Abstract
Context
Given the higher risk of AIDS-defining malignancies that include Kaposi sarcoma (KS), certain non-Hodgkin lymphomas (NHLs), and cervical cancer in persons with human immunodeficiency virus (HIV) infection, the HIV epidemic has likely contributed to the overall numbers of these cancers in the United States.
Objective
To quantify the proportions of KS, AIDS-defining NHLs, and cervical cancer in the United States that occurred among persons with AIDS from 1980 to 2007.
Design, Setting, and Participants
The HIV/AIDS Cancer Match Study (1980–2007) linked data from 16 US HIV/AIDS and cancer registries to identify cases with and without AIDS for KS, AIDS-defining NHLs (ie, diffuse large B-cell lymphoma [DLBCL], Burkitt lymphoma [BL], and central nervous system [CNS] lymphoma), and cervical cancer. Using linked data, we derived cancer rates for persons with and without AIDS. To estimate national counts, the rates were applied to national AIDS surveillance and US Census data.
Main Outcome Measure
Proportion of AIDS-defining malignancies in the United States occurring in persons with AIDS.
Results
In the United States, an estimated 79.0% (95% confidence interval [CI], 78.6%–79.4%) of 85 922 KS cases, 5.5% (95% CI, 5.3%–5.6%) of 383 095 DLBCL cases, 19.4% (95% CI, 17.8%–21.1%) of 17 780 BL cases, 26.2% (95% CI, 25.2%–27.1%) of 28 259 CNS lymphoma cases, and 0.41% (95% CI, 0.36%–0.46%) of 386 166 cervical cancer cases occurred among persons with AIDS during 1980–2007. The proportion of KS and AIDS-defining NHLs in persons with AIDS peaked in the early 1990s (1990–1995: KS, 89.0% [95%CI, 88.6%–89.3%]; DLBCL, 9.5% [95%CI, 9.2%–9.8%]; BL, 27.4% [95% CI, 25.0%–29.7%]; and CNS lymphoma, 47.2% [95% CI, 45.7%–48.7%]; all P<.001 [compared with 1980–1989]) and then declined (2001–2007: KS, 67.0% [95% CI, 64.5%–69.4%]; DLBCL, 4.3% [95% CI, 3.9%–4.6%]; BL, 20.8% [95% CI, 17.2%–24.3%]; and CNS lymphoma, 12.3% [95% CI, 10.1%–14.4%]; all P<.001 [compared with 1990–1995]). The proportion of cervical cancers in persons with AIDS increased overtime (1980–1989: 0.11% [95% CI, 0.08%–0.13%]; 2001–2007: 0.69% [95% CI, 0.49%–0.89%]; P<.001).
Conclusions
In the United States, the estimated proportions of AIDS-defining malignancies that occurred among persons with AIDS were substantial, particularly for KS and some NHLs. Except for cervical cancer, the proportions of AIDS-defining malignancies occurring among persons with AIDS peaked in the mid-1990s and then declined.
Persons with human immunodeficiency virus (HIV) infection are at risk for numerous medical complications, particularly AIDS, which can result in death. Early in the HIV epidemic, persons with AIDS had markedly increased risks of Kaposi sarcoma (KS) (50 000-fold risk compared with the general population), cervical cancer (8-fold), and 3 subtypes of non-Hodgkin lymphoma (NHL): diffuse large B-cell lymphoma (DLBCL) (98-fold), Burkitt lymphoma (BL) (57-fold), and central nervous system (CNS) lymphoma (5000-fold).1 These 5 malignancies are included in the 1993 US Centers for Disease Control and Prevention (CDC) AIDS definition.2 Diagnostic classification of lymphomas has evolved during recent years, and immunoblastic lymphoma as noted in the CDC definition is now considered a variant of DLBCL.3
Highly active antiretroviral therapy (HAART) was introduced in 1996, leading to improved immune status and decreased AIDS risk in persons with HIV. Despite a 70% decline in incidence of AIDS-defining cancers (ADCs), particularly KS and NHL,4,5 risk remains elevated in persons with HIV.1 Although AIDS prevalence in the US general population is low (0.15% in 2007),6 the risk of certain ADCs is so strong that the HIV epidemic has likely influenced national cancer counts and trends. During the 1980s and early 1990s, US general population rates of KS increased 30-fold, NHL rates doubled, and CNS lymphoma rates increased 17-fold.7–9 Incidence rates of these cancers peaked in the early 1990s and then declined, mirroring AIDS incidence trends.7–9
The contribution of the HIV epidemic to the total numbers of ADCs in the United States has not been previously quantified. A better understanding of this association can provide information on the epidemiology and etiology of these cancers and help target HIV testing and clinical care.10 The objective of our study was to estimate the proportions of KS, DLBCL, BL, CNS lymphoma, and cervical cancer in the United States that occurred among persons with AIDS from 1980 to 2007.
METHODS
We estimated the total number of US cases of ADCs during 1980–2007 in 2 steps (eFigure, available at http://www.jama.com). First, we calculated cancer incidence rates in persons with and without AIDS. Second, we applied these rates to national estimates of the AIDS and non-AIDS populations.
