Abstract
Runt-related transcription factor 3, or RUNX3, is a tumor suppressor in gastric cancer. Inactivation of RUNX3 is causally associated with the genesis of gastric cancer, since RUNX3 is frequently inactivated in gastric cancers by hemizygous deletion, hypermethylation of its promoter, or protein mislocalization. Infection with Helicobacter pylori is the strongest risk factor for the development of gastric cancer. Recent studies have indicated that H. pylori infection plays an important role in the inactivation of RUNX3, and that this inactivation contributes to the pathogenesis of H. pylori. Here we summarize these recent advances and discuss their significances in understanding the initiation and development of gastric cancer.
Keywords: GASTRIC CANCER, H. PYLORI, RUNX3, TUMOR SUPPRESSOR
INTRODUCTION
Worldwide, gastric cancer is the fourth most diagnosed cancer and the second most common cause of cancer-related death. It is estimated that 10,570 people will die from gastric cancer in 2010 in the United States alone [Altekruse et al., 2009]. Two main subtypes of gastric cancers exist. Intestinal-type adenocarcinomas, which occur primarily in the distal regions of the stomach, predominate in countries where gastric cancer is more common. Diffuse-type adenocarcinomas occur more frequently in younger populations [Vauhkonen et al., 2006]. Adenocarcinomas of the stomach are often not diagnosed until they have metastasized to other tissues, which makes their prognoses very poor [Bornschein et al., 2009]. Gastric carcinogenesis is a complex, multistep and multifactorial event, and like all cancers, many etiological factors contribute to the development and progression of gastric cancer. These can include the activation of oncogenes, inactivation of tumor suppressors, diet, tobacco use, and infection with Helicobacter pylori [Peek and Blaser, 2002].
RUNX3 is a transcription factor that regulates lineage-specific gene expression in developmental processes and is involved in the formation of a variety of cancers [Ito, 2004]. RUNX3 elicits its tumor suppressor functions by controlling the expression of many genes involved in the growth, apoptosis, and differentiation of gastric epithelial cells [Chi et al., 2005; Yamamura et al., 2006; Yano et al., 2006] as well as genes involved in angiogenesis and cell junctions [Chang et al., 2009; Peng et al., 2006]. RUNX3 is expressed in glandular stomach epithelial cells, and loss of expression of RUNX3 is causally related to the genesis and progression of gastric cancer and also correlates with differentiation, metastasis, and poor prognosis of gastric cancer [Hsu et al., 2009; Li et al., 2002; Sugiura et al., 2008; Wei et al., 2005]. A study in 2002 found that knocking out runx3 in mice lead to hyperplasia of the gastric epithelium, an early developmental step in carcinogenesis [Li et al., 2002]. Furthermore, it was found that 82% of human gastric cancer samples showed reduced RUNX3 activity due to hemizygous deletion, hypermethylation of its promoter, or protein mislocalization. [Ito et al., 2005; Li et al., 2002]. The inactivation of RUNX3 appears to occur both at an early stage as well as during progression of gastric cancer [Ito et al., 2005; Li et al., 2002], and its expression correlates with the stage of the cancer; fewer late-stage tumors expressed RUNX3 than early-stage tumors [Li et al., 2002]. While emerging evidence suggests that RUNX3 is a tumor suppressor whose inactivation is involved in the initiation and progression of gastric cancer, the trigger for RUNX3 inactivation within the cells is largely unknown.
