Abstract
The transglutaminase (TGase) is present in the pollen tube where it most likely participates in the regulation of different activities including the organization of cytoskeletal elements (microtubules and actin filaments). In addition to a cytosolic form of TGase, new data suggest the existence of TGase forms associated with the internal membranes and with the cell wall of pollen tubes. This different localization extends the functional range of pollen TGase but also raises the question how TGase can be precisely (and in harmony with the pollen tube growth) redistributed in different cellular compartments. The discovery that TGase exists as different isoforms may suggest a pathway to achieve this result.
Keywords: transglutaminase, secretion, pollen tube, cytoskeleton
In a recent work, Del Duca and coworkers1 have analyzed the distribution of transglutaminase (TGase) in the pollen tube of pear tree, mainly in relation to the dynamics of the cytoskeleton and membrane traffic. The enzyme was localized in association with different cellular compartments, including the cytosol, the internal membrane system and the cell wall. In view of the fact that TGase is associated with several cellular compartments, it is important to understand why and how the enzyme is differentially distributed, if a relationship exists between the TGase forms present in different districts and if each form of TGase performs a specific activity. In fact, TGase often have more features depending on their cellular localization.2 In addition, by enzymatically adding amino groups to different substrates, TGases can exert different roles, from regulatory mechanisms to processes involving programmed cell death. The specific role of TGase in pollen tube is not yet clear. Several publications have highlighted that TGase can enzymatically modify both tubulin and actin of pollen tubes, suggesting that it is involved in the regulation of the dynamics of actin filaments and microtubules. This function could be exerted by the cytosolic TGase that, presumably after activation by changes in Ca2+ concentration, could thus introduce post-translational modifications in both actin and tubulin monomers. It is not yet clear if these modifications are reversible; otherwise, the role of TGase would consequently be restricted to processes related to the disorganization of the cytoskeletal apparatus, as those occurring during the response to self-incompatibility. Currently, other potential cytoplasmic substrates of pollen TGase are unknown and it is consequently difficult to make further assumptions about its role. Quite different is the possible role of membrane TGase and, more generally, of non-cytosolic TGase, including therefore also the cell wall (extracellular) TGase. Data published in some papers indicated consistently that the pollen TGase is also associated with the membrane compartments of pollen tubes, including the Golgi apparatus and plasma membrane.1,3 Some papers report the presence of TGase in association with the chloroplast fraction of plant cells (in which the enzyme could exert protective functions on the photosystems of the photosynthetic apparatus).4 However, this feature has to be excluded in pollen tubes in view of the limited number of plastidial elements and absence of photosynthetic metabolic functions. The association of TGase with the Golgi apparatus, apart from suggesting that TGase is transported to the plasma membrane or toward the vacuolar compartment, also suggests that the enzyme is secreted outside the cell via the vesicular system. Consequently, the Golgi-TGase association could be the first step of a secretory process that leads TGase in the extracellular matrix (cell wall).
Association of TGase with the membrane system seems to require the post-translational modification of the enzyme. Two-dimensional electrophoretic analysis of TGase extracted from different cellular compartments showed that TGase exhibits an isoforms pattern typical for each compartment. The use of different isoforms in dependence of different compartments is not an atypical feature, but it seems commonly used to mark the same protein in different environments. A relatively recent example is sucrose synthase, a key enzyme of the plant metabolism that can exist in differently phosphorylated forms depending on the localization.5 We do not know the type of post-translational modification (and even where and when it occurs) to which TGase is subjected; nevertheless, it seems clear that a specific kind of post-translational modification is a prerequisite to fix the enzyme in different localizations.