To calculate cancer rates, we used data from the HIV/AIDS Cancer Match (HACM) Study, which links 16 US population-based HIV/AIDS and cancer registries during 1980–2007.11,12 A probabilistic algorithm matches records by name, Social Security number, sex, birth date, death date, and race. Anonymized data only are retained. The HACM Study received approval from institutional review boards at participating registries or received exemption. Each institutional review board waived the need for informed consent. The study was exempted from human subjects review by the National Cancer Institute. Race/ethnicity information is collected by cancer and AIDS registries for the purpose of public health surveillance either passively from the sources who report cases or through active case finding in which demographic information is abstracted from medical records.
Data were included from the following registries and time periods: Colorado (1988–2007); Connecticut (1980–2005); Florida (1981–2002); Georgia (1995–2007); Illinois (1986–2004); Massachusetts (1982–2002); Maryland (1992–2007); Michigan (1985–2003); New Jersey (1980–2007); Texas (1995–2003); New York, New York (1980–2000); Los Angeles (1980–2002), San Diego (1988–2000), and San Francisco (1980–2001), California; Seattle, Washington (1980–2005); and Washington, DC (1996–2006).11 Because it is standard practice for cancer registries to delay the release of data until complete surveillance is confirmed, more recent cancer registry data are not available in the HACM Study. Only 1 of the registries included in the HACM Study had data from 2008, and rates estimated from this registry alone would be based on sparse and unrepresentative data; thus, we selected 2007 as our last year of analysis.
Statistical Analysis
The cancer registries in the HACM Study provided data on all incident cancers occurring in each registry area. Through the linkage, we identified persons with ADCs reported to the cancer registries who also had AIDS reported to the HIV/AIDS registries. Because we focused on ADCs, all individuals who matched to the HIV/AIDS registry had AIDS by definition. For each cancer of interest, we calculated the number of cases with and without AIDS in strata of calendar year, age group (0–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years), race, and sex.
Because the sensitivity of the match between HACM HIV/AIDS and cancer registries is imperfect, the number of cancer cases with AIDS is an underestimate. We estimated sensitivity to correct for this underestimation. In the calendar period before the AIDS epidemic (1975–1979), zero KS cases occurred among 20- to 29-year-olds in data from the US Surveillance, Epidemiology, and End Results (SEER) program.13 Based on these results, we expected 100% of KS cases occurring in 20- to 29-year-olds during the HIV epidemic to have AIDS. However, among 20- to 29-year-olds in the HACM Study, only 81.1% of KS cases during 1980–2007 matched to HIV/AIDS registries. Therefore, we estimated the sensitivity of our match to be 81.1%. For each cancer, we corrected for imperfect sensitivity by dividing the number of cases with AIDS by 0.811 in each cell defined by the preceding demographic characteristics. We then subtracted the corrected number of cases with AIDS from the total number of cases in that cell to estimate the corrected number of cases without AIDS.
To calculate cancer incidence rates for persons with AIDS, we used the corrected number of cases with AIDS and divided by the person-time among persons with AIDS (total, 2.07 million person-years), based on HIV/AIDS registry data, in cells defined by sex, age group, race, calendar year, and HIV transmission risk group (men who have sex with men [MSM], injection drug users [IDUs], MSM/IDUs, heterosexual contact, and other). For each person with AIDS, person-time began at the latter of the date of AIDS diagnosis or the start of AIDS and cancer registry coverage (beginning date of the linked data) and ended at the earliest of date of death, end of registry coverage, or December 31, 2007.
We note that it is difficult to define cancer incidence rates in persons with AIDS when the ADC is the AIDS-defining event. However, it is appropriate to include cancers that occurred as AIDS-defining conditions and those that occurred subsequently in persons with AIDS in our calculations because both fractions contribute to the cancer burden. We treated the cancer counts and person-time in exactly the same manner both in calculating rates and, as described later in this section, in assessing the AIDS population to whom we applied the rates, so our estimated counts of cancers in persons with AIDS are not biased by the inclusion of cancers occurring at AIDS onset.
For estimating incidence rates for persons without AIDS, the corrected number of cases without AIDS was divided by the person-time among persons without AIDS, in cells of sex, age group, race, and calendar year (total, 2.17 billion person-years). Person-time without AIDS was estimated by subtracting the person-time in persons with AIDS from person-time in the general population in the cancer registry areas (obtained from the US Census).14 Person-time in the general population was estimated annually from the first year of AIDS and cancer registry coverage to the last year of registry coverage or 2007.
To quantify the number of persons living with AIDS in the United States during 1980–2007, we used data from the CDC HIV/AIDS surveillance system.15 AIDS cases have been reported to the CDC by 50 US states and the District of Columbia since the onset of the epidemic. The CDC estimated the number of persons diagnosed and living with AIDS at the end of each calendar year, with statistical adjustments made for reporting delays and cases with missing risk factor information.16,17 To quantify the number of persons living without AIDS, we subtracted annual CDC AIDS estimates from US Census estimates14 for the US population during 1980–2007.
To estimate cancer counts in persons with AIDS in the United States, we then applied cancer incidence rates in persons with AIDS to CDC counts of persons with AIDS, stratified by sex, age group, race, calendar year, and HIV transmission risk group. Likewise, to estimate cancer counts in persons without AIDS, we applied cancer incidence rates in persons without AIDS to counts of persons without AIDS in the United States, stratified by sex, age group, race, and calendar year. We estimated the proportion of cancers in the United States occurring in persons with AIDS by dividing the cases with AIDS by the total number of cases. Cancer incidence rates were estimated separately for each cancer type, and 1 person could contribute cases to the overall US count for more than 1 ADC. For example, the estimated US total for KS includes both persons who only had KS and persons who had both KS and another ADC, but these 2 groups are not distinguished. Of the 42 891 persons with AIDS who had an ADC in the HACM Study, 5.7% had more than 1 type of ADC during follow-up.
