated with %WMEN after adjusting for FFMI. We also found a significant effect of ethnicity on the interaction between FFMI and CAL and %WMEN. The directionality of the relationship, however, was the same in both Native Americans and non-natives and was actually slightly weaker in the Native Americans. In a subsample of 127 individuals with 24 hour measurement of energy expenditure (EE), we demonstrated that total EE and the unexplained variance of EE (i.e. after adjusting for age, sex, ethnicity, FFM, FM), is positively associated with food and macronutrient intake and also %WMEN. Associations of total EE with %CHO and %FAT were similar to the observations made with FFMI and FMI.
Similar to the recent studies (12;13), our results indicate that FFM predicts food intake in humans. We also found that BMI (and total body weight) is a positive predictor of ad-libitum food intake in humans even though these associations were entirely explained by FFMI. That BMI was positively associated with food intake in our study may be due to our larger sample size with larger variances in weight and BMI. When separating total body mass into FFM and FM it becomes evident that food intake largely depends on FFM independent of FM. It is well known that FFM and not FM is the major determinant of RMR and EE (10;11). Thus, as proposed by Blundell et al., this positive relationship between food intake and FFM most likely reflects a physiological response to the increased energy needs in individuals with higher FFM in order to preserve body mass. Fat mass, when adjusted for FFM, was a negative determinant of food intake, and this would seem to be consistent with adipose tissue’s primary biological function of storing energy. When sufficient energy is stored, the relative amount of adipose tissue might provide signals to decrease energy intake. However, at least in our analysis, it is clear that FFM is a more powerful driver of caloric intake.
Higher FFM was associated with increased intake of all macronutrients. It has been proposed by Millward et al (18) that dietary protein intake is the key to maintaining a stable whole body protein mass (protein-stat mechanism). We did not observe differences in strength between the associations of FFMI with protein, carbohydrate and fat intake (tested by fisher-z transformation of correlation coefficients; data not shown). Indeed, higher FFMI and to a greater degree FMI were associated with increased caloric intake due to relatively higher proportion of fat versus carbohydrate intake, indicating a preference of heavier subjects for high-fat foods compared with carbohydrate dense foods. This is consistent with a large study of 508 male and 1293 female obese subjects seeking weight loss treatment showing a positive relationship between baseline fat intake and BMI, although men seemed to prefer high-fat/high-protein foods while females tended to consume more high-fat/high-carbohydrate foods (19). Similar findings had been reported in a study of weight-discordant monozygotic twins, demonstrating a higher fat-preference for obese versus their lean siblings, indicating that the increased caloric intake which may have lead to and is needed to maintain the increased weight is primarily due to preference for high-fat foods (20).
The reason why FFMI (adjusted for FMI) and EE are associated not only with mean caloric intake but also with %WMEN, thus indicating a propensity to overeat is not clear. In a food scarce environment, the drive to overeat may represent a protective biological mechanism to prevent an individual from subsequent food scarcity. In today’s obesogenic environment, where palatable and high-caloric foods are readily available at any time, this propensity might result in a chronic positive energy balance. FMI, when adjusted for FFMI, was negatively associated with food intake and with %WMEN. Adipose tissue could promote negative energy balance via several mechanisms. Leptin is released by adipocytes, with plasma concentrations rising proportionally to adipose tissue mass (7). Leptin may act primarily in signaling starvation rather than mediating satiety (21;22). However leptin administration has been shown to decrease food intake in animals (23) and humans (24). However, it is not clear whether leptin could explain the negative association of FMI with food intake when accounting for FFMI. Apart from leptin other adipocyte-derived hormones have been discussed as potential modulators of energy balance. These include the adipokines adiponectin and resistin, estrogens and glucocorticoids. The individual functions of these hormones and their effect on food intake still remain to be established (25). Nevertheless, this is a particularly interesting finding in this analysis that warrants further research suggesting that FFMI is not just sensing energy needs but somehow may also underpin the drive to overeat.
While there is a general lack of knowledge on how increases in FFM and associated increases in total energy needs result in increased energy intake, there is no clear biological mechanism known that could explain the associations of FFM with food intake.