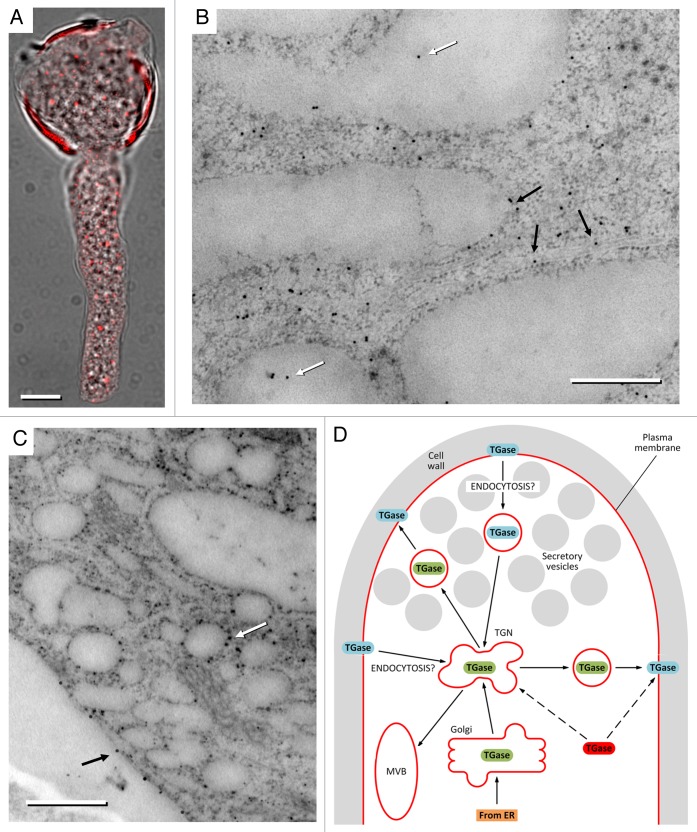
The association between TGase and the membrane system is an issue not easy to decipher. The optimal isolation of single membrane compartments is not technically simple and can therefore result in interpretations not completely accurate. However, the association between TGase and Golgi was demonstrated by immunological and enzyme assays.1 Conventional immunofluorescence microscopy showed that TGase distributes as dots in the cytoplasm of growing pollen tubes (Fig. 1A), a labeling pattern that generally evokes the distribution of organelles and, more specifically, of Golgi bodies. A more detailed analysis by immuno-microscopy allowed us to detect the presence of TGase in association with other membrane compartments of the pollen tube of pear tree, including the rough endoplasmic reticulum (Fig. 1B). In addition to the presence of TGase in the internal membranes of pollen tubes, the enzyme was also localized in association with the membrane of hypothetical secretory vesicles (Fig. 1C). The association of TGase with the membrane of vesicular structures suggests that part of the enzyme is transported to the plasma membrane where it can exert its specific function. Examples of TGase in association with the plasma membrane are numerous (at least in animal cells) and the functions performed by these enzymes at the plasma membrane can be quite diverse.6
Figure 1. Analysis by immuno-electron microscopy of TGase in pear pollen tube. (A) Immunofluorescence microscopy of TGase (red) in the pollen tube of pear. The fluorescence image is superimposed on the same pollen tube shown in bright field microscopy. Bar: 10 μm. (B) Localization of TGase by immuno-gold in not-apical regions of the pollen tube. The enzyme is shown within membrane structures (white arrows) but also in association with the surface of internal membranes (possibly including the endoplasmic reticulum, black arrows). Bar: 500 nm. (C) Distribution of TGase near the cell cortex. TGase can be observed in association with the surface of vesicular structures (white arrow) and with the plasma membrane of pollen tubes (black arrows). Bar: 500 nm. (D) Model of the hypothetical secretory process of TGase. The enzyme (in green) may be secreted through a conventional mechanism that starts in the endoplasmic reticulum (ER), goes through the Golgi and the Trans Golgi Network (TGN), ending up with the secretory vesicle-mediated exocytosis. Secretory vesicles may not necessarily fuse with the apical membrane. In the cell wall, TGase would be subject to post-translational modifications that mark and/or activate the enzyme (in blue). Either excess or functionally useless TGase may be removed by endocytotic processes to be degraded in the Multi Vesicular Body (MVB). Considering data in the literature, we cannot exclude that the cytosolic TGase (in red) is directly incorporated into the membranes and then secreted.
