Abstract
H. pylori infection causes chronic gastritis and peptic ulceration and is the strongest risk factor for the development of gastric cancer. The pathogenesis of H. pylori is believed to be associated with infection-initiated chronic gastritis, which is characterized by enhanced expression of many inflammatory genes. H. pylori utilizes various virulence factors, targeting different cellular proteins, to modulate the host inflammatory response. In this review, we explore the many different ways by which H. pylori initiates inflammation, leveling many “hits” on the gastric mucosa which can lead to the development of cancer. We also discuss some recent findings in understanding the pathogen-host interactions and the role of transcription factor NF-κB in H. pylori-induced inflammation.
Keywords: Helicobacter pylori, inflammation, gastric cancer, NF-κB
Many factors are known to contribute to the development of cancer, and gastric cancer development is no different. Chronic infections, tobacco smoke, diet, and obesity are all major factors which play into the development of cancer [Aggarwal and Gehlot, 2009]. There is a single mechanism which underlies many of these risk factors: inflammation.
The link between inflammation and cancer was proposed by Virchow in the 19th century with his observation that tumors often arose at sites of chronic inflammation and that inflammatory cells were present in tumor samples [Balkwill and Mantovani, 2001]. Inflammation has been observed in many infection-triggered cancers, and approximately 15% of human cancers are associated with chronic infection and inflammation [Karin and Greten, 2005]. For example, Hepatitis B or C virus infections, given their names because of their ability to cause chronic liver inflammation, can lead to hepatocellular carcinoma. Infections with many types of human papillomaviruses are recognized as key risk factors for cervical cancer, and the inflammation caused by persistent infection acts as a “cofactor” in carcinogenesis. A protein product of human T-cell leukemia/lymphotrophic virus type 1 (HTLV-1) “hijacks” host inflammation-regulating pathways to induce inflammation and transform T-cells, leading to the development of adult T-cell leukemia [Sun and Yamaoka, 2005]. Inflammation has become a new hallmark for cancer [Hanahan and Weinberg, 2011].
Helicobacter pylori and gastric cancer
Infection with Helicobacter pylori and the resulting chronic inflammation is a major step in the initiation and development of gastric cancer. H. pylori is a Gram-negative spirochete which infects more than half of the world’s population. Infections are very common in undeveloped countries, likely due to water contamination and less sanitary living conditions. The bacterium colonizes the stomach of its host, where it attaches to the mucosal epithelia. Infection persists often for the lifetime of the host unless treatment is received. H. pylori is susceptible to most antibiotics, although resistance is becoming more common and triple or quadruple therapy consisting of two antibiotics, a proton pump inhibitor and bismuth is now used to eradicate the bacteria [Chuah et al., 2011].
H. pylori has been classified as a Group I carcinogen by the International Agency for Research on Cancer since 1994. Numerous studies have been done to elicit the link between H. pylori infection and gastric cancer. A pooled reanalysis by the Helicobacter and Cancer Collaborative Group combined results from twelve studies and found that the matched odds ratio for the association of H. pylori infection and non-cardia cancer was 2.97 (95% CI: 2.34–3.77) [2001]. Both intestinal and diffuse types of gastric carcinomas are associated with H. pylori infection, but only cancers found distal to the cardia have been linked to H. pylori infection [Peek, 2005]. Besides gastric adenocarcinoma, infection with the bacterium has also been linked to gastric ulcers, gastritis, and MALT (mucosa-associated lymphoid tissue) lymphoma. Worldwide, gastric cancer is the fourth most diagnosed cancer and the second most common cause of cancer-related death, and H. pylori is the causative agent in approximately 63% of these cancers [Peek, 2005].
Pathogenicity of Helicobacter pylori
Many proposed mechanisms for the pathogenicity of H. pylori exist, including changes in host gene expression, infection-induced cell proliferation, epithelial cell elongation and loss of polarity, degradation of cell-cell junctions, and decreased gastric acid secretion [Yamaoka, 2010]. The pathogenicity of H. pylori is attributed largely to its various virulence components, including flagella, lipopolysaccharide (LPS), vacuolating toxin VacA, and cytotoxin-associated gene pathogenicity island (cagPAI) [Yamaoka, 2010]. Of all these, cagPAI is the most potent and the most studied virulence component.
The cagPAI is a 40 kbp fragment of DNA containing 27 potential coding regions which is found in many strains of H. pylori [Peek, 2005]. It encodes a type 4 secretion system (T4SS) and the virulence factor CagA. CagA, a 125–140 kDa protein, is injected into H. pylori-infected host epithelial cells where it is often activated by tyrosine phosphorylation by the host src kinase and targets host proteins to modify cellular responses [Hatakeyama, 2008; Peek, 2005]. Overwhelming evidence ties the presence of CagA to an increased risk of non-cardia gastric carcinoma over the risk caused by infection with CagA-negative H. pylori [Hatakeyama, 2008; Peek, 2005].