Our approach shows that the proportion of total US cases with AIDS is influenced by 3 main components: cancer incidence rates in persons with AIDS, cancer incidence rates in persons without AIDS, and the proportion of the US population with AIDS (ie, AIDS prevalence). We tabulated these components across calendar periods, age groups, and sex to illustrate how their variation was associated with the proportion of cancer cases with AIDS. Calendar periods were based on the availability of antiretroviral therapies: 1980–1989 (largely no therapy), 1990–1995 (monotherapy and combination therapy), 1996–2000 (early HAART), and 2001–2007 (late HAART). AIDS prevalence was estimated in strata by dividing the total number of person-years with AIDS from CDC surveillance data15 by the total number of person-years in the United States from US Census data.14
Cancer incidence rates for persons with and without AIDS were standardized to the 2000 US population by sex, age group, and race, based on US Census estimates.14 For persons with AIDS, we excluded the period immediately after AIDS onset (0–3 months) in the rates presented. Because ADCs are AIDS-defining events, cases occurring at AIDS onset can lead to artificially high incidence rates, which would overestimate relative risks in persons with AIDS compared with the general population.12 Also, cancers may be reported to cancer registries after a short delay; thus, cancers that are AIDS-defining events may appear to follow the AIDS diagnosis by a few months. Further, persons with HIV may undergo increased medical surveillance at the time of AIDS diagnosis, which leads to an increased number of prevalent cancers diagnosed during this period. The exclusion of the AIDS onset period from incidence rate calculations is similar to an approach used previously.12,18 Nonetheless, as noted earlier in this section, the cases at AIDS onset were appropriately included in calculating total cancer counts.
In a subanalysis, the evaluation of KS, DLBCL, and CNS lymphoma was restricted to those diagnosed in 2001–2007, with the proportion of cancer cases with AIDS being compared across age groups (BL and cervical cancer were not included). The purpose of this analysis was to assess whether the same age patterns observed in our overall analysis were similar to those in the most recent calendar period, as the number of older persons with HIV is increasing.
We calculated Poisson-based confidence intervals (CIs) for incidence rates and cancer counts in persons with AIDS. We used Poisson process theory to obtain variances of the estimated number of cancers in persons without AIDS, the total number of cancers, and the proportions. All CIs were 2-sided with an a priori significance level of P<.05. For comparison of differences between calendar periods, age groups, and sexes, we calculated χ2 P values for differences in proportions using asymptotic normal theory. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina).
RESULTS
In the United States during 1980–2007, there were an estimated 85 922 cases of KS (95% CI, 84 917–86 927), 383 095 cases of DLBCL (95% CI, 380 621–385 568), 17 780 cases of BL (95% CI, 17 340–18 220), 28 259 cases of CNS lymphoma (95% CI, 27 840–28 678), and 386 166 cases of cervical cancer (95% CI, 382 777–389 554). The estimated proportions of total US cases of ADCs that occurred among persons with AIDS were 79.0% (95% CI, 78.6%–79.4%) for KS, 5.5% (95% CI, 5.3%–5.6%) for DLBCL, 19.4% (95% CI, 17.8%–21.1%) for BL, 26.2% (95% CI, 25.2%–27.1%) for CNS lymphoma, and 0.41% (95% CI, 0.36%–0.46%) for cervical cancer.
