Abstract
Cysteine-rich proteins seem to play important regulatory roles in Medicago truncatula/Sinorhizobium meliloti symbiosis. In particular, a large family of nodule-specific cysteine-rich (NCR) peptides is crucial for the differentiation of nitrogen-fixing bacteroids. The Medicago truncatula N5 protein (MtN5) is currently the only reported non-specific lipid transfer protein necessary for successful rhizobial symbiosis; in addition, MtN5 shares several characteristics with NCR peptides: a small size, a conserved cysteine-rich motif, an N-terminal signal peptide for secretion and antimicrobial activity. Unlike NCR peptides, MtN5 expression is not restricted to the root nodules and is induced during the early phases of symbiosis in root hairs and nodule primordia. Recently, MtN5 was determined to be involved in the regulation of root tissue invasion; while, it was dispensable for nodule primordia formation. Here, we discuss the hypothesis that MtN5 participates in linking the progression of bacterial invasion with restricting the competence of root hairs for infection.
Keywords: non-specific lipid transfer proteins, MtN5, rhizobial symbiosis, root infection, Medicagotruncatula
The symbiotic interaction between plants belonging to the Fabaceae family and rhizobacteria involves two independent but coordinated processes: bacterial infection and nodule organogenesis. The infection process starts at the root epidermis and requires the recognition of rhizobia by the host plant. This phase is mediated by species/strain-specific nodulation factors (NFs) secreted by rhizobia, which provoke physiological and biochemical responses in the root hair cells.1-4 Other bacterial effector molecules (i.e., exopolysaccharides) are required with the NFs for the subsequent root hair curling and formation of infection threads (ITs), which enable the penetration of the symbionts into the root cortex.5,6 It has been suggested that these molecules act as specific suppressors of plant defense responses.
The perception of rhizobia occurs at the root epidermis; while, formation of the nodule is controlled at the level of the root cortex. The study of nodulation defective mutants highlighted that the two processes can be separated since bacterial infection can take place without nodule organogenesis and nodule primordia can develop in the absence of bacteria.7-11 Nevertheless, epidermal and cortical events must be synchronized to form functional nodules. Two plant hormones (i.e., auxin and cytokinin) play a pivotal role in determining nodule primordia development and modulation of their levels and ratios is believed to be coordinated with symbiotic epidermal events.6,12,13 Nodule formation is also controlled by shoot-derived signals implicated in the so-called autoregulation of nodulation (AON).14 Just as nodule formation is strictly regulated, it is plausible that the invasion of the nodule is also a critical check point for the progression of infection and that the cross-talk between epidermal and cortical tissue occurs in two directions: from root hairs/epidermis to cortical cells during the first phases of pre-infection/infection and from cortical cells/nodule primordia to the epidermis during IT progression and invasion.
The endosymbiosis between leguminous plants bearing indeterminate nodules and rhizobia (e.g., the Medicago truncatula-Sinorhizobium meliloti interaction) can be described as a chronic infection characterized by tight host control over the bacteria.15 Although rhizobia are only initially perceived as intruders and progression of the infection is associated with inactivation of a general defense response by the plant, confinement or regulation of the metabolism and multiplication of bacteria is probably maintained locally where bacteria are hosted.15 An example is the leguminous plants belonging to the inverted repeat lacking clade (IRLC). After release of the bacteria into the target cells of nodule primordia, their differentiation into nitrogen-fixing bacteroids is controlled by a large family of nodule-specific cysteine-rich (NCR) peptides produced by the host plant. Some of these peptides are similar to defensins and possess antimicrobial activity in vitro.16,17
Another cysteine-rich protein induced during rhizobial symbiosis is MtN5, which is required for optimal bacterial infection and nodule invasion.18 MtN5 displays several features common to NCR peptides such as a small size, a conserved N-terminal region that includes a secretion signal peptide and in vitro antimicrobial activity.19,20 The C-terminal cysteine-rich region of MtN5 contains an 8-cysteine domain that is characteristic of the non-specific lipid transfer protein (ns-LTP) family; whereas, NCR peptides have four or six conserved cysteines.21,22 Plant ns-LTPs are a heterogeneous group of small basic proteins that can associate with different types of phospholipids and seem to display various biological functions.