It is well know that CagA binds and activates SHP-2 phosphatase, disrupting cell focal adhesions [Hatakeyama, 2008]. CagA also inhibits the polarity regulator PAR1b/MARK2 kinase, which causes a loss of epithelial cell polarity and plays a role in the disruption of normal epithelial architecture [Hatakeyama, 2008]. Another hypothesized mechanism for disruption of adherens junctions within gastric epithelial cells is a H. pylori surface protein-TLR (toll-like receptor) 2 induced activation of the protease calpain, which cleaves E-cadherin and allows for increased β-catenin signaling [O'Connor et al., 2011].
Recently, H. pylori was shown to inactivate the gastric tumor suppressor RUNX3 by proteasome-mediated degradation induced by the H. pylori virulence factor CagA [Tsang et al., 2010], or by gene silencing via promoter hypermethylation of runx3 [Kitajima et al., 2008; Tsang et al., 2011]. In a similar vein, CagA has also been shown to induce proteasome-mediated degradation of p53 by binding a modulator of p53 function and tumor suppressor, ASPP2 [Buti et al., 2011]. CagA is also known to bind E-cadherin, thus interfering with the normal regulation of β-catenin, a protein whose dysregulation has been shown to cause transdifferentiation of numerous cell lineages and increased cell proliferation [Murata-Kamiya et al., 2007].
H. pylori infection can also inhibit gastric acid secretion. This is partially due to the inhibition of proton pump gene expression by the cagPAI genes in parietal cells [Saha et al., 2010]. Hypochlorhydria can lead to gastric colonization by other, more potent bacterial inducers of inflammation, and this increased inflammation leads to development of adenocarcinoma in mice [Zavros et al., 2002].
H. pylori infection has been shown to stimulate bone marrow-derived mesenchymal stem cell (BMDC) migration to the gastrointestinal tract via induction of cytokine production, a phenomenon postulated to provide transdifferentiable cells from which adenocarcinomas may arise [Ferrand et al., 2011]. In fact, a recent study showed that nearly a quarter of dysplastic lesions in the gastric mucosa of H. pylori-infected mice were derived from BMDCs [Varon et al., 2012].
All together, these studies suggest many different mechanisms by which H. pylori infection may lead to a host of changes in the stomach epithelia leading eventually to the genesis of cancer. While many “hits” are required to induce carcinogenesis, one of the most important mechanisms is likely the induction of chronic inflammation by H. pylori.
Inflammation induced by Helicobacter pylori
H. pylori infection leads to inflammation through a variety of pathways, induced both in the gastric epithelial cells which they first contact and in circulating immune cells recruited to the site of infection. Inflammatory molecules found to be upregulated in the stomachs of H. pylori-infected patients include IL-1, IL-6, IL-8, TNF-α, and RANTES [McGee and Mobley, 2000].
H. pylori utilizes many different mechanisms for the induction of pro-inflammatory cytokines. Peptidoglycan can enter host epithelial cells via the cagPAI-encoded T4SS and stimulate the intracellular pathogen-recognition receptor Nod1, which in turn signals to activate NF-κB and AP-1 for the induction of cytokines such as IL-8 [Allison et al., 2009; Viala et al., 2004]. OipA and BabA, two outer membrane adhesion proteins, were shown by knock-out studies to be important in the induction of IL-6, IL-8 and IL-11 production, though the mechanism is as yet unclear [Sugimoto et al., 2011; Yamaoka et al., 2000]. VacA appears to be primarily for immunosuppression of T cell activation, allowing persistence of the bacterial infection, but evidence also shows that the toxin can induce some NF-κB activity within targeted T cells [Takeshima et al., 2009].
The virulence factor CagA has been studied extensively, especially its role in H. pylori-induced inflammation, and many studies suggest that it plays a role in NF-κB activation and IL-8 production. Bacteria which translocate CagA into host cells induce higher levels of IL-8 production, and activate NF-κB, AP-1 and NFAT [Backert and Naumann, 2010]. NF-κB nuclear translocation and IL-8 production were induced in gastric epithelial cells by ectopic expression of CagA [Backert and Naumann, 2010]. Additionally, NF-κB activation and inflammation was markedly less in the gastric antra of Mongolian gerbils infected with cagA-deficient H. pylori as compared to infection with wild-type H. pylori [Shibata et al., 2006]. However, CagA does not seem to be a major player in H. pylori-induced IL-12 production [Viala et al., 2004] and the ability of CagA to activate NF-κB and IL-8 production appears to be H. pylori strain-specific [Lamb et al., 2010].