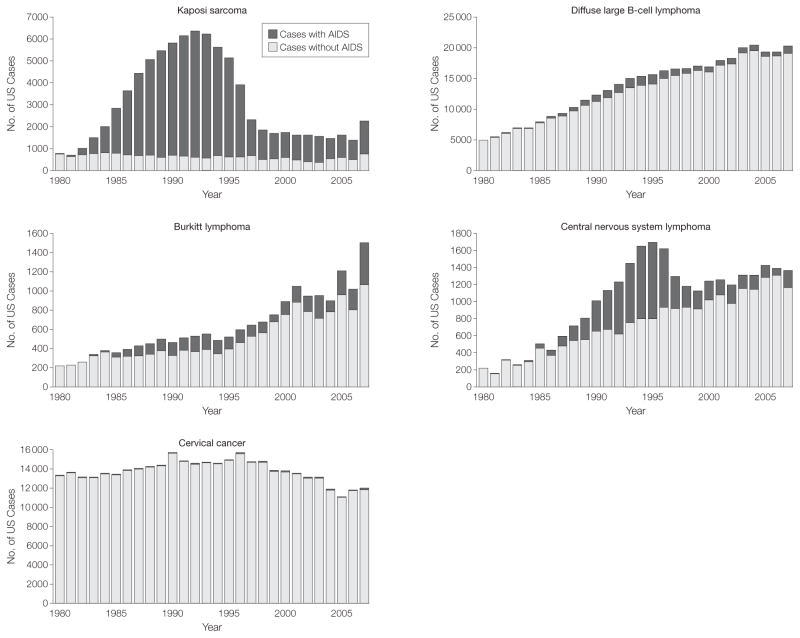
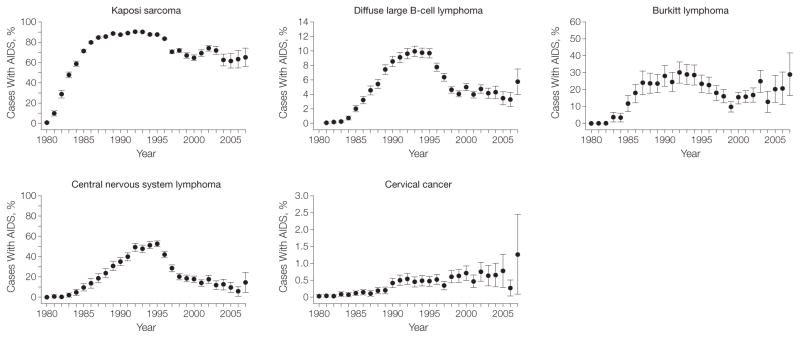
Across calendar years, the estimated proportion of US KS cases with AIDS rose steeply from 0.83% (95% CI, 0.02%–1.7%) in 1980 to 89.0% (95% CI, 88.6%–89.3%) during 1990–1995 (P<.001), before declining to 67.0% (95% CI, 64.5%–69.4%; P<.001 [compared with 1990–1995]) in the most recent calendar period (TABLE 1, FIGURE 1, and FIGURE 2). As shown in Table 1, the peak in the proportion of KS cases with AIDS was caused by the high KS incidence rate in persons with AIDS during 1990–1995 (incidence, 1100 per 100 000 person-years), and the 8-fold increase in AIDS prevalence that occurred between 1980–1989 and 1990–1995. Despite a continued rise in AIDS prevalence, the subsequent decline in the proportion of KS cases with AIDS was driven by a drop in KS incidence among persons with AIDS (incidence, 83.0 per 100 000 person-years in 2001–2007). DLBCL, BL, and CNS lymphoma demonstrated similar patterns, peaking in 1990–1995 with 9.5% (95% CI, 9.2%–9.8%; P<.001 [compared with 1980–1989]), 27.4% (95% CI, 25.0%–29.7%; P<.001 [compared with 1980–1989]), and 47.2% (95% CI, 45.7%–48.7%; P<.001 [compared with 1980–1989]) of cases occurring among persons with AIDS, respectively (Table 1, Figure 1, and Figure 2). An additional factor contributing to the pattern for NHLs was the increase over time in the incidence among persons without AIDS. The proportion of cervical cancer cases with AIDS remained low across calendar years (Figure 1 and Figure 2) but increased over time from 0.11% (95% CI, 0.08%–0.13%) in 1980–1989 to 0.69% (95% CI, 0.49%–0.89%) in 2001–2007 (P<.001 for 1980–1989 compared with 2001–2007), likely resulting in part from rising AIDS prevalence.
Table 1.
Estimated Proportion of US Cases of Kaposi Sarcoma, Diffuse Large B-Cell Lymphoma, Burkitt Lymphoma, Central Nervous System Lymphoma, and Cervical Cancer Among Persons With AIDS by Calendar Period
| Average US AIDS Prevalence, % | Cancer Incidence Rate per 100 000 Person-Years (95% CI)a
|
Estimated Total US Cancer Cases, No. (95% CI)b
|
Proportion of Cancer Cases With AIDS, % (95% CI) | P Valuec | |||
|---|---|---|---|---|---|---|---|
| Persons With AIDS | Persons Without AIDS | Persons With AIDS | Persons Without AIDS | ||||
| Kaposi sarcoma | |||||||
| 1980–1989 | 0.007 | 2363 (2029–2697) | 0.36 (0.34–0.38) | 20 194 (19 782–20 606) | 7298 (7085–7510) | 73.5 (72.9–74.0) | <.001 |
|
| |||||||
| 1990–1995 | 0.06 | 1100 (1053–1147) | 0.27 (0.26–0.29) | 31 449 (30 910–31 988) 3907 (3721–4092) | 89.0 (88.6–89.3) | [Reference] | |
|
| |||||||
| 1996–2000 | 0.10 | 230.0 (171.3–288.8) | 0.22 (0.21–0.24) | 8520 (8254–8786) | 3014 (2865–3163) | 73.9 (73.0–74.7) | <.001 |
|
| |||||||
| 2001–2007 | 0.14 | 83.0 (71.41–94.58) | 0.17 (0.15–0.18) | 7731 (7086–8377) | 3809 (3574–4045) | 67.0 (64.5–69.4) | <.001 |
|
| |||||||
| DLBCL | |||||||
| 1980–1989 | 0.007 | 446.2 (329.2–563.1) | 3.59 (3.52–3.66) | 2383 (2246–2519) | 76 032 (74 889–77 174) | 3.0 (2.9–3.2) | <.001 |
|
| |||||||
| 1990–1995 | 0.06 | 542.2 (457.9–626.6) | 5.18 (5.11–5.25) | 8130 (7868–8391) | 77 495 (76 682–78 308) | 9.5 (9.2–9.8) | [Reference] |
|
| |||||||
| 1996–2000 | 0.10 | 213.9 (175.6–252.3) | 5.77 (5.70–5.84) | 4641 (4448–4834) | 78 805 (78 010–79 599) | 5.6 (5.3–5.8) | <.001 |
|
| |||||||
| 2001–2007 | 0.14 | 118.1 (85.7–150.6) | 6.15 (6.06–6.23) | 5787 (5255–6319) | 129 823 (128 001–131 644) | 4.3 (3.9–4.6) | <.001 |