H. pylori is the only bacterium classified by the World Health Organization as a Type I carcinogen, with an estimated 63% of all stomach cancers caused by infection with the bacterium [Peek and Blaser, 2002]. The gram-negative spirochete persistently colonizes the mucosa of the stomach, where it may attach directly to the epithelial cells. Over half the world’s population is infected with H. pylori, though the wide majority of these infections are symptomless. However, significant pathologies are found in the minority, including chronic gastritis, peptic ulcer disease, and gastric cancers [Peek and Crabtree, 2006]. Infection is most commonly associated with intestinal-type gastric adenocarcinoma, though infection can also lead to the development of non-Hodgkin’s or MALT lymphomas [Peek and Crabtree, 2006]. Many proposed mechanisms for the pathogenicity of H. pylori exist, including infection-induced cell proliferation, epithelial cell elongation and loss of polarity, and degradation of cell-cell junctions. Recent studies indicate that H. pylori infection is important in the inactivation of RUNX3 by both protein degradation and promoter hypermethylation [Kitajima et al., 2008; Tsang et al., 2010], which may represent another critical element for the pathogenesis of H. pylori.
H. PYLORI AND INACTIVATION OF RUNX3 BY PROTEASOME-MEDIATED DEGRADATION
Ubiquitination of Runt family proteins have long been described, but the physiological significance of this process is not well defined [Huang et al., 2001]. Recent studies indicate that ubiquitination-dependent proteolytic degradation of RUNX3 is an important regulatory mechanism for controlling its tumor suppressor activity [Bae and Lee, 2006]. Ubiquitination occurs via a series of reactions mediated by three different enzymes: E1, E2 and E3. Catalysis is initiated by ubiquitin activating enzyme E1, which uses energy from ATP to activate a ubiquitin molecule. Activated ubiquitin is transferred to a ubiquitin conjugating enzyme E2 and is then transferred to the substrate by a ubiquitin E3 ligase. Of all three enzymes, only the E3 ligase confers substrate specificity [Weissman, 2001]. Several E3 ligases for RUNX3 ubiquitination have been identified. For example, RUNX3 is ubiquitinated by Smurfs (Smad ubiquitin regulator factors), and Smurf-mediated ubiquitination of RUNX3 reduces the stability and activity of RUNX3 [Jin et al., 2004]. Additionally, RUNX3 has been shown to be a target of MDM2, an E3 ligase known for regulating the function of the tumor suppressor p53 [Chi et al., 2009].
Our recent studies indicate that H. pylori infection induces the ubiquitination and degradation of RUNX3 [Tsang et al., 2010]. Infection with H. pylori leads to reduced cellular levels of RUNX3 in cultured gastric epithelial cells as well as in gastric epithelial cells of infected mice [Tsang et al., 2010]. The reduced expression of RUNX3 appears to be a result of H. pylori-induced ubiquitination and degradation of RUNX3 [Tsang et al., 2010]. Interestingly, H. pylori-induced degradation of RUNX3 depends upon the virulence factor CagA, since the wild-type but not the cagA-deficient H. pylori strains down-regulate the cellular levels of RUNX3 [Tsang et al., 2010].
Virulence factor CagA is encoded by the cag pathogenicity island (cagPAI) of H. pylori, which also encodes a type IV secretion system. The 120–140 kDa CagA is injected into host epithelial cells via the type IV secretion system [Peek and Blaser, 2002]. Within the cells, CagA interacts with many different intracellular host proteins to elicit its many roles in the pathogenesis of H. pylori. For example, CagA associates with and activates the cytoplasmic protein tyrosine phosphatase SHP-2, resulting in cytoskeletal reorganization, cell elongation and cell scattering, and the “hummingbird” phenotype [Higashi et al., 2002a; Higashi et al., 2002b]. CagA also associates with TAK1 to activate NF-κB and regulate the NF-κB-dependent inflammatory response [Lamb et al., 2009]. Through its association with various host proteins, H. pylori CagA is actively involved in H. pylori-induced gastric carcinogenesis.
The ability of H. pylori to induce the degradation of RUNX3 also relies on the specific interaction of CagA with RUNX3. CagA directly interacts with RUNX3 in vivo and in vitro. More importantly, the interaction is mediated by the specific recognition of the PPxY (Py) motif of RUNX3 by the WW domain of CagA. Blocking the interaction either by the mutation of the Py motif or by deletion of the WW domain reduces the ability of CagA to induce the ubiquitination and degradation of RUNX3, emphasizing the importance of this interaction in the degradation of RUNX3 [Tsang et al., 2010].