Besides activating cytokine release, H. pylori also stimulates the production of growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and inflammation modulators such as cyclooxygenase-2 (COX-2) and reactive oxygen/nitrogen species (ROS/RNS). H. pylori is found to stimulate production GM-CSF from cultured antral biopsies and a human gastric epithelial cell line [Beales and Calam, 1997]. H. pylori infection activates AP-1 to induce COX-2 and inducible nitric oxide synthase (iNOS) transcription, which in turn produce prostaglandin E2 and nitric oxide [Cho et al., 2010]. LPS of H. pylori seems to play a role in the upregulation of iNOS, leading to an increased NO release [Cavallo et al., 2011]. Interestingly, NO synthesis can be modulated by H. pylori arginases and induced host arginase II, and this coupled with activation of heat shock factor-1 by infection result in decreased anti-inflammatory regulator heme oxygenase-1 expression [Gobert et al., 2011]. Additionally, VacA was shown to induce ROS production and mitochondrial DNA mutation in gastric epithelial cells [Huang et al., 2011]. H. pylori sonicates were observed to induce oxidative bursts from neutrophils and monocytes in culture [Hansen et al., 1999] via a mechanism involving the virulence factor H. pylori neutrophil-activating protein (HP-NAP), a secreted protein which can pass through the epithelial layer into the lamina propria to attract leukocytes [D'Elios et al., 2007]. HP-NAP induces ROS release from neutrophils [Evans et al., 1995] and causes the recruitment of other leukocytes to the site of infection by stimulating the production of chemokines such as CXCL8, CCL3, and CCL4 [Polenghi et al., 2007]. Mast cells, recruited by these chemokines, are stimulated to degranulate by HP-NAP, likely through a Gi protein-coupled receptor [Montemurro et al., 2002].
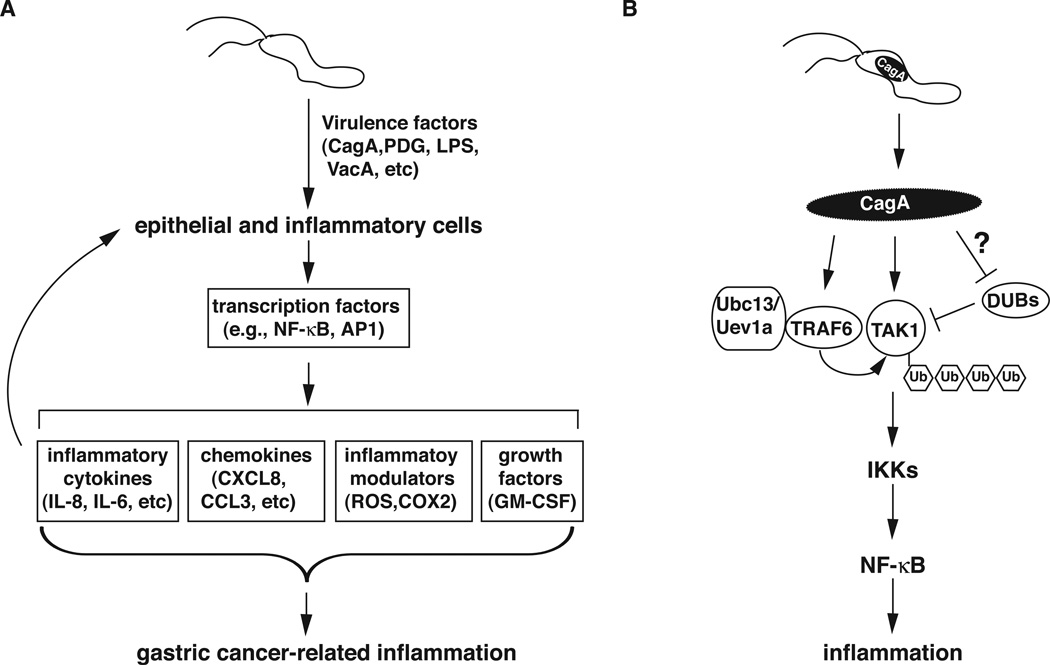
Another area that is currently being explored is the modulation of T cell responses by H. pylori virulence factors such as VacA, γ-glutamyl transpeptidase, and cholesterol α-glucosides, among others. Studies currently indicate that Th1, Treg and Th17 types predominate in response to H. pylori infection, a mixture that is believed to encourage the chronicity of the infection and infection-mediated gastritis is considered a Th1-mediated disease [Beigier-Bompadre et al., 2011]. All together, these mechanisms create an inflammatory environment conducive to the initiation and progression of cancer (Figure 1A).
Figure 1.