According to the classification of ns-LTPs recently proposed by Wang et al.,22 MtN5 would cluster, based on the cysteine motif, with a very small group of ns-LTPs (Type III). Type III ns-LTPs consist of three other members, including A. thaliana DIR1 with a role in pathogen systemic defense (Table 1, section A). MtN5 is currently the only ns-LTP implicated in symbiosis.23 Interestingly, we identified two other Type III putative ns-LTPs in the M. truncatula genome (Medtr7g052640.1 and Medtr3g055250.1) (Table 1, section B).
Table 1. The 8 cysteine motif and number of flanking amino acid residues in Type III ns-LTPs.
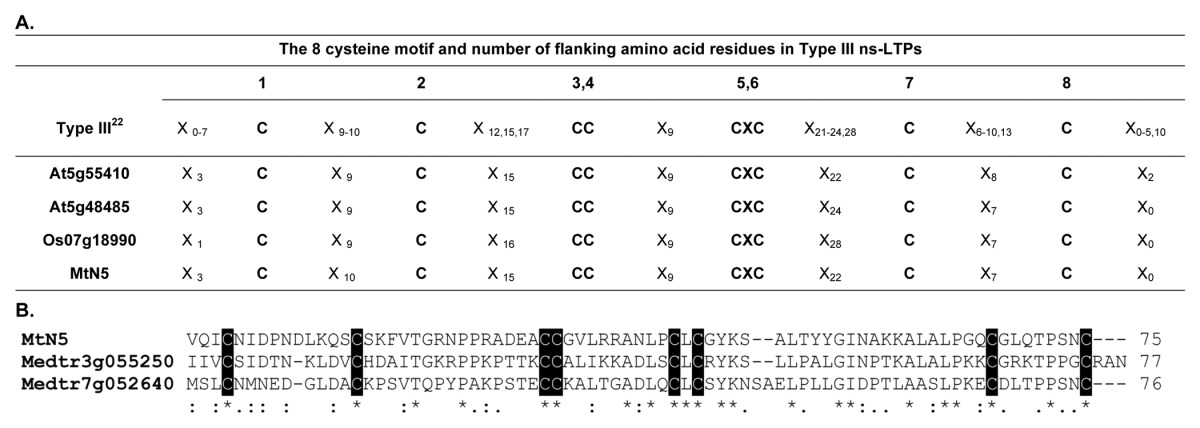
The amino acid sequences of mature MtN5 and two other type III ns-LTPs identified in the M. truncatula genome were aligned using the ClustalW2 (www.ebi.ac.uk/Tools/msa/clustalw2) program using default alignment parameters, selecting BLOSUM for the protein matrix, and choosing the input order of the sequences for the output option. Consensus symbols: *, identical residues; :, residues with strongly similar properties; ., residues with weakly similar properties. The conserved cysteine residues are shown in black boxes.
In contrast to the NCR peptides, MtN5 expression is not confined to the nodule; rather, it is induced at a very early stage of symbiosis in the epidermis and later on in primordia and young nodules. In the mature nodule, expression of MtN5 appears to be restricted to the apical part corresponding to the invasion zone.18 The pre-infection stage is characterized by expression of the early nodulin gene ENOD11, which is strongly induced in MtN5-silenced roots. The NIN transcription factor, which may be involved in the coordination of epidermal infection and nodule organogenesis, is not affected by the lack of MtN5 function. These data suggest MtN5 is involved in the early stages of symbiosis. This is further confirmed by the observation of increased curled root hairs (by about 100%), reduced colonization (by about 80%) of nodule primordia and decreased FLOT4 expression in MtN5-silenced roots, without any impairment in nodule primordia formation.18 FLOT4 function is required for proper formation and growth of ITs.24
The common symbiotic pathway, based on DMI1, DMI2 and DMI3 and the so-called parallel NF-mediated signaling pathway may work in combination to regulate the development of ITs.12 IT development also involves the activation of phospholipase enzymes (PLC and PLD) at the plasma membrane and the generation of diffusible secondary messengers.12 Through the use of specific inhibitors and M. truncatula insertional mutants, it was determined that MtN5 expression requires PLD activity but not DMI2, suggesting MtN5 is involved in the parallel NF-mediated signaling pathway that controls rhizobial infection (Fig. 1).18

Figure 1. Model depicting the putative role of MtN5 in rhizobial infection. MtN5 is implicated in the molecular events occurring at the epidermis after perception of nodulation factors (NFs) and phospholipase D (PLD) activation and acts upstream of FLOTILLIN 4 (FLOT4). The function of MtN5 seems independent of DMI1 and NIN. Studies with MtN5-silenced roots indicated MtN5 is required for rhizobial infection, but not for nodule primordia formation. It is not yet established whether this protein could act either on the initiation and progression of infection threads (ITs) or on nodule invasion. The impaired nodule infection observed in MtN5-silenced roots was accompanied by an enhanced responsiveness of root hairs to rhizobia, suggesting MtN5 could participate either directly or indirectly in a mechanism that restricts the competence of root hairs for infection.