Then how does the binding of CagA to RUNX3 trigger its ubiquitination and degradation? The WW domain has been found in a variety of E3 ligases and is involved in the binding of these E3 ligases to their substrates [Yang and Kumar, 2010]. One possibility is that CagA could contain intrinsic E3 ligase activity. However, a cysteine residue, the amino acid which is essential for an E3 ligase to form a thioester bond with ubiquitin [Pickart, 2001], is not found within CagA. Another possibility is that CagA might function as a scaffold protein to recruit an E3 ubiquitin ligase for the ubiquitination and degradation of RUNX3. Supporting this, we found that CagA immunoprecipitates from transfected cells were able to ubiquitinate RUNX3 in vitro (unpublished data, Tsang and Chen). Furthermore, it should be noted that two WW domains, defined as WW1 and WW2, were identified within the N-terminal region of CagA. Although both WW domains of CagA are involved in the ubiquitination and degradation of RUNX3, only WW2 of CagA is essential for CagA’s interaction with the Py motif of RUNX3. Currently, it is not clear why CagA needs two WW domains for the ubiquitination and degradation of RUNX3 but only one WW domain for the interaction with RUNX3. It is possible that WW2 is involved in the interaction with RUNX3 while WW1 is involved in the recruitment of an E3 ligase. However, the identity of the E3 ligase remains to be established. Several E3 ligases, including Smurfs and MDM2, have been identified for the ubiquitination of RUNX3 [Jin et al., 2004], and it will be interesting to investigate whether any one of them is actually involved in the CagA-induced degradation of RUNX3.
In addition to the recruitment of a potential E3 ligase for RUNX3, binding of CagA to RUNX3 might facilitate the cytoplasmic localization of RUNX3, which seems to be a critical step in the degradation of RUNX3 [Kim et al., 2009]. Jun-activation domain-binding protein 1 (Jab/CSN5) induces the cytoplasmic localization and degradation of RUNX3 [Kim et al., 2009]. In addition, histone methyltransferase G9a has also been shown to promote the nuclear export and induce the degradation of RUNX3 in response to hypoxia [Lee et al., 2009]. Similarly, since CagA is a cell membrane-associated protein and RUNX3 shuttles between the nucleus and the cytoplasm, CagA might promote down-regulation of RUNX3 by sequestering it in the cytoplasm where it can be ubiquitinated and degraded by the 26S proteasome.
H. PYLORI AND SILENCING OF RUNX3 VIA HYPERMETHYLATION OF THE runx3 PROMOTER
Aberrant DNA methylation of CpG islands in promoters is one of the major mechanisms for inactivating tumor suppressor genes and is closely involved in the formation of cancer [Jones and Baylin, 2007]. DNA methylation is mediated by a class of enzymes called DNA methyltransferases (DNMT) that catalyze the transfer of the methyl group from Sadenosyl- methionine onto cytosine. DNA methylation often occurs on CpG dinucleotides which are concentrated in large clusters called CpG islands [Kulis and Esteller, 2010]. CpG islands are found primarily in the promoter and/or the first exon region of genes, and their methylation results in the inactivation of gene expression [Kulis and Esteller, 2010]. In cancer cells, global DNA hypomethylation is accompanied by region-specific hypermethylation, especially of the promoters of tumor suppressors, leading to heritable transcriptional silence and to carcinogenesis [Kulis and Esteller, 2010].