H. pylori induces gastric cancer-related inflammatory response. A) Schematic model for H. pylori-induced inflammation. H. pylori infection activates various transcription factors via its different virulence factors in gastric epithelial cells or inflammatory cells such as T cells and macrophages. Activated transcription factors then induce the expression of genes encoding pro-inflammatory cytokines, chemokines, inflammatory modulators and growth factors, creating an inflammatory microenvironment that facilitates the transformation of gastric epithelial cells. B) Modulation of TAK1 ubiquitination and activation by H. pylori CagA. After translocation into the epithelial cells, CagA enhances TRAF6- and Ubc13-mediated K63-linked polyubiquitination of TAK1 by directly associating with TRAF6 and TAK1. The enhanced ubiquitination of TAK1 might result from a stimulation of the activity of TRAF6 or Ubc13 or from an inhibition of an unidentified deubiquitinase (DUB) of TAK1. Polyubiquitinated TAK1 is then activated, leading to the activation of IKKs and NF-κB, and the subsequent inflammatory response.
Activation of NF-κB, the master regulator of inflammation, by Helicobacter pylori
Transcription factor NF-κB is a master regulator of immune and inflammatory responses and regulates many cellular processes important in carcinogenesis, including transformation, proliferation, angiogenesis and metastasis [Orlowski and Baldwin, 2002]. Due to its essential role in inflammation and immunity, NF-κB activation and modulation by H. pylori have been topics of great interest to many investigators. NF-κB can be activated by numerous pro-inflammatory stimuli, ranging from TLR activation by pathogen products to cytokines released by other cells, through the canonical and the non-canonical pathways [Hayden and Ghosh, 2008]. In the canonical pathway, the binding of various ligands to their receptors leads to the activation of the IκB kinase (IKK) complex, which consists of IKKα, IKKβ and NEMO/IKKγ. This kinase complex in turn phosphorylates and induces the degradation of the inhibitor of IκB, leading to the nuclear translocation of prototypical NF-κB heterodimer of p50 and RelA/p65, and the activation of its target genes involved in inflammation, cell growth and survival [Chen and Greene, 2004]. In the non-canonical pathway, signaling from receptors activates NF-κB-inducing kinase (NIK) and IKKα. Activated IKKα then phosphorylates p100 and induces its proteasomal processing to p52, which pairs with RelB to form the transcriptionally active complex that activates target genes involved in lymphoid organogenesis, B cell survival and maturation, and bone metabolism [Sun, 2011].
H. pylori infection activates NF-κB through both the canonical and the non-canonical pathways in a cell type-specific fashion [Lamb et al., 2010]. In epithelial cells, H. pylori infection activates NF-κB via the canonical pathway. But in immune cells, such as B lymphocytes, H. pylori can activate NF-κB through both the canonical and the non-canonical pathways [Ohmae et al., 2005]. While it is clear that H. pylori infection activates NF-κB, the precise mechanics of the H. pylori-NF-κB pathway interface have been difficult to resolve and appear to be numerous. Three bacterial products are currently thought to be particularly important for the activation of NF-κB by H. pylori: LPS, peptidoglycan, and CagA [Lamb et al., 2010].
H. pylori LPS has been shown to be recognized by host cells either by binding to TLR2 or TLR4 and has a role in H. pylori-induced NF-κB activation and inflammatory response [Ishihara et al., 2004; Kawahara et al., 2001]. However, many of these studies are done in cells such as HEK293 or gastric epithelial cells in which TLRs are overexpressed, raising questions about their roles in vivo [Ishihara et al., 2004]. Consistently, H. pylori LPS has been found to be a very weak activator of TLRs [Mandell et al., 2004], which may aid the bacteria’s persistence and its sustenance of a low-grade inflammation within the stomach without being a major player in the initial induction of inflammation. Nevertheless, H. pylori LPS might be especially important in activation of inflammatory pathways in leukocytes responding to the bacterial colonization, since studies looking at monocytes and macrophages show the importance of TLRs in their response but not in the response elicited in gastric epithelial cells [Maeda et al., 2001; Obonyo et al., 2007].
Another H. pylori bacterial effector molecule, peptidoglycan, is recognized by gastric epithelial cells through the intracellular nucleotide binding and oligomerization domain 1 (NOD1). The delivery mechanism of peptidoglycan into the host cells is proposed to be through either the T4SS pili or via outer membrane vesicles which bind to lipid rafts on the host cell [Grubman et al., 2010; Viala et al., 2004]. Peptidoglycan-induced NOD1 signaling activates MAPKs in both the NF-κB and the AP-1 pathways, leading to cytokine release [Allison et al., 2009].