Skeletal muscle tissue accounts for ~50% of a healthy individual’s FFM and approximately 30–40% of total body weight. Emerging evidence indicates that skeletal muscle tissue has endocrine functions. Myokines (e.g. mysostatin, IL-6, IGF-1) are secreted in response to physical activity and muscle contraction and might have important roles in the pathophysiology of diseases that are associated with sedentary behavior (e.g. type 2 diabetes, cardiovascular diseases, cancer) (26). It is possible, therefore, that some of the cytokines released by muscle tissue may actually decrease food intake. However, exercise induced increases in Il-6 have been linked to reduced appetite (27), thus not yet providing a plausible mechanism for the positive associations of FFM with food intake. Human behavior, including food intake, is ultimately regulated by the central nervous system and also organs and tissues that comprise FFM need to be centrally controlled and represented. In order to survive, integration of behavioral actions (i.e. food intake) with internal needs (i.e. energy needs) is mandatory. Our group recently showed in a sample of 76 largely obese subjects, that FFM but not FM is strongly negatively associated with gray matter volume of brain regions that are believed to be involved in the regulation of homeostatic and autonomic bodily functions including food intake (28). This could potentially represent a central adaptive mechanism in response to increases in FFM and associated increases in total energy needs. While some of the regions that were associated with FFM (even after adjustment for FM) were implicated in decision-making and inhibitory control, these changes itself might perpetuate unhealthy eating behaviors, thus possibly contributing to a chronic positive energy balance.
Several limitations must be acknowledged. Most of our subjects overate during the three days of ad-libitum food intake measurement, which in part could be attributed to the rather mundane environment of a research facility. However, while the mean intake on the vending machines is 155±45% of WMEN in this cohort, the range is actually 53.7 –298.7% of WMEN. Metabolic parameters, particularly adipokines, hunger and satiety hormones (e.g. leptin, ghrelin, PYY, PP, GLP-1, CKY) that might have explained some of the variance in food intake were not considered in this study. Nevertheless, in a subsample of our study population (n=69) plasma ghrelin concentrations were negatively associated with body mass and adiposity as recently published (17) and no independent associations with FFMI have been observed (data not shown). Total caloric intake and macronutrient content was calculated based on the information provided by the manufacturer of the individual foods and there is variation in the accuracy of food product labels (29). For the longitudinal analysis of future fat gain, no information is available on these individual’s eating habits and physical activity levels between the observation time points. Although FFM is also elevated in body builders and athletes, results of this study should be seen in the context of obesity and sedentary behavior. FFM and FM are different biological compartments but do co-vary strongly. Thus a cautious interpretation of these results is required, particularly when adjustments were made for the respective other compartment. We report results from a large number of statistical tests, however no adjustments for multiple comparisons have been made because of the strong interrelatedness of food intake variables and measures of body composition/energy expenditure, thus adding to the limitations of this study.
Conclusions
We show that in humans, fat-free mass and 24 hour energy expenditure are positively associated with ad-libitum food intake and intake of individual macronutrients. We furthermore show that 24-hour energy expenditure and fat-free mass (adjusted for fat mass) are positively associated with overeating. Overall, these findings indicate that FFM primarily promotes positive and FM (after adjustment for FFM) negative energy balance. In this scenario, FFM-related increases in energy expenditure must be compensated by increased caloric intake via peripheral or central mechanisms. As humans are prone to positive energy balance, in our food rich environment, this sensing of increased energy requirements also leads to overconsumption.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
ClinicalTrials.gov Identifier: NCT00342732
The authors have no conflict of interests to declare.