Not surprisingly, methylation of the runx3 promoter represents a major mechanism for the inactivation of RUNX3 in gastric cancer. Hypermethylation of the runx3 promoter is found in many gastric cancer cell lines and gastric cancer samples as well as in, with less frequency, non-cancerous gastric diseases including chronic gastritis, intestinal metaplasia and gastric adenoma [Guo et al., 2002; Kim et al., 2004]. Several factors, including H. pylori infection, inflammation, and oxidative stress, have been indicated to be involved in the epigenetic inactivation of RUNX3 [Chuang and Ito, 2010]. Among these, H. pylori infection appears to be the most critical factor, since H. pylori infection triggers inflammation and oxidative stress in gastric tissues [Farinati et al., 2008]. Consistent with the high levels of aberrant methylation of several CpG islands in H. pylori-infected gastric mucosa [Maekita et al., 2006], H. pylori infection has been shown to be an independent risk factor for runx3 promoter methylation in gastric cancer [Kitajima et al., 2008]. Furthermore, H. pylori infection positively correlates with the methylation of the runx3 promoter in gastric cancer as well as in gastric atrophy and intestinal metaplasia [Kitajima et al., 2008]. Supporting the role of H. pylori infection in the methylation of the runx3 promoter, eradication of H. pylori increases the expression of RUNX3 in the glandular epithelial cells of the corpus [Suzuki et al.].
It is still not clear how H. pylori initiates or maintains the hypermethylation of the runx3 promoter. H. pylori-induced runx3 promoter hypermethylation might be due to its ability to directly regulate DNA methyltransferases, or the bacteria may induce hypermethylation indirectly via the inflammatory response it generates. Up-regulation of DNA maintenance methylation enzyme DNMT1 has been reported to be responsible for hypermethylation of the promoters of some tumor suppressors [Hodge et al., 2005]. It is plausible to assume that a similar mechanism might account for H. pylori infection-initiated DNA methylation. Surprisingly, this doesn’t seem to be the case for the runx3 promoter, since H. pylori infection does not up-regulate the mRNA levels of dnmts in infected Mongolian gerbils [Niwa et al., 2010]. However, it is still possible that H. pylori might alter the distribution or the activity of DNMTs, since DNMT1 is essential for maintaining the hypermethylation of the runx3 promoter. For example, in human gastric cancer cells, depletion of DNMT1 leads to decreased DNA methylation of the runx3 promoter and the re-expression of RUNX3 [Jung et al., 2007].
In addition to the possible involvement of DNMTs, H. pylori-induced methylation of the runx3 promoter might be caused by infection-associated inflammatory mediators (e.g., reactive oxygen species or nitric oxide). Studies from Katayama et al demonstrate that H. pylori infection induces the production of nitric oxide in macrophages and that macrophage-derived nitric oxide causes the methylation of the runx3 promoter and the loss of RUNX3 expression in epithelial cells [Katayama et al., 2009]. The production of nitric oxide is likely a result of the enhanced expression of iNOS induced by H. pylori-associated inflammation [Jaiswal et al., 2001]. Intriguingly, virulence factor CagA appears not to be involved in the inflammation-initiated methylation of the runx3 promoter [Kitajima et al., 2008], although CagA plays a key role in the H. pylori-induced inflammatory response [Lamb et al., 2010]. The role of CagA in H. pylori-induced runx3 promoter hypermethylation or in general DNA methylation merits further investigation.
PATHOLOGICAL FUNCTIONS OF H. PYLORI-MEDIATED INACTIVATION OF RUNX3
It is well documented that RUNX3 functions as a tumor suppressor in the TGF-β signaling pathway through the attenuation of cell growth and the induction of apoptosis [Ito and Miyazono, 2003]. For example, RUNX3 suppresses gastric epithelial cell growth by inducing cell cycle regulator p21(WAF1/Cip1) expression and induces apoptosis of gastric epithelial cells by up-regulating proapoptotic factor Bim [Chi et al., 2005; Yano et al., 2006]. Therefore, inactivation of RUNX3 by H. pylori might result in enhanced gastric epithelial cell proliferation and survival, which in turn may facilitate H. pylori colonization [Mimuro et al., 2007].
Additionally, inactivation of RUNX3 by H. pylori might contribute to the disruption of the proper architecture of the gastric epithelium. Claudin-1, a main component of the tight junction family of proteins, is a direct target of RUNX3 transcription activation in gastric epithelial cells [Chang et al., 2009]. runx3−/− mice-derived gastric epithelial cells have reduced levels of claudin-1 and increased tumorigenic potential when compared with the runx3+/+ cells [Chang et al., 2009]. Disruption of the tight junctions between epithelial cells might allow H. pylori to invade intercellularly, which may facilitate the induction of cell motility and elongation and expression of mitotic genes involved in cell proliferation.