The presence of TGase in the cell wall of pollen tubes reinforces the idea that TGase is synthesized on the endoplasmic reticulum, exported to Golgi and then to the vesicular system of secretion. However, the secretory process could not end exactly as expected for the pollen tube system. Generally, it is believed that the reticulum-Golgi transport system produces secretory vesicles that accumulate at the pollen tube apex (the so-called clear zone) and that fuse with the plasma membrane. This linear pathway can be disturbed by the application of inhibitory substances, such as Brefeldin A (BFA), that induce the formation of a membrane complex in the pollen tube sub-apex (the so-called BFA compartment).7 In our tests with BFA, we never observed the formation of such a complex containing TGase suggesting that TGase (if secreted through the reticulum-Golgi pathway) may use a vesicular system different from that usually used in pollen tubes. The possibility that TGase uses a different secretory pathway is also evidenced by further data of electron microscopy (Fig. 1C) revealing the association of TGase with vesicular structures of different sizes, but not localized in the apical region of pollen tubes. These vesicles could be a vehicle for transporting TGase to areas of the plasma membrane separate from the apical region. A common finding with other proteins is the fact that the transport of TGase is still dependent on the integrity of actin filaments. However, this evidence is reasonable and not unexpected because the coordination of membrane trafficking in plant cells requires the dynamic activity of actin filaments. It is therefore possible to hypothesize alternative mechanisms of secretion. Non-Golgi-based secretion of TGase is a documented alternative secretion pathway. The so-called mechanism of “membrane blebbing or microvesicle shedding” provides that cytosolic TGase is packaged directly into membrane-derived protrusions; these protrusions come off and the formerly cytosolic components are released when the vesicular protrusions break.8 Based on these observations (and on information available in the literature), we can hypothesize the following mechanism for TGase secretion in the pollen tube (Fig. 1D). The membrane TGase (green) could be classically synthesized in the endoplasmic reticulum and exported to the Golgi apparatus and then to the Trans-Golgi Network (TGN) from where it would be packaged into secretory vesicles. In this way, TGase is secreted externally in the cell wall and the new position is stabilized by a further post-translational modification (blue TGase, which could also serve to activate the enzyme). Once in the cell wall, TGase could be released outside in the pollen-style extracellular matrix, as suggested in apple,3 but TGase could be also eventually recycled by endocytotic events that may occur both in the apical region of pollen tubes and in more distal regions. The TGN-recycled TGase, according to the most reliable models, might be either secreted again or directed toward the Multi-Vesicular-Body (MVB) for degradation.9 Currently we cannot discriminate between recycling and degradation of TGase, although we believe that the latter is more likely because in line with what found in animal cells and because the degradation process would allow maintaining constant the levels of TGase in the apical and subapical region. Considering the uncertainties still existing on this route, it is also plausible and intriguing to believe that cytosolic TGase (in red) is directly incorporated and packaged within secretory structures that deliver TGase to the extracellular matrix.
Regardless of the type of secretion, the presence of extracellular TGase asks the question about the function exerted locally by this enzyme. The cell wall of pollen tubes contains, like other plant cells, polysaccharides and proteins; the latter can be generally categorized into two main groups: enzymes that modify the cell wall (such as pectin methyl-esterase) and proteins combined with polysaccharide chains of variable length, such as arabinogalactan proteins (AGPs). Identifying the specific target of TGase is not simple. It is clear that the cell wall proteins may represent an ideal target for the enzyme suggesting that such protein substrates are consequently controlled by TGase. Currently, there is no evidence in favor of this hypothesis. The identification of protein substrates would require locating the products of the TGase activity, for example by enzymatically combining tagged-substrates (for example, labeled polyamines) to protein targets and then using these tags for “fish” the target proteins from cell wall extracts. The protein substrates may also be represented by the protein skeleton of AGPs. In tomato, one AGP (LeAGP-1) was identified and isolated as a trimer, whose assembly was supposedly caused by a TGase activity.10 In pear pollen tube, double labeling assays showed that TGase co-localized (although partially) with MAC207-labeled AGPs.1 The substrates of TGase may also be represented by polysaccharides of the cell wall. As already mentioned,1 the pectin skeleton could theoretically be a plausible target since previous works showed that (acid) pectins can interact with (basic) polyamines, the latter being natural substrates of TGase.11 Although these findings do not prove that pectins are targets of TGase, they suggest that this enzyme may play a role in cell wall organization.