Root hair curling and rhizobia penetration occur in a restricted area of the root. Several M. truncatula mutants showing simultaneous perturbation of IT development and nodule organogenesis also have excessive curling, suggesting the existence of a feed-back control over the competence of root hairs for infection.25 The phenotype of MtN5-silenced roots is of particular interest because the root hairs seem to have an increased competence for infection along with reduced nodule invasion, but without any impairment in nodule primordia formation (Fig. 1). Thus, MtN5-silenced roots could be used to evaluate the effect of bacterial penetration on the feedback control of root hair curling. Future investigations should be performed to assess whether MtN5 plays a role in IT development or in nodule primordia invasion. These studies should shed light on the different check-points occurring during rhizobial infection.
The control of bacterial infection by antimicrobial peptides seems to be a common phenomenon in symbiosis.17 The root nodules of M. truncatula produce a large number of NCR peptides, which are crucial for bacteroid differentiation. It is possible that other cysteine-rich peptides, such as the ns-LTP MtN5, are engaged in the localized control of rhizobia in epidermal cells or during cortical cell invasion. Due to its capacity to bind lipid molecules, MtN5 might either interact with the rhizobia plasma membrane or participate in the signaling between rhizobia and host cells.
Glossary
Abbreviations:
- NCR
nodule-specific cysteine rich
- NFs
nodulation factors
- ITs
infection threads
- AON
autoregulation of nodulation
- IRLC
inverted repeat lacking clade
- ns-LTP
non-specific lipid transfer protein
- DIR1
defective in induced resistance 1
- ENOD11
early nodulin 11
- NIN
nodule inception
- FLOT4
flotillin4
- DMI
doesn’t make infections
- PLC
phospholipase C
- PLD
phospholipase D
- CM
cysteine motif
- LYK3
lysin motif receptor-like kinase 3
- NFP
nodulation factor perception
- NSP
nodulation-signaling pathway
- CRE1
cytokinin response1
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24836
References
- 1.Timmers ACJ, Auriac MC, Truchet G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development. 1999;126:3617–28. doi: 10.1242/dev.126.16.3617. [DOI] [PubMed] [Google Scholar]
- 2.Sieberer BJ, Timmers AC, Emons AM. Nod factors alter the microtubule cytoskeleton in Medicago truncatula root hairs to allow root hair reorientation. Mol Plant Microbe Interact. 2005;18:1195–204. doi: 10.1094/MPMI-18-1195. [DOI] [PubMed] [Google Scholar]
- 3.Cárdenas L, Thomas-Oates JE, Nava N, López-Lara IM, Hepler PK, Quinto C. The role of nod factor substituents in actin cytoskeleton rearrangements in Phaseolus vulgaris. Mol Plant Microbe Interact. 2003;16:326–34. doi: 10.1094/MPMI.2003.16.4.326. [DOI] [PubMed] [Google Scholar]
- 4.de Ruijter NCA, Bisseling T, Emons AMC. Rhizobium Nod factors induce an increase in subapical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol Plant Microbe Interact. 1999;12:829–32. doi: 10.1094/MPMI.1999.12.9.829. [DOI] [Google Scholar]
- 5.Oldroyd GE, Murray JD, Poole PS, Downie JA. The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet. 2011;45:119–44. doi: 10.1146/annurev-genet-110410-132549. [DOI] [PubMed] [Google Scholar]
- 6.Oldroyd GE, Downie JA. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol. 2008;59:519–46. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 7.Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–4. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- 8.Gleason C, Chaudhuri S, Yang T, Muñoz A, Poovaiah BW, Oldroyd GE. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature. 2006;441:1149–52. doi: 10.1038/nature04812. [DOI] [PubMed] [Google Scholar]