Finally, since hypermethylation of the runx3 promoter is also found in precancerous conditions such as chronic gastritis and intestinal metaplasia [Kim et al., 2004], silencing RUNX3 might alter how frequently the gastric epithelial cells near the site of infection are converted to intestinal-type cells [Tsuji et al., 2006]. H. pylori infection-initiated promoter methylation of runx3 and the resulting inactivation of RUNX3, combined with other factors, triggers the transdifferentiation of gastric epithelial cells into intestinal-type cells, followed by intestinal hyperplasia, which leads to the development of gastric cancer [Fukamachi, 2006; Kitajima et al., 2008].
CONCLUDING REMARKS
In this review, we summarize the recent findings that define the important role for H. pylori in the inactivation of gastric tumor suppressor RUNX3. Multiple lines of evidence demonstrate that various pathways and factors are involved in the H. pylori-mediated inactivation of RUNX3 (Fig. 1). While the degradation of RUNX3 might represent one of the first results of the host-pathogen interaction, quickly inducing the proliferation and survival of gastric epithelial cells and improving the growth conditions for the H. pylori, the infection-mediated long-term silencing of RUNX3 at the epigenetic level might allow for the transdifferentiation of gastric epithelial cells into intestinal-type cells. H. pylori infection is a major risk factor for the development of gastric cancer and its precursor lesions [Peek and Blaser, 2002], and H. pylori eradication with triple therapy has been an effective approach for the prevention of gastric cancer [Graham and Fischbach; Tsuji et al., 2006]. However, due to increased antibiotic resistance and newly recognized beneficial effects of H. pylori infection, alternative medical approaches to H. pylori infections must be considered [Cover and Blaser, 2009]. New approaches should rely on a better understanding of the molecular and cellular mechanisms for H. pylori-induced gastric cancer. Although the detailed mechanisms for the H. pylori-mediated inactivation of RUNX3 remain to be determined, this line of research promises to yield new insights into the pathogenesis of H. pylori and gastric cancer. Since inactivation of RUNX3 is closely associated with the pathogenesis of H. pylori, specifically blocking the interaction between H. pylori CagA and RUNX3 in H. pylori-infected patients or reactivation of RUNX3 in gastric lesions or gastric cancers by reducing the methylation of the runx3 promoter might reduce the tumorigenic potential of H. pylori while at the same time retaining its beneficial effects.
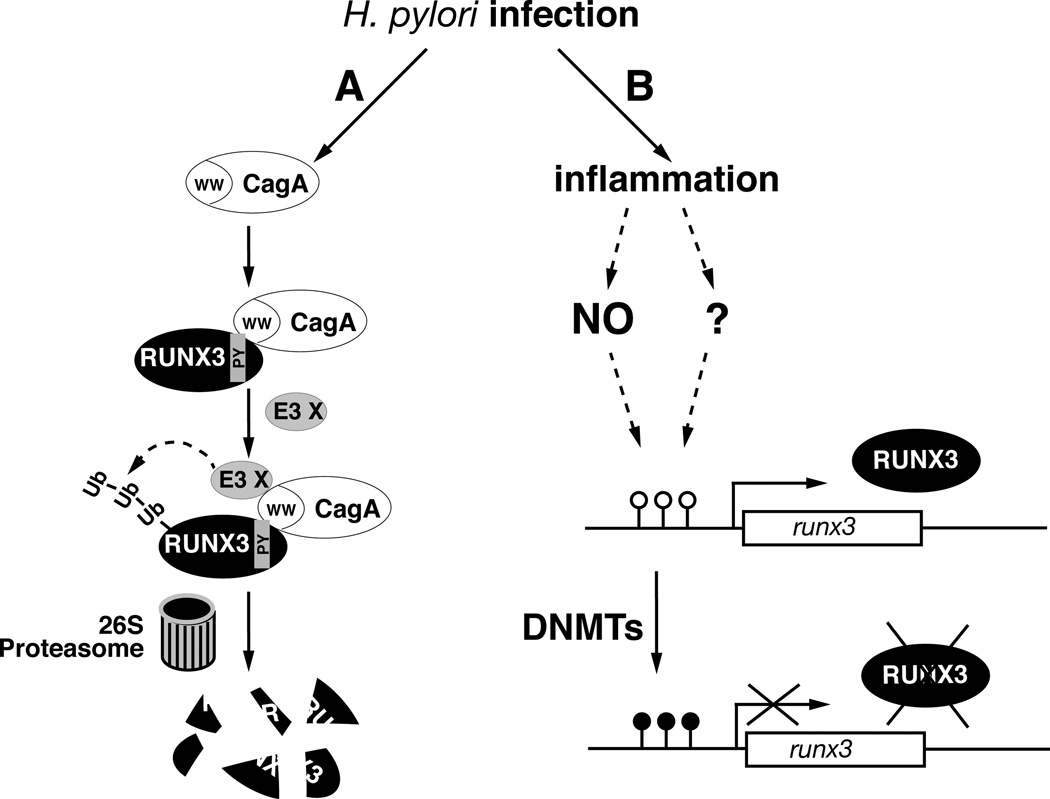
Fig. 1.
Inactivation of tumor suppressor RUNX3 by H. pylori infection. (A) CagA-dependent inactivation of RUNX3 by proteolytic degradation. CagA is injected into host epithelial cells after H. pylori infection. Within the cells, it interacts with the Py motif of RUNX3 via its WW domain. Binding of CagA to RUNX3 recruits an unidentified E3 ubiquitin ligase and triggers the proteasome-mediated degradation of RUNX3. This rapid inactivation of RUNX3 during the early stage of infection might