Reference List
- 1.Ogden C, Carroll M, Kit B, Flegal K. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 82. 2012 DHHS. Ref Type: Pamphlet. [Google Scholar]
- 2.Finkelstein E, Trogden J, Cohen J, Dietz W. Annual Medical Spending Attributable To Obesity: Payer-And Service-Specific Estimates. Health Aff. 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Flegal K, Carroll M, Ogden C, Johnson C. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Flegal K, Carroll M, Ogden C, Curtin L. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson D, Rotherberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 6.Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67:1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5. [DOI] [PubMed] [Google Scholar]
- 7.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- 8.Gautron L, Elmquist J. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121:2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoud H, Morrison C. The Brain, Appetite, and Obesity. Ann Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 10.Ravussin E, Lillioja S, Anderson T, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravussin E, Burnand B, Schutz Y, Jequier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr. 1982;35:566–573. doi: 10.1093/ajcn/35.3.566. [DOI] [PubMed] [Google Scholar]
- 12.Blundell J, Caudwell P, Gibbons C, Hopkinds M, Naslund E, King N, et al. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr. 2012;107:445–449. doi: 10.1017/S0007114511003138. [DOI] [PubMed] [Google Scholar]
- 13.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr. 2013;37:7–14. doi: 10.3945/ajcn.111.029975. [DOI] [PubMed] [Google Scholar]
- 14.Venti C, Votruba S, Franks P, Krakoff J, Salbe A. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr. 2010;91(2):343–348. doi: 10.3945/ajcn.2009.28315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53(6):1368–1371. doi: 10.1093/ajcn/53.6.1368. [DOI] [PubMed] [Google Scholar]
- 16.VanItallie T, Yang M, Heymsfield S, Funk R, Boileau R. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 17.Votruba S, Kirchner H, Tschop M, Salbe A, Krakoff J. Morning ghrelin concentrations are not affected by short-term overfeeding and do not predict ad libitum food intake in humans. Am J Clin Nutr. 2009;89:801–806. doi: 10.3945/ajcn.2008.27011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millward D. Metabolic demands for amino acids and the human dietary requirement: Millward and Rivers (1988) revisited. J Nutr. 1998;(128/suppl):S2563–S2576. doi: 10.1093/jn/128.12.2563S. [DOI] [PubMed] [Google Scholar]
- 19.Linde J, Utter J, Jeffrey R, Sherwood N, Pronk N, Boyle R, et al. Specific food intake, fat and fiber intake, and behavioral correlates of BMI among overweight and obese members of a managed care organization. Int J Behavior Nutr Physical Act. 2006;3:42. doi: 10.1186/1479-5868-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rissanen A, Hakala P, Lissner L, Mattlar C-E, Koskenvuo M, Ronnemaa T. Acquired preference especially for dietary fat and obesity: a study of weight404 discordant monozygotic twin pairs. Int J Obes. 2002;26(7):973–977. doi: 10.1038/sj.ijo.0802014. [DOI] [PubMed] [Google Scholar]
- 21.Ahima R, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 22.Chan J, Heist K, DePaoli A, Veldhuis J, Mantzoros C. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang-Christensen M, Havel P, Jacobs R, Larsen P, Cameron JAG. Central administration of leptin inhibits food intake and activates the sympathetic nervous system in rhesus macaques. J Clin Endocrinol Metab. 1999;84:711–717. doi: 10.1210/jcem.84.2.5458. [DOI] [PubMed] [Google Scholar]
- 24.Heymsfield S, Greenberg A, Fujioka K, Dixon R, Kushner R, Hunt T, et al. Recombinant Leptin for Weight Loss in Obese and Lean Adults: A Randomized, Controlled, Dose-Escalation Trial. JAMA. 1999;282(16):1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 25.Henry B, Clarke I. Adipose Tissue Hormones and the Regulation of Food Intake. J Neuroendocrinology. 2008;20:842–849. doi: 10.1111/j.1365-2826.2008.1730.x. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen B, Febbraio M. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 27.Ropelle E, Flores M, Cintra D, Rocha G, Pauli J, Morari J, et al. IL-6 and IL-10 Anti- Inflammatory Activity Links Exercise to Hypothalamic Insulin and Leptin Sensitivity through IKK? and ER Stress Inhibition. PLoS Biol. 2010;8:8. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Weise C, Thiyyagura P, Reiman E, Krakoff J. Fat-free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. Neuroimage. 2013;64:712–721. doi: 10.1016/j.neuroimage.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jumpertz R, Venti C, Le D, Michaels J, Parrington S, Krakoff J, et al. Food label accuracy of common snack foods. Obesity. 2013 doi: 10.1002/oby.20185. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]