- 9.Tirichine L, Imaizumi-Anraku H, Yoshida S, Murakami Y, Madsen LH, Miwa H, et al. Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature. 2006;441:1153–6. doi: 10.1038/nature04862. [DOI] [PubMed] [Google Scholar]
- 10.Tirichine L, James EK, Sandal N, Stougaard J. Spontaneous root-nodule formation in the model legume Lotus japonicus: a novel class of mutants nodulates in the absence of rhizobia. Mol Plant Microbe Interact. 2006;19:373–82. doi: 10.1094/MPMI-19-0373. [DOI] [PubMed] [Google Scholar]
- 11.Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, et al. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315:104–7. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- 12.Murray JD. Invasion by invitation: rhizobial infection in legumes. Mol Plant Microbe Interact. 2011;24:631–9. doi: 10.1094/MPMI-08-10-0181. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Rizzo S, Crespi M, Frugier F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell. 2006;18:2680–93. doi: 10.1105/tpc.106.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortier V, Holsters M, Goormachtig S. Never too many? How legumes control nodule numbers. Plant Cell Environ. 2012;35:245–58. doi: 10.1111/j.1365-3040.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 15.Soto MJ, Domínguez-Ferreras A, Pérez-Mendoza D, Sanjuán J, Olivares J. Mutualism versus pathogenesis: the give-and-take in plant-bacteria interactions. Cell Microbiol. 2009;11:381–8. doi: 10.1111/j.1462-5822.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- 16.Haag AF, Baloban M, Sani M, Kerscher B, Pierre O, Farkas A, et al. Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biol. 2011;9:e1001169. doi: 10.1371/journal.pbio.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haag AF, Arnold MF, Myka KK, Kerscher B, Dall’angelo S, Zanda M, et al. Molecular insights into bacteroid development during Rhizobium-legume symbiosis. FEMS Microbiol Rev. 2012 doi: 10.1111/1574-6976.2012.12003. [DOI] [PubMed] [Google Scholar]
- 18.Pii Y, Molesini B, Masiero S, Pandolfini T. The non-specific lipid transfer protein N5 of Medicago truncatula is implicated in epidermal stages of rhizobium-host interaction. BMC Plant Biol. 2012;12:233. doi: 10.1186/1471-2229-12-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pii Y, Astegno A, Peroni E, Zaccardelli M, Pandolfini T, Crimi M. The Medicago truncatula N5 gene encoding a root-specific lipid transfer protein is required for the symbiotic interaction with Sinorhizobium meliloti. Mol Plant Microbe Interact. 2009;22:1577–87. doi: 10.1094/MPMI-22-12-1577. [DOI] [PubMed] [Google Scholar]
- 20.Marshall E, Costa LM, Gutierrez-Marcos J. Cysteine-rich peptides (CRPs) mediate diverse aspects of cell-cell communication in plant reproduction and development. J Exp Bot. 2011;62:1677–86. doi: 10.1093/jxb/err002. [DOI] [PubMed] [Google Scholar]
- 21.Boutrot F, Chantret N, Gautier MF. Genome-wide analysis of the rice and Arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genomics. 2008;9:86. doi: 10.1186/1471-2164-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang NJ, Lee CC, Cheng CS, Lo WC, Yang YF, Chen MN, et al. Construction and analysis of a plant non-specific lipid transfer protein database (nsLTPDB) BMC Genomics. 2012;13(Suppl 1):S9. doi: 10.1186/1471-2164-13-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature. 2002;419:399–403. doi: 10.1038/nature00962. [DOI] [PubMed] [Google Scholar]
- 24.Haney CH, Long SR. Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc Natl Acad Sci USA. 2010;107:478–83. doi: 10.1073/pnas.0910081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauser L, Roussis A, Stiller J, Stougaard J. A plant regulator controlling development of symbiotic root nodules. Nature. 1999;402:191–5. doi: 10.1038/46058. [DOI] [PubMed] [Google Scholar]