promote the proliferation and survival of gastric epithelial cells and the growth of the H. pylori. (B) Inactivation of RUNX3 by hypermethylation of the runx3 promoter. H. pylori infection induces the hypermethylation of the runx3 promoter. This epigenetic-level inactivation of RUNX3 is associated with H. pylori infection-induced inflammation, which results in abnormal activity of DNMTs and the production of nitric oxide (NO). Solid arrow: known interaction or inactivation; arrow with question mark: proposed interaction; dashed arrow: activation through multiple steps. ○ =CpG, ●=mCpG.
ACKNOWLEDGEMENTS
This work is supported in part by NIH grant DK-085158 (to L.F.C.). Y.H.T. is an A*STAR-Illinois Partnership fellow.
REFERENCES
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SC, Lee YH. Phosphorylation, acetylation and ubiquitination: the molecular basis of RUNX regulation. Gene. 2006;366:58–66. doi: 10.1016/j.gene.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Bornschein J, Rokkas T, Selgrad M, Malfertheiner P. Helicobacter pylori and clinical aspects of gastric cancer. Helicobacter. 2009;14(Suppl 1):41–45. doi: 10.1111/j.1523-5378.2009.00695.x. [DOI] [PubMed] [Google Scholar]
- Chang TL, Ito K, Ko TK, Liu Q, Salto-Tellez M, Yeoh KG, Fukamachi H, Ito Y. Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Chi XZ, Kim J, Lee YH, Lee JW, Lee KS, Wee H, Kim WJ, Park WY, Oh BC, Stein GS, Ito Y, van Wijnen AJ, Bae SC. Runt-related transcription factor RUNX3 is a target of MDM2-mediated ubiquitination. Cancer Res. 2009;69:8111–8119. doi: 10.1158/0008-5472.CAN-09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, Li QL, Kim HR, Cha EJ, Lee YH, Kaneda A, Ushijima T, Kim WJ, Ito Y, Bae SC. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperation with transforming growth factor {beta}-activated SMAD. Mol Cell Biol. 2005;25:8097–8107. doi: 10.1128/MCB.25.18.8097-8107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LS, Ito Y. RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene. 2010;29:2605–2615. doi: 10.1038/onc.2010.88. [DOI] [PubMed] [Google Scholar]
- Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinati F, Cardin R, Cassaro M, Bortolami M, Nitti D, Tieppo C, Zaninotto G, Rugge M. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev. 2008;17:195–200. doi: 10.1097/CEJ.0b013e3282f0bff5. [DOI] [PubMed] [Google Scholar]
- Fukamachi H. Runx3 controls growth and differentiation of gastric epithelial cells in mammals. Dev Growth Differ. 2006;48:1–13. doi: 10.1111/j.1440-169X.2006.00832.x. [DOI] [PubMed] [Google Scholar]
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- Guo WH, Weng LQ, Ito K, Chen LF, Nakanishi H, Tatematsu M, Ito Y. Inhibition of growth of mouse gastric cancer cells by Runx3, a novel tumor suppressor. Oncogene. 2002;21:8351–8355. doi: 10.1038/sj.onc.1206037. [DOI] [PubMed] [Google Scholar]
- Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, Hatakeyama M. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci U S A. 2002a;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002b;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA, Munroe DJ, Farrar WL. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 2005;65:4673–4682. doi: 10.1158/0008-5472.CAN-04-3589. [DOI] [PubMed] [Google Scholar]