One of the most studied H. pylori effectors, CagA, has been shown to have a myriad of effects within gastric epithelial cells, one of which is the activation of NF-κB. After translocation into host gastric epithelial cells, CagA can interact with the intramembrane hepatocyte growth factor receptor Met, causing sustained activation of PI3K and Akt which leads in turn to β-catenin and NF-κB activation [Suzuki et al., 2009]. Multimerization of CagA seems to be important for Met-PI3K-Akt-mediated NF-κB activation [Suzuki et al., 2009]. Additionally, CagA was found to interact with TNF receptor associated factor 6 (TRAF6) and TGF-β-activating kinase 1 (TAK1) for the activation of TAK1, which is responsible for phosphorylation and activation of the IKK complex leading to NF-κB activation [Lamb et al., 2009]. This finding will be discussed further below.
Targeting of TAK1 by Helicobacter pylori CagA
TAK1 is a key regulator of signal transduction cascades leading to the activation of IKK in response to various stimuli including cytokines and pathogen infections [Adhikari et al., 2007]. TAK1 activates IKK2 by phosphorylation of two serine residues in the activation loop [Wang et al., 2001]. K63-linked ubiquitination has long been shown to be involved in the activation of TAK1 and IKKs [Wang et al., 2001]. For example, ubiquitination of IL-1β receptor-associated kinase 1 (IRAK1) is important for IKK activation in the IL-1 pathway [Conze et al., 2008; Windheim et al., 2008]. Auto-ubiquitination of TRAF6 has also been shown to be a critical determinant of TAK1 and IKK activation [Lamothe et al., 2007]. Recent studies indicate that TRAF6-mediated K63-linked ubiquitination of TAK1 at specific lysines is essential for its auto-phosphorylation and activation (Fan et al, 2010; Yamazaki et al, 2009; Sorrentino et al, 2008). TRAF6-mediated K63-linked ubiquitination at lysine 34 of TAK1 has been reported to induce TGF-β-mediated p38 and JNK activation [Sorrentino et al., 2008]. Ubiquitination of TAK1 at a different lysine, lysine-158, is required for TNF-α or IL-1β-induced TAK1 activation and IKK/NF-κB activation [Fan et al., 2010].
We have recently reported that TRAF6-mediated ubiquitination of TAK1 is also essential for the H. pylori-induced NF-κB activation and production of pro-inflammatory cytokines. Activation of TRAF6 typically takes place near the plasma membrane, where membrane-associated proteins transfer signals from transmembrane receptors such as TLRs or TNFR [Jiang and Chen, 2012]. CagA has recently been shown to be a membrane-associate protein which binds phosphatidylserine in the inner leaflet and acts as a scaffold protein [Murata-Kamiya et al., 2010], which may explain its ability to interact with and activate TRAF6. H. pylori CagA is critical for the stimulation of TAK1 ubiquitination and activation. An isogenic cagA-deficient H. pylori mutant failed to induce the ubiquitination and auto-phosphorylation of TAK1 in AGS cells. CagA physically associates with TAK1 and TRAF6 to enhance the activity of TAK1 and TAK1-induced NF-κB activation via the TRAF6-mediated K63-linked ubiquitination of TAK1 [Lamb et al., 2009]. The mechanism by which CagA enhances the ubiquitination and activation of TAK1, however, remains largely unclear. Several lysines within TAK1, including K34, K158 and K209, have been shown to be K63-linked ubiquitinated [Fan et al., 2010; Sorrentino et al., 2008; Yamazaki et al., 2009]. Of all these lysines, K158, but not K34 or K209, appears to be involved in the CagA-mediated TAK1 ubiquitination since mutation of K158 blocks CagA-facilitated ubiquitination and activation of TAK1 (Lamb and Chen, unpublished results).
Ubiquitination is an ATP-dependent three-step enzymatic cascade involving an ubiquitin-activating enzyme (E1), an ubiquitin-conjugating enzyme (E2) and an ubiquitin-protein ligase (E3) [Adhikari et al., 2007]. While it is clear that TRAF6 is the E3 ligase for K63-linked ubiquitination and activation of TAK1 in response to H. pylori infection, the identity of the E2 for the ubiquitination remains to be identified. Biochemical studies reveal that Ubc13/Uev1A are the E2 conjugating enzymes for TRAF6-catalyzed K63-linked polyubiquitination and activates TAK1 kinase activity in vitro [Wang et al., 2001]. Consistently, Ubc13/Uev1A has been shown to be essential for the ubiquitination of TRAF6 in the IL-1R/TLR signaling [Yamazaki et al., 2009]. Our preliminary data also suggest that Ubc13/Uev1A is involved in H. pylori-mediated TAK1 ubiquitination and activation (Lamb and Chen, unpublished results).
It remains unclear how CagA enhances TAK1 ubiquitination and how TAK1 ubiquitination activates its kinase activity. One possibility is that CagA functions as a scaffold protein to bring TAK1 to the proximity of TRAF6 or other components of ubiquitination machinery, since CagA binds to both TAK1 and TRAF6 (Figure 1B). Binding of CagA to TRAF6 might also facilitate the dimerization of TRAF6, a step critical for the activation of TRAF6. Furthermore, the K63-linked ubiquitin chain of TAK1 might serve as an adaptor for the recruitment of ubiquitin-binding molecules such as TAB2/3 or NEMO, resulting in the stabilization of the signaling complex and the activation of down-stream IKK2.