Acknowledgments
We sincerely thank Mrs Claudia Faleri (Dipartimento Scienze della Vita, University of Siena) for permission to use the immunofluorescence and immuno-gold microscopy images.
Glossary
Abbreviations:
- tgase
transglutaminase
- MVB
multi vesicular body
- TGN
trans golgi network
- BFA
brefeldin A
- AGP
arabino-galactan proteins
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24446
References
- 1.Del Duca S, Faleri C, Iorio RA, Cresti M, Serafini-Fracassini D, Cai G. Distribution of transglutaminase in pear pollen tubes in relation to cytoskeleton and membrane dynamics. Plant Physiol. 2013 doi: 10.1104/pp.112.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beninati S, Piacentini M. The transglutaminase family: an overview: minireview article. Amino Acids. 2004;26:367–72. doi: 10.1007/s00726-004-0091-7. [DOI] [PubMed] [Google Scholar]
- 3.Di Sandro A, Del Duca S, Verderio E, Hargreaves AJ, Scarpellini A, Cai G, et al. An extracellular transglutaminase is required for apple pollen tube growth. Biochem J. 2010;429:261–71. doi: 10.1042/BJ20100291. [DOI] [PubMed] [Google Scholar]
- 4.Della Mea M, Di Sandro A, Dondini L, Del Duca S, Vantini F, Bergamini C, et al. A Zea mays 39-kDa thylakoid transglutaminase catalyses the modification by polyamines of light-harvesting complex II in a light-dependent way. Planta. 2004;219:754–64. doi: 10.1007/s00425-004-1278-6. [DOI] [PubMed] [Google Scholar]
- 5.Persia D, Cai G, Del Casino C, Faleri C, Willemse MTM, Cresti M. Sucrose synthase is associated with the cell wall of tobacco pollen tubes. Plant Physiol. 2008;147:1603–18. doi: 10.1104/pp.108.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–9. doi: 10.1016/S0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 7.Parton RM, Fischer-Parton S, Trewavas AJ, Watahiki MK. Pollen tubes exhibit regular periodic membrane trafficking events in the absence of apical extension. J Cell Sci. 2003;116:2707–19. doi: 10.1242/jcs.00468. [DOI] [PubMed] [Google Scholar]
- 8.Aumuller G, Wilhelm B, Seitz J. Apocrine secretion - fact or artifact? Ann Anatomy - Anat Anz. 1999;181:437–46. doi: 10.1016/S0940-9602(99)80020-X. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Zhuang XH, Hillmer S, Robinson DG, Jiang LW. Vacuolar sorting receptor (VSR) proteins reach the plasma membrane in germinating pollen tubes. Mol Plant. 2011;4:845–53. doi: 10.1093/mp/ssr011. [DOI] [PubMed] [Google Scholar]
- 10.Gao M, Kieliszewski MJ, Lamport DTA, Showalter AM. Isolation, characterization and immunolocalization of a novel, modular tomato arabinogalactan-protein corresponding to the LeAGP-1 gene. Plant J. 1999;18:43–55. doi: 10.1046/j.1365-313X.1999.00428.x. [DOI] [PubMed] [Google Scholar]
- 11.D’Orazi D, Bagni N. In vitro interactions between polyamines and pectic substances. Biochem Biophys Res Commun. 1987;148:1259–63. doi: 10.1016/S0006-291X(87)80268-1. [DOI] [PubMed] [Google Scholar]