- Hsu PI, Hsieh HL, Lee J, Lin LF, Chen HC, Lu PJ, Hsiao M. Loss of RUNX3 expression correlates with differentiation, nodal metastasis, and poor prognosis of gastric cancer. Ann Surg Oncol. 2009;16:1686–1694. doi: 10.1245/s10434-009-0428-2. [DOI] [PubMed] [Google Scholar]
- Huang G, Shigesada K, Ito K, Wee HJ, Yokomizo T, Ito Y. Dimerization with PEBP2beta protects RUNX1/AML1 from ubiquitin-proteasome-mediated degradation. EMBO J. 2001;20:723–733. doi: 10.1093/emboj/20.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A, Hiong KC, Peh BK, Han HC, Ito T, Teh M, Yeoh KG, Ito Y. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743–7750. doi: 10.1158/0008-5472.CAN-05-0743. [DOI] [PubMed] [Google Scholar]
- Ito Y. Oncogenic potential of the RUNX gene family: 'overview'. Oncogene. 2004;23:4198–4208. doi: 10.1038/sj.onc.1207755. [DOI] [PubMed] [Google Scholar]
- Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003;13:43–47. doi: 10.1016/s0959-437x(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G626–G634. doi: 10.1152/ajpgi.2001.281.3.G626. [DOI] [PubMed] [Google Scholar]
- Jin YH, Jeon EJ, Li QL, Lee YH, Choi JK, Kim WJ, Lee KY, Bae SC. Transforming growth factor-beta stimulates p300-dependent RUNX3 acetylation, which inhibits ubiquitination-mediated degradation. J Biol Chem. 2004;279:29409–29417. doi: 10.1074/jbc.M313120200. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Park J, Kim TY, Park JH, Jong HS, Im SA, Robertson KD, Bang YJ. Potential advantages of DNA methyltransferase 1 (DNMT1)-targeted inhibition for cancer therapy. J Mol Med. 2007;85:1137–1148. doi: 10.1007/s00109-007-0216-z. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Takahashi M, Kuwayama H. Helicobacter pylori causes runx3 gene methylation and its loss of expression in gastric epithelial cells, which is mediated by nitric oxide produced by macrophages. Biochem Biophys Res Commun. 2009;388:496–500. doi: 10.1016/j.bbrc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi JK, Cinghu S, Jang JW, Lee YS, Li YH, Goh YM, Chi XZ, Lee KS, Wee H, Bae SC. Jab1/CSN5 induces the cytoplasmic localization and degradation of RUNX3. J Cell Biochem. 2009;107:557–565. doi: 10.1002/jcb.22157. [DOI] [PubMed] [Google Scholar]
- Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW, Bang YJ, Kang GH. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest. 2004;84:479–484. doi: 10.1038/labinvest.3700060. [DOI] [PubMed] [Google Scholar]
- Kitajima Y, Ohtaka K, Mitsuno M, Tanaka M, Sato S, Nakafusa Y, Miyazaki K. Helicobacter pylori infection is an independent risk factor for Runx3 methylation in gastric cancer. Oncol Rep. 2008;19:197–202. [PubMed] [Google Scholar]
- Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- Lamb A, Yamaguchi H, Chen LF. The many roads traveled by Helicobacter pylori to NF-kappaB activation. Gut Microbes. 2010;1:109–113. doi: 10.4161/gmic.1.2.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, Peek RM, Blanke SR, Chen LF. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–1249. doi: 10.1038/embor.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim J, Kim WH, Lee YM. Hypoxic silencing of tumor suppressor RUNX3 by histone modification in gastric cancer cells. Oncogene. 2009;28:184–194. doi: 10.1038/onc.2008.377. [DOI] [PubMed] [Google Scholar]
- Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe T, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae SC, Ito Y. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, Tamura G, Saito D, Sugimura T, Ichinose M, Ushijima T. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- Mimuro H, Suzuki T, Nagai S, Rieder G, Suzuki M, Nagai T, Fujita Y, Nagamatsu K, Ishijima N, Koyasu S, Haas R, Sasakawa C. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe. 2007;2:250–263. doi: 10.1016/j.chom.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Niwa T, Tsukamoto T, Toyoda T, Mori A, Tanaka H, Maekita T, Ichinose M, Tatematsu M, Ushijima T. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–1440. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- Peng Z, Wei D, Wang L, Tang H, Zhang J, Le X, Jia Z, Li Q, Xie K. RUNX3 inhibits the expression of vascular endothelial growth factor and reduces the angiogenesis, growth, and metastasis of human gastric cancer. Clin Cancer Res. 2006;12:6386–6394. doi: 10.1158/1078-0432.CCR-05-2359. [DOI] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Mori Y, Ogawa R, Katada T, Harata K, Fujii Y. Decreased expression of RUNX3 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep. 2008;19:713–719. [PubMed] [Google Scholar]
- Suzuki M, Suzuki H, Minegishi Y, Ito K, Nishizawa T, Hibi T. H. pylori-Eradication Therapy Increases RUNX3 Expression in the Glandular Epithelial Cells in Enlarged-Fold Gastritis. J Clin Biochem Nutr. 46:259–264. doi: 10.3164/jcbn.09-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang YH, Lamb A, Romero-Gallo J, Huang B, Ito K, Peek RM, Jr, Ito Y, Chen LF. Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for proteasome-mediated degradation. Oncogene. 2010;29:5643–5650. doi: 10.1038/onc.2010.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S, Tsujii M, Murata H, Nishida T, Komori M, Yasumaru M, Ishii S, Sasayama Y, Kawano S, Hayashi N. Helicobacter pylori eradication to prevent gastric cancer: underlying molecular and cellular mechanisms. World J Gastroenterol. 2006;12:1671–1680. doi: 10.3748/wjg.v12.i11.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauhkonen M, Vauhkonen H, Sipponen P. Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:651–674. doi: 10.1016/j.bpg.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang L, Le X, Yao J, Wu TT, Huang S, Xie K. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005;65:4809–4816. doi: 10.1158/0008-5472.CAN-04-3741. [DOI] [PubMed] [Google Scholar]
- Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281:5267–5276. doi: 10.1074/jbc.M512151200. [DOI] [PubMed] [Google Scholar]
- Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010;17:68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, Inoue K, Ida H, Bouillet P, Strasser A, Bae SC, Ito Y. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2006;26:4474–4488. doi: 10.1128/MCB.01926-05. [DOI] [PMC free article] [PubMed] [Google Scholar]