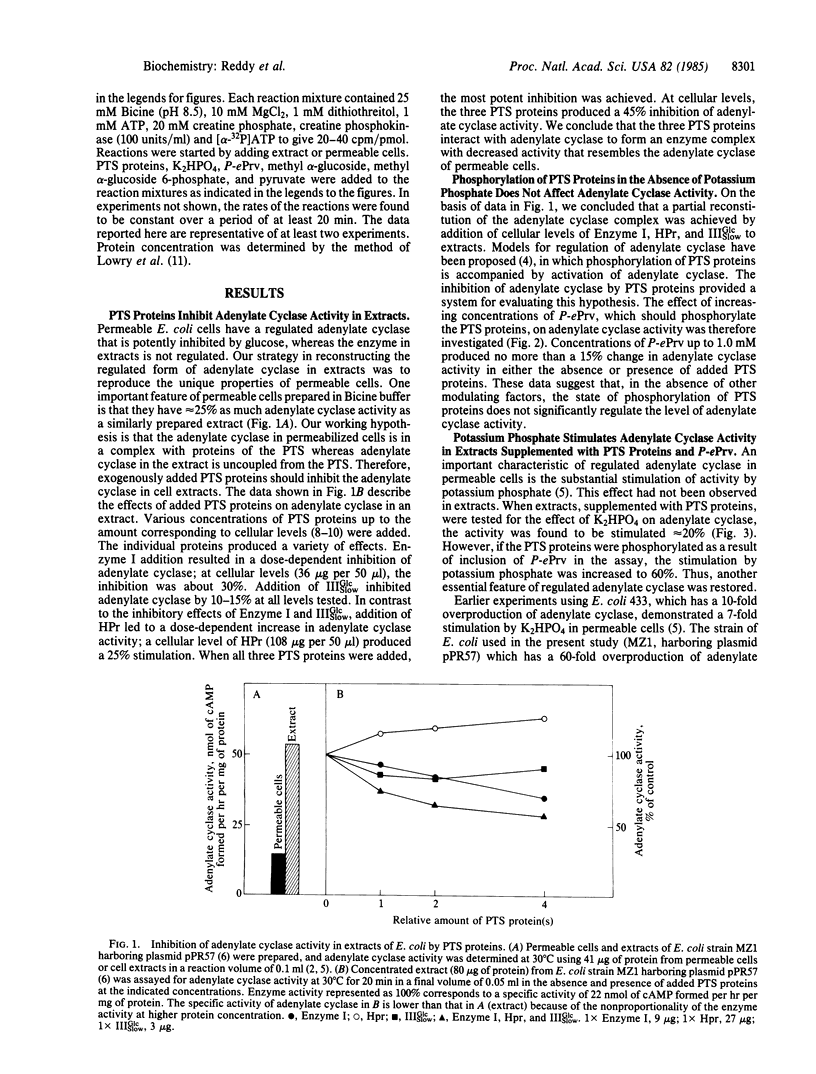
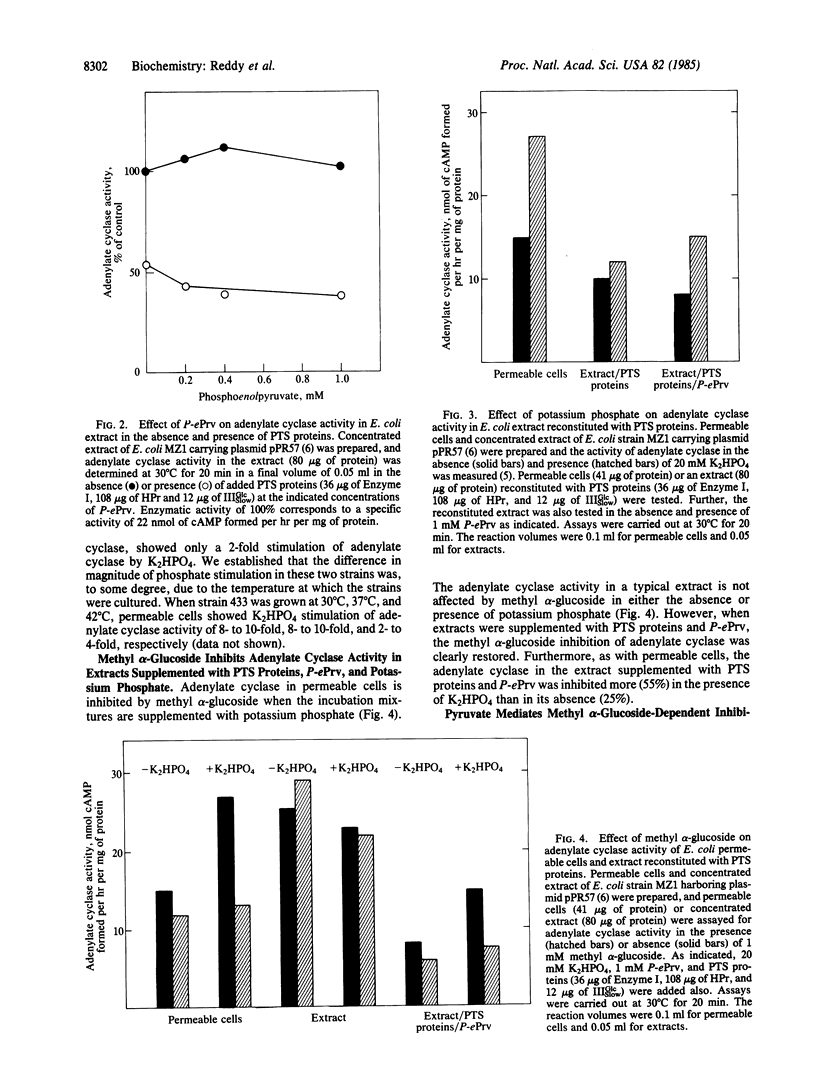
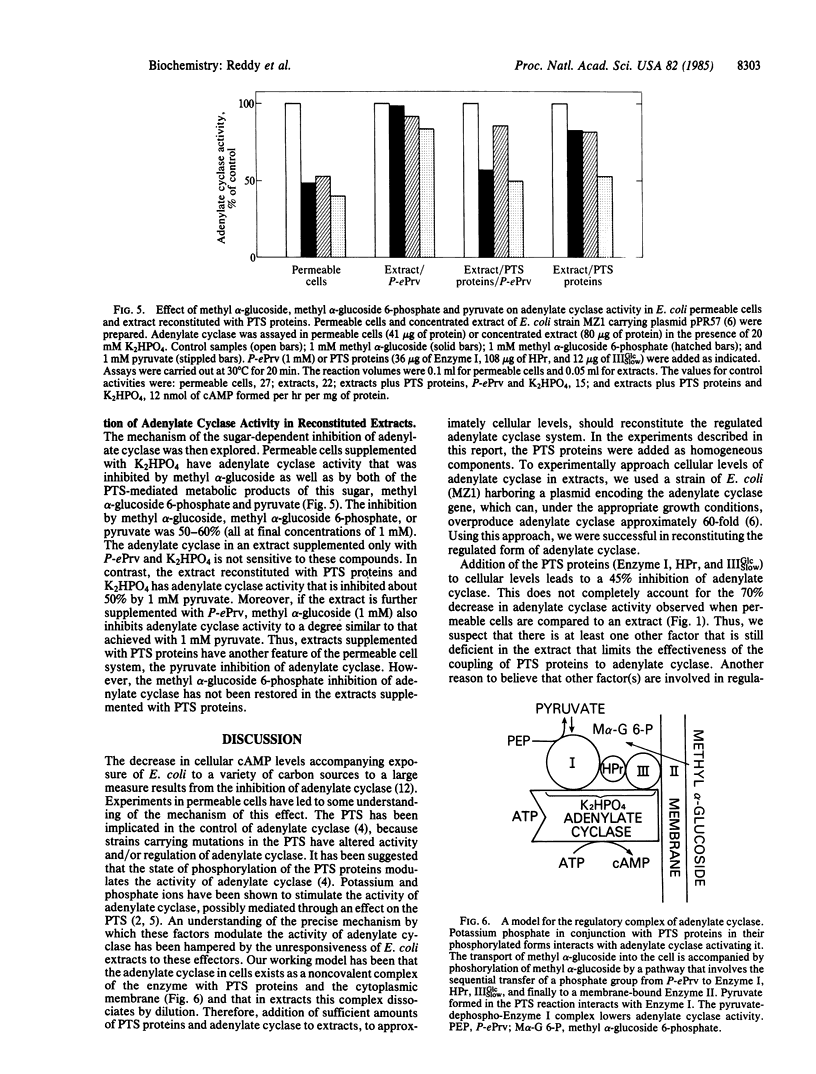
Abstract
The inhibition of adenylate cyclase activity of Escherichia coli by methyl alpha-glucoside has been demonstrated in intact or in permeable cells but not in cell-free extracts. In intact or permeable cells, this inhibition is demonstrable only in strains expressing the genes for proteins of the phosphoenolpyruvate:glycose phosphotransferase system (PTS); in permeable cells, the inhibition also requires potassium phosphate. Using homogeneous proteins of the PTS, we have reconstituted in cell-free extracts many of the features of the regulated form of adenylate cyclase: (i) In the absence of K2HPO4, permeable cells have lower adenylate cyclase activity than extracts; addition of homogeneous PTS proteins to the extracts brings adenylate cyclase activity close to the level observed in permeable cells. (ii) The low activity observed in permeable cells is stimulated by potassium phosphate; this stimulation is also observed in extracts supplemented with PTS proteins and phosphoenolpyruvate. (iii) In permeable cells, potassium phosphate-stimulated adenylate cyclase activity is inhibited by methyl alpha-glucoside or pyruvate; extracts behaved similarly when supplemented with PTS proteins, K2HPO4, and phosphoenolpyruvate. Thus, the regulated form of adenylate cyclase has been reconstituted in cell-free extracts by addition of homogeneous PTS proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beneski D. A., Nakazawa A., Weigel N., Hartman P. E., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a phosphocarrier protein HPr from wild type and mutants of Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14492–14498. [PubMed] [Google Scholar]
- Harwood J. P., Peterkofsky A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J Biol Chem. 1975 Jun 25;250(12):4656–4662. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liberman E., Reddy P., Gazdar C., Peterkofsky A. The Escherichia coli adenylate cyclase complex. Stimulation by potassium and phosphate. J Biol Chem. 1985 Apr 10;260(7):4075–4081. [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. The Escherichia coli adenylate cyclase complex: activation by phosphoenolpyruvate. J Supramol Struct. 1978;9(2):219–230. doi: 10.1002/jss.400090207. [DOI] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weigel N., Waygood E. B., Kukuruzinska M. A., Nakazawa A., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of enzyme I from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14461–14469. [PubMed] [Google Scholar]