|
| |||||||
| Burkitt lymphoma | |||||||
| 1980–1989 | 0.007 | 91.44 (0–195.9) | 0.13 (0.12–0.14) | 467 (406–527) | 3092 (2968–3215) | 13.1 (11.5–14.7) | <.001 |
|
| |||||||
| 1990–1995 | 0.06 | 62.91 (29.63–96.18) | 0.15 (0.13–0.16) | 840 (755–925) | 2229 (2089–2369) | 27.4 (25.0–29.7) | [Reference] |
|
| |||||||
| 1996–2000 | 0.10 | 18.97 (6.07–31.86) | 0.22 (0.21–0.23) | 570 (502–638) | 2996 (2884–3108) | 16.0 (14.3–17.7) | <.001 |
|
| |||||||
| 2001–2007 | 0.14 | 31.68 (1.57–61.79) | 0.29 (0.27–0.30) | 1575 (1247–1903) | 6012 (5793–6231) | 20.8 (17.2–24.3) | .002 |
|
| |||||||
| CNS lymphoma | |||||||
| 1980–1989 | 0.007 | 229.9 (137.0–322.7) | 0.17 (0.16–0.18) | 666 (592–740) | 3673 (3553–3793) | 15.4 (13.8–16.9) | <.001 |
|
| |||||||
| 1990–1995 | 0.06 | 292.8 (241.5–344.0) | 0.29 (0.27–0.30) | 3859 (3675–4043) | 4320 (4155–4485) | 47.2 (45.7–48.7) | [Reference] |
|
| |||||||
| 1996–2000 | 0.10 | 90.14 (68.42–111.9) | 0.35 (0.33–0.36) | 1729 (1611–1847) | 4739 (4593–4885) | 26.7 (25.3–28.2) | <.001 |
|
| |||||||
| 2001–2007 | 0.14 | 26.11 (16.66–35.57) | 0.38 (0.36–0.40) | 1138 (916–1360) | 8134 (7944–8325) | 12.3 (10.1–14.4) | <.001 |
|
| |||||||
| Cervical cancerd | |||||||
| 1980–1989 | 0.001 | 13.02 (0–27.41) | 12.34 (12.22–12.46) | 147 (115–179) | 136 576 (135 542–137 610) | 0.11 (0.08–0.13) | <.001 |
|
| |||||||
| 1990–1995 | 0.02 | 55.72 (31.42–80.02) | 11.62 (11.45–11.79) | 433 (378–488) | 89 166 (87 920–90 412) | 0.48 (0.42–0.54) | [Reference] |
|
| |||||||
| 1996–2000 | 0.04 | 61.40 (41.82–80.98) | 10.41 (10.29–10.53) | 411 (354–468) | 72 595 (71 788–73 402) | 0.56 (0.49–0.64) | .11 |
|
| |||||||
| 2001–2007 | 0.06 | 48.76 (29.80–67.72) | 8.55 (8.41–8.70) | 600 (427–773) | 86 238 (83 378–89 098) | 0.69 (0.49–0.89) | .05 |
Abbreviations: CI, confidence interval; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; KS, Kaposi sarcoma; NHL, non-Hodgkin lymphoma.
In persons with AIDS, incidence rates were estimated starting at 4 months after AIDS onset by dividing case counts in persons with AIDS by person-time in persons with AIDS. In persons without AIDS, incidence rates were estimated by dividing case counts in persons without AIDS by person-time in persons without AIDS. All rates were estimated with data from the HIV/AIDS Cancer Match Study and were standardized to the 2000 US population by sex, age, and race.
Cancer counts in persons with AIDS were estimated by applying cancer incidence rates in persons with AIDS to national AIDS population estimates from the CDC HIV/AIDS surveillance system. Cancer counts in persons without AIDS were estimated by applying cancer incidence rates in persons without AIDS to the US population without AIDS, derived from the US Census after subtracting AIDS cases.
For each cancer, P values were calculated with the χ2 test; the proportion with AIDS was compared with the 1990–1995 calendar period.
AIDS prevalence estimates and cervical cancer incidence rates were restricted to females.
Figure 1.
Number of AIDS-defining Cancer Cases in the United States in Persons With and Without AIDS by Calendar Year
Estimated counts of each malignancy that occurred among persons with and without AIDS in the United States by calendar year during 1980–2007.
Figure 2.
Proportion of US AIDS-defining Cancer Cases With AIDS by Calendar Year
Estimated proportion of cases of each malignancy that occurred among persons with AIDS in the United States by calendar year during 1980–2007. Error bars indicate 95% confidence intervals.