Concluding remarks and future prospects
Helicobacter pylori infection is one of the leading factors for the development of gastric carcinoma. Infection with this common bacterium is often chronic, thriving for decades in the stomach of its host. Such a long incubation time allows for many changes to accumulate as a result of this interaction, and even small changes, such as subclinical inflammation, can cause great problems over a lifetime. Studying the pathways through which H. pylori virulence factors cause cellular or tissue changes, or activate signaling pathways such as the NF-κB, will be instrumental in better understanding host-pathogen interactions and also developing a better understanding of the ways in which inflammatory pathways are regulated.
Activation of NF-κB is a double-edged sword. It is essential for the activation of innate and adaptive immune responses against pathogens. However, sustained and constitutive NF-κB activation results in the pathogenesis of many diseases, including chronic inflammation, infectious diseases, and cancer [Hayden and Ghosh, 2008]. Therefore, activated NF-κB needs to be turned off properly to avoid prolonged and detrimental inflammatory responses. Cells utilize many mechanisms at multiple levels for the termination of NF-κB signaling. For example, physiologic NF-κB signaling induces down-regulation of its own activity, via IκBα resynthesis and cylandromatosis (CYLD) expression, mechanisms which are often lost or overpowered in cancer. Additionally, direct ubiquitination and degradation of nuclear NF-κB, is another means by which NF-κB can be turned off [Yang et al., 2009]. While current studies largely focus on how H. pylori utilizes its various components to interact with different cellular signaling molecules to activate NF-κB or other transcription factors for the induction of inflammation, little is known regarding whether NF-κB termination signals could also be targeted for H. pylori to maintain sustained NF-κB activation and inflammation. Activation of NF-κB by H. pylori might be only part of the story. How those normal checks and balances are overcome on the road to carcinogenesis, and seemingly must remain impaired throughout the life of a cancer, remains to be investigated.
NF-κB activity is many-faceted; it has a role in every aspect of cancer, from development to metastasis, and studying the induction of its activity is only the beginning. Can H. pylori not only establish an environment conducive to the promotion of carcinogenesis, but also direct signaling within cells and tumors towards angiogenesis, proliferation, and even metastasis, all regulated by the NF-κB pathway? Many questions remain in our search to understand the pathogenesis of H. pylori in cancer, but it seems that many could be answered with a better appreciation of the ways in which H. pylori activates NF-κB. A better understanding of the molecular mechanism for H. pylori-mediated NF-κB activation and inflammation would allow the identification of agents that suppress the inflammatory response for the prevention and treatment of gastric cancer.
Acknowledgements
This work is supported in part by NIH grant DK-085158 (to L.F.C.)
Footnotes
Conflict of interest
The authors declare no conflict of interest
Reference
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison CC, Kufer TA, Kremmer E, Kaparakis M, Ferrero RL. Helicobacter pylori Induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol. 2009;183:8099–8109. doi: 10.4049/jimmunol.0900664. [DOI] [PubMed] [Google Scholar]
- Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends in Microbiology. 2010;18:479–486. doi: 10.1016/j.tim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Beales IL, Calam J. Helicobacter pylori stimulates granulocyte-macrophage colony-stimulating factor (GM-CSF) production from cultured antral biopsies and a human gastric epithelial cell line. European Journal of Gastroenterology and Hepatology. 1997;9:451–455. doi: 10.1097/00042737-199705000-00008. [DOI] [PubMed] [Google Scholar]
- Beigier-Bompadre M, Moos V, Belogolova E, Allers K, Schneider T, Churin Y, Ignatius R, Meyer TF, Aebischer T. Modulation of the CD4+ T-cell response by Helicobacter pylori depends on known virulence factors and bacterial cholesterol and cholesterol alpha-glucoside content. Journal of Infectious Diseases. 2011;204:1339–1348. doi: 10.1093/infdis/jir547. [DOI] [PubMed] [Google Scholar]
- Buti L, Spooner E, Van der Veen AG, Rappuoli R, Covacci A, Ploegh HL. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc Natl Acad Sci U S A. 2011;108:9238–9243. doi: 10.1073/pnas.1106200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo P, Cianciulli A, Mitolo V, Panaro MA. Lipopolysaccharide (LPS) of helicobacter modulates cellular DNA repair systems in intestinal cells. Clin Exp Med. 2011;11:171–179. doi: 10.1007/s10238-010-0118-1. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Cho SO, Lim JW, Kim KH, Kim H. Involvement of Ras and AP-1 in Helicobacter pylori-induced expression of COX-2 and iNOS in gastric epithelial AGS cells. Digestive Diseases and Sciences. 2010;55:988–996. doi: 10.1007/s10620-009-0828-y. [DOI] [PubMed] [Google Scholar]
- Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011;17:3971–3975. doi: 10.3748/wjg.v17.i35.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol. 2008;28:3538–3547. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elios MM, Amedei A, Cappon A, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunology and Medical Microbiology. 2007;50:157–164. doi: 10.1111/j.1574-695X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR. Characterization of a Helicobacter pylori neutrophil-activating protein. Infection and Immunity. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, Zhang H, Fu S, Qin J, Yang J. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem. 2010;285:5347–5360. doi: 10.1074/jbc.M109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand J, Lehours P, Schmid-Alliana A, Megraud F, Varon C. Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-kappaB-dependent pathway. PLoS One. 2011;6:e29007. doi: 10.1371/journal.pone.0029007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert AP, Asim M, Piazuelo MB, Verriere T, Scull BP, de Sablet T, Glumac A, Lewis ND, Correa P, Peek RM, Jr, Chaturvedi R, Wilson KT. Disruption of nitric oxide signaling by Helicobacter pylori results in enhanced inflammation by inhibition of heme oxygenase-1. Journal of Immunology. 2011;187:5370–5379. doi: 10.4049/jimmunol.1102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group HaCC. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman A, Kaparakis M, Viala J, Allison C, Badea L, Karrar A, Boneca IG, Le Bourhis L, Reeve S, Smith IA, Hartland EL, Philpott DJ, Ferrero RL. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell Microbiol. 2010;12:626–639. doi: 10.1111/j.1462-5822.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hansen PS, Go MF, Varming K, Andersen LP, Graham DY, Nielsen H. Proinflammatory activation of neutrophils and monocytes by Helicobacter pylori is not associated with cagA, vacA or picB genotypes. APMIS. 1999;107:1117–1123. [PubMed] [Google Scholar]
- Hatakeyama M. SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol. 2008;11:30–37. doi: 10.1016/j.mib.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Huang XW, Luo RH, Zhao Q, Shen ZZ, Huang LL, An XY, Zhao LJ, Wang J, Huang YZ. Helicobacter pylori induces mitochondrial DNA mutation and reactive oxygen species level in AGS cells. Int J Med Sci. 2011;8:56–67. doi: 10.7150/ijms.8.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara S, Rumi MA, Kadowaki Y, Ortega-Cava CF, Yuki T, Yoshino N, Miyaoka Y, Kazumori H, Ishimura N, Amano Y, Kinoshita Y. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. Journal of Immunology. 2004;173:1406–1416. doi: 10.4049/jimmunol.173.2.1406. [DOI] [PubMed] [Google Scholar]
- Jiang X, Chen ZJ. The role of ubiquitylation in immune defence and pathogen evasion. Nat Rev Immunol. 2012;12:35–48. doi: 10.1038/nri3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kawahara T, Kuwano Y, Teshima-Kondo S, Kawai T, Nikawa T, Kishi K, Rokutan K. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. Journal of Medical Investigation. 2001;48:190–197. [PubMed] [Google Scholar]
- Kitajima Y, Ohtaka K, Mitsuno M, Tanaka M, Sato S, Nakafusa Y, Miyazaki K. Helicobacter pylori infection is an independent risk factor for Runx3 methylation in gastric cancer. Oncol Rep. 2008;19:197–202. [PubMed] [Google Scholar]
- Lamb A, Yamaguchi H, Chen LF. The many roads traveled by Helicobacter pylori to NF-kappaB activation. Gut Microbes. 2010;1:109–113. doi: 10.4161/gmic.1.2.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, Peek RM, Blanke SR, Chen LF. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–1249. doi: 10.1038/embor.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe B, Webster WK, Gopinathan A, Besse A, Campos AD, Darnay BG. TRAF6 ubiquitin ligase is essential for RANKL signaling and osteoclast differentiation. Biochem Biophys Res Commun. 2007;359:1044–1049. doi: 10.1016/j.bbrc.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Akanuma M, Mitsuno Y, Hirata Y, Ogura K, Yoshida H, Shiratori Y, Omata M. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J Biol Chem. 2001;276:44856–44864. doi: 10.1074/jbc.M105381200. [DOI] [PubMed] [Google Scholar]
- Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infection and Immunity. 2004;72:6446–6454. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee DJ, Mobley HL. Pathogenesis of Helicobacter pylori infection. Curr Opin Gastroenterol. 2000;16:24–31. doi: 10.1097/00001574-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. European Journal of Immunology. 2002;32:671–676. doi: 10.1002/1521-4141(200203)32:3<671::aid-immu671>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe. 2010;7:399–411. doi: 10.1016/j.chom.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Jr, Azuma T, Hatakeyama M. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- O'Connor PM, Lapointe TK, Jackson S, Beck PL, Jones NL, Buret AG. Helicobacter pylori activates calpain via toll-like receptor 2 to disrupt adherens junctions in human gastric epithelial cells. Infection and Immunity. 2011;79:3887–3894. doi: 10.1128/IAI.05109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonyo M, Sabet M, Cole SP, Ebmeyer J, Uematsu S, Akira S, Guiney DG. Deficiencies of myeloid differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4 produce specific defects in macrophage cytokine secretion induced by Helicobacter pylori. Infect Immun. 2007;75:2408–2414. doi: 10.1128/IAI.01794-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmae T, Hirata Y, Maeda S, Shibata W, Yanai A, Ogura K, Yoshida H, Kawabe T, Omata M. Helicobacter pylori activates NF-kappaB via the alternative pathway in B lymphocytes. J Immunol. 2005;175:7162–7169. doi: 10.4049/jimmunol.175.11.7162. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- Peek RM., Jr Orchestration of aberrant epithelial signaling by Helicobacter pylori CagA. Sci STKE. 2005;2005:pe14. doi: 10.1126/stke.2772005pe14. [DOI] [PubMed] [Google Scholar]
- Polenghi A, Bossi F, Fischetti F, Durigutto P, Cabrelle A, Tamassia N, Cassatella MA, Montecucco C, Tedesco F, de Bernard M. The neutrophil-activating protein of Helicobacter pylori crosses endothelia to promote neutrophil adhesion in vivo. Journal of Immunology. 2007;178:1312–1320. doi: 10.4049/jimmunol.178.3.1312. [DOI] [PubMed] [Google Scholar]
- Saha A, Hammond CE, Beeson C, Peek RM, Jr, Smolka AJ. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut. 2010;59:874–881. doi: 10.1136/gut.2009.194795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata W, Hirata Y, Maeda S, Ogura K, Ohmae T, Yanai A, Mitsuno Y, Yamaji Y, Okamoto M, Yoshida H, Kawabe T, Omata M. CagA protein secreted by the intact type IV secretion system leads to gastric epithelial inflammation in the Mongolian gerbil model. J Pathol. 2006;210:306–314. doi: 10.1002/path.2040. [DOI] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landstrom M. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Helicobacter pylori outer membrane proteins on gastric mucosal interleukin 6 and 11 expression in Mongolian gerbils. Journal of Gastroenterology and Hepatology. 2011;26:1677–1684. doi: 10.1111/j.1440-1746.2011.06817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Research. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Yamaoka S. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene. 2005;24:5952–5964. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D, Berg DE, Sasakawa C. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Takeshima E, Tomimori K, Takamatsu R, Ishikawa C, Kinjo F, Hirayama T, Fujita J, Mori N. Helicobacter pylori VacA activates NF-kappaB in T cells via the classical but not alternative pathway. Helicobacter. 2009;14:271–279. doi: 10.1111/j.1523-5378.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- Tsang YH, Lamb A, Chen LF. New insights into the inactivation of gastric tumor suppressor RUNX3: the role of H. pylori infection. J Cell Biochem. 2011;112:381–386. doi: 10.1002/jcb.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang YH, Lamb A, Romero-Gallo J, Huang B, Ito K, Peek RM, Jr, Ito Y, Chen LF. Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for proteasome-mediated degradation. Oncogene. 2010;29:5643–5650. doi: 10.1038/onc.2010.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon C, Dubus P, Mazurier F, Asencio C, Chambonnier L, Ferrand J, Giese A, Senant-Dugot N, Carlotti M, Megraud F. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology. 2012;142:281–291. doi: 10.1053/j.gastro.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Windheim M, Stafford M, Peggie M, Cohen P. Interleukin-1 (IL-1) induces the Lys63-linked polyubiquitination of IL-1 receptor-associated kinase 1 to facilitate NEMO binding and the activation of IkappaBalpha kinase. Mol Cell Biol. 2008;28:1783–1791. doi: 10.1128/MCB.02380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A. 2000;97:7533–7538. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, Akira S, Hayashi H, Su B, Inoue J. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal. 2009;2:ra66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- Yang XD, Huang B, Li M, Lamb A, Kelleher NL, Chen LF. Negative regulation of NF-kappaB action by Set9-mediated lysine methylation of the RelA subunit. EMBO J. 2009;28:1055–1066. doi: 10.1038/emboj.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Hypergastrinemia in response to gastric inflammation suppresses somatostatin. Am J Physiol Gastrointest Liver Physiol. 2002;282:G175–G183. doi: 10.1152/ajpgi.00287.2001. [DOI] [PubMed] [Google Scholar]