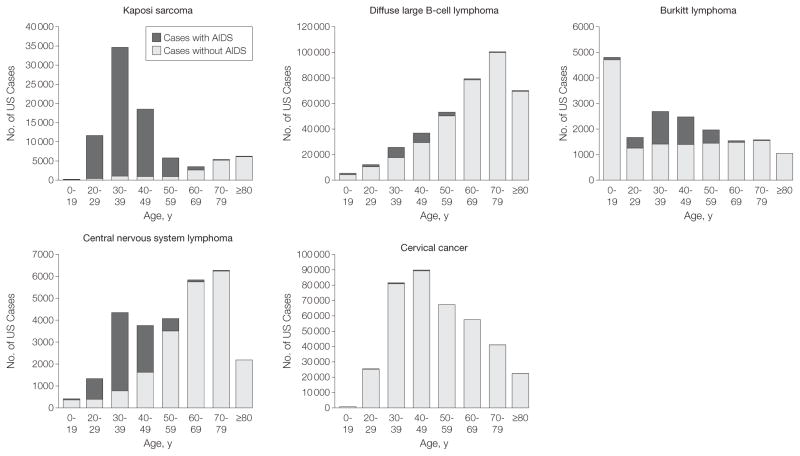
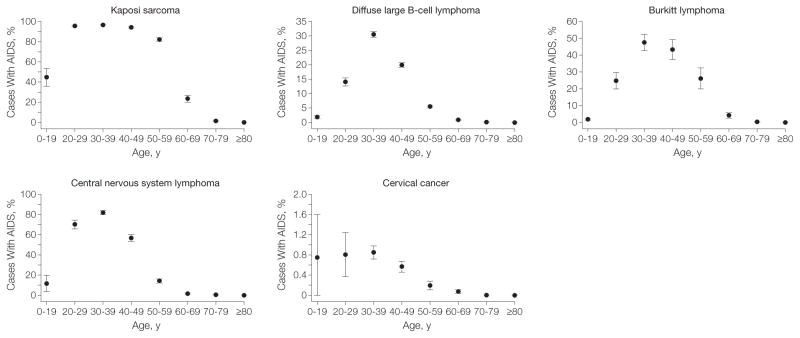
Across age groups, individuals 60 years and older had the lowest proportions with AIDS for each malignancy (all P values <.001 [compared with 30-to 59-year-olds]) (TABLE 2, FIGURE 3, and FIGURE 4). This pattern was due to the low prevalence of AIDS among older persons in the United States (0.02% averaged across calendar years), rising cancer rates with age in persons without AIDS, and decreasing rates with age in persons with AIDS for some cancers (ie, KS, BL, and CNS lymphoma). Similarly higher proportions of cases with AIDS occurred among 0- to 29-year-olds and 30- to 59-year-olds for KS (94.6% [95% CI, 93.9%–95.4%] and 94.5% [95% CI, 94.2%–94.9%], respectively; P=.81), and CNS lymphoma (56.4% [95% CI, 52.6%–60.2%] and 51.5% [95% CI, 49.8%–53.1%]), respectively; P= .02). Although AIDS prevalence in 0- to 29-year-olds is low (0.01%), the incidence of KS and CNS lymphoma was higher among persons with AIDS in this age group, and close to zero in persons without AIDS, resulting in a proportion with AIDS similar to that in 30- to 59-year-olds, who had a higher AIDS prevalence (0.15%).
Table 2.
Estimated Proportion of US Cases of Kaposi Sarcoma, Diffuse Large B-Cell Lymphoma, Burkitt Lymphoma, Central Nervous System Lymphoma, and Cervical Cancer Among Persons With AIDS by Age Group
| Average US AIDS Prevalence, % | Cancer Incidence Rate per 100 000 Person-Years (95% CI)a
|
Estimated Total US Cancer Cases, No. (95% CI)b
|
Proportion of Cancer Cases With AIDS, % (95% CI) | P Valuec | |||
|---|---|---|---|---|---|---|---|
| Persons With AIDS | Persons Without AIDS | Persons With AIDS | Persons Without AIDS | ||||
| Kaposi sarcoma | |||||||
| 0–29 y | 0.01 | 457.7 (417.6–497.7) | 0.020 (0.016–0.023) | 11 245 (10 807–11 683) | 636 (523–750) | 94.6 (93.9–95.4) | .82 |
|
| |||||||
| 30–59 y | 0.15 | 672.1 (656.3–687.9) | 0.11 (0.10–0.12) | 55 734 (54 877–56 590) | 3217 (2919–3514) | 94.5 (94.2–94.9) | [Reference] |
|
| |||||||
| ≥60 y | 0.02 | 251.7 (78.9–424.5) | 1.24 (1.22–1.27) | 916 (767–1065) | 14 175 (13 938–14 411) | 6.1 (5.1–7.0) | <.001 |
|
| |||||||
| DLBCL | |||||||
| 0–29 y | 0.01 | 187.4 (144.7–230.1) | 0.49 (0.47–0.50) | 1826 (1641–2011) | 15 810 (15 422–16 198) | 10.4 (9.4–11.3) | <.001 |
|
| |||||||
| 30–59 y | 0.15 | 331.2 (315.0–347.4) | 3.51 (3.44–3.58) | 18 222 (17 631–18 814) | 97 784 (96 009–99 559) | 15.7 (15.3–16.2) | [Reference] |
|
| |||||||
| ≥60 y | 0.02 | 256.5 (164.9–348.2) | 21.2 (21.1–21.3) | 892 (739–1045) | 248 560 (246 943–250 178) | 0.36 (0.30–0.42) | <.001 |
|
| |||||||
| Burkitt lymphoma | |||||||
| 0–29 y | 0.01 | 56.27 (23.30–89.25) | 0.18 (0.18–0.19) | 505 (402–609) | 5975 (5819–6132) | 7.8 (6.3–9.3) | <.001 |
|
| |||||||
| 30–59 y | 0.15 | 28.03 (22.91–33.15) | 0.14 (0.14–0.15) | 2874 (2540–3209) | 4266 (4016–4515) | 40.3 (37.0–43.5) | [Reference] |
|
| |||||||
| ≥60 y | 0.02 | 13.31 (3.79–22.83) | 0.33 (0.33–0.34) | 72 (45–98) | 4088 (3995–4181) | 1.7 (1.1–2.4) | <.001 |
|
| |||||||
| CNS lymphoma | |||||||
| 0–29 y | 0.01 | 145.1 (103.3–186.9) | 0.024 (0.021–0.026) | 985 (869–1100) | 760 (691–830) | 56.4 (52.6–60.2) | .02 |
|
| |||||||
| 30–59 y | 0.15 | 152.0 (141.9–162.1) | 0.21 (0.20–0.22) | 6280 (5987–6574) | 5923 (5664–6181) | 51.5 (49.8–53.1) | [Reference] |
|
| |||||||
| ≥60 y | 0.02 | 30.66 (13.05–48.27) | 1.18 (1.17–1.20) | 127 (71–183) | 14 184 (14 017–14 351) | 0.89 (0.50–1.28) | <.001 |
|
| |||||||
| Cervical cancerd | |||||||
| 0–29 y | 0.009 | 23.75 (10.66–36.85) | 1.56 (1.52–1.59) | 210 (99–322) | 25 974 (25 291–26 658) | 0.80 (0.38–1.2) | .27 |
|
| |||||||
| 30–59 y | 0.06 | 92.22 (74.00–110.4) | 16.7 (16.6–16.9) | 1336 (1181–1491) | 237 367 (234 101–240 634) | 0.56 (0.49–0.62) | [Reference] |
|
| |||||||
| ≥60 y | 0.006 | 42.59 (0.29–84.89) | 17.7 (17.6–17.8) | 45 (20–69) | 121 233 (120 672–121 794) | 0.04 (0.02–0.06) | <.001 |
Abbreviations: CI, confidence interval; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; KS, Kaposi sarcoma; NHL, non-Hodgkin lymphoma.
In persons with AIDS, incidence rates were estimated starting at 4 months after AIDS onset by dividing case counts in persons with AIDS by person-time in persons with AIDS. In persons without AIDS, incidence rates were estimated by dividing case counts in persons without AIDS by person-time in persons without AIDS. All rates were estimated with data from the HIV/AIDS Cancer Match Study and were standardized to the 2000 US population by sex, age, and race.
Cancer counts in persons with AIDS were estimated by applying cancer incidence rates in persons with AIDS to national AIDS population estimates from the CDC HIV/AIDS surveillance system. Cancer counts in persons without AIDS were estimated by applying cancer incidence rates in persons without AIDS to the US population without AIDS, derived from the US Census after subtracting AIDS cases.
For each cancer, P values were calculated with the χ2 test; the proportion with AIDS was compared with the 30- to 59-year-old age group.
AIDS prevalence estimates and cervical cancer incidence rates were restricted to females.
Figure 3.
Number of AIDS-defining Cancer Cases in the United States in Persons With and Without AIDS, by Age Group
Estimated counts of each malignancy that occurred among persons with and without AIDS in the United States by age group.
Figure 4.
Proportion of US AIDS-defining Cancer Cases With AIDS by Age Group
Estimated proportion of cases of each malignancy that occurred among persons with AIDS in the United States by age group. Error bars indicate 95% confidence intervals.
For DLBCL and BL, the highest proportion with AIDS occurred in 30-to 59-year-olds (15.7% [95% CI, 15.3%–16.2%; P<.001 compared with 0- to 29-year-olds and with ≥60-year-olds] and 40.3% [95% CI, 37.0%–43.5%, respectively; P<.001 compared with 0- to 29-year-olds and with ≥60-year-olds]). Although the proportion of cervical cancers with AIDS was higher in younger than older females (P<.001 for ≥60-year-olds vs 30- to 59-year-olds), this proportion was low even among 0-to 29-year-olds and 30- to 59-year-olds (0.80% [95% CI, 0.38%–1.2%] and 0.56% [95% CI, 0.49%–0.62%], respectively).
When we restricted our analysis to 2001–2007, we observed age patterns similar to those of the overall analysis. The proportion of cases with AIDS was similar among 0- to 29-year-olds and 30-to 59-year-olds for KS (87.4% [95% CI, 81.6%–93.1%] and 85.5% [95% CI, 82.9%–88.2%], respectively; P=.57) and CNS lymphoma (30.7% [95% CI, 17.0%–44.3%] and 29.2% [95% CI, 24.5%–33.9%], respectively; P = .84). For DLBCL, the proportion of cases with AIDS was greater among 30- to 59-year-olds (12.5% [95% CI, 11.4%–13.6%]) than 0- to 29-year-olds (6.1% [95% CI, 3.4%–8.7%]; P<.001). Despite a temporal increase in AIDS prevalence among those 60 years and older (1980–1989: 0.0008%; 2001–2007: 0.05%), we did not observe a corresponding increase in the proportion of malignancies with AIDS in this age group: eg, for KS, 6.5% (95% CI, 2.1%–11.0%) with AIDS in 2001–2007; DLBCL, 0.42% (95% CI, 0.26%–0.57%); and CNS lymphoma, 0.79% (95% CI, 0.0%–1.7%).
Stratified by sex (TABLE 3), the proportion of malignancies with AIDS was higher in males than females for KS (84.2% [95% CI, 83.9%–84.6%] vs 17.0% [95% CI, 15.6%–18.3%]; P<.001), DLBCL (9.4% [95% CI, 9.2%–9.7%] vs 0.93% [95% CI, 0.84%–1.0%]; P<.001), BL (24.4% [95% CI, 22.3%–26.5%] vs 5.3% [95% CI, 3.9%–6.8%]; P<.001), and CNS lymphoma (40.3% [95% CI, 39.0%–41.6%] vs 5.4% [95% CI, 4.7%–6.2%]; P<.001). These differences were largely driven by US AIDS prevalence, which was nearly 4 times higher in males than females (0.11% vs 0.03%, respectively). Further, for each malignancy, the incidence in persons with AIDS was much higher among males than females.
Table 3.
Estimated Proportion of US Cases of Kaposi Sarcoma, Diffuse Large B-Cell Lymphoma, Burkitt Lymphoma, Central Nervous System Lymphoma, and Cervical Cancer Among Persons With AIDS by Sex
| Average US AIDS Prevalence, % | Cancer Incidence Rate per 100 000 Person-Years (95% CI)a
|
Estimated Total US Cancer Cases, No. (95% CI)b
|
Proportion of Cancer Cases With AIDS, % (95% CI) | P Valuec | |||
|---|---|---|---|---|---|---|---|
| Persons With AIDS | Persons Without AIDS | Persons With AIDS | Persons Without AIDS | ||||
| Kaposi sarcoma | |||||||
| Male | 0.11 | 927.4 (895.2–959.7) | 0.37 (0.35–0.38) | 66 763 (65 796–67 731) | 12 494 (12 112–12 877) | 84.2 (83.9–84.6) | [Reference] |
|
| |||||||
| Female | 0.03 | 114.2 (56.35–172.1) | 0.16 (0.15–0.16) | 1131 (1027–1236) | 5533 (5427–5639) | 17.0 (15.6–18.3) | <.001 |
|
| |||||||
| DLBCL | |||||||
| Male | 0.11 | 323.8 (295.1–352.6) | 5.36 (5.32–5.39) | 19 266 (18 652–19 881) | 184 633 (182 595–186 671) | 9.4 (9.2–9.7) | [Reference] |
|
| |||||||
| Female | 0.03 | 195.4 (156.4–234.3) | 4.88 (4.84–4.93) | 1675 (1502–1847) | 177 521 (176 193–178 849) | 0.93 (0.84–1.0) | <.001 |
|
| |||||||
| Burkitt lymphoma | |||||||
| Male | 0.11 | 52.13 (29.07–75.18) | 0.27 (0.26–0.28) | 3203 (2859–3546) | 9920 (9630–10 211) | 24.4 (22.3–26.5) | [Reference] |
|
| |||||||
| Female | 0.03 | 23.49 (6.81–40.17) | 0.12 (0.11–0.12) | 249 (176–321) | 4408 (4304–4512) | 5.3 (3.9–6.8) | <.001 |
|
| |||||||
| CNS lymphoma | |||||||
| Male | 0.11 | 161.5 (141.3–181.7) | 0.29 (0.28–0.30) | 6771 (6464–7079) | 10 045 (9769–10 321) | 40.3 (39.0–41.6) | [Reference] |
|
| |||||||
| Female | 0.03 | 98.33 (68.08–128.6) | 0.29 (0.29–0.30) | 621 (532–709) | 10 822 (10 670–10 974) | 5.4 (4.7–6.2) | <.001 |
|
| |||||||
| Cervical cancer | |||||||
| Female | 0.03 | 55.43 (43.41–67.46) | 10.77 (10.71–10.84) | 1591 (1398–1784) | 384 575 (381 191–387 959) | 0.41 (0.36–0.46) | |
Abbreviations: CI, confidence interval; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; KS, Kaposi sarcoma; NHL, non-Hodgkin lymphoma.
In persons with AIDS, incidence rates were estimated starting at 4 months after AIDS onset by dividing case counts in persons with AIDS by person-time in persons with AIDS. In persons without AIDS, incidence rates were estimated by dividing case counts in persons without AIDS by person-time in persons without AIDS. All rates were estimated with data from the HIV/AIDS Cancer Match Study and were standardized to the 2000 US population by sex, age, and race.
Cancer counts in persons with AIDS were estimated by applying cancer incidence rates in persons with AIDS to national AIDS population estimates from the CDC HIV/AIDS surveillance system. Cancer counts in persons without AIDS were estimated by applying cancer incidence rates in persons without AIDS to the US population without AIDS, derived from the US Census after subtracting AIDS cases.
For each cancer, P values were calculated with the χ2 test; the proportion with AIDS was compared between men and women.
COMMENT
Despite an average prevalence of AIDS in the United States of only 0.07% during 1980–2007, the HIV epidemic has contributed substantially to the total number of certain malignancies. We quantified the proportion of KS, DLBCL, BL, CNS lymphoma, and cervical cancer cases that occurred among persons with AIDS in the United States during this time period. By far, AIDS has had the largest influence on US counts of KS, contributing 79% of all cases. Six percent of DLBCL cases, 19% of BL cases, and 26% of CNS lymphomas also occurred among persons with AIDS. The AIDS epidemic has had less effect on cervical cancer, with 0.4% of cases occurring among women with AIDS.
Changes in the AIDS epidemic over time have substantially influenced US national trends for KS and AIDS-defining subtypes of NHL. The proportion of cases of these cancers with AIDS peaked during 1990–1995, when incidence of KS and NHL among HIV-infected persons was quite high. Subsequent declines in the proportion with AIDS were associated with large decreases in cancer incidence rates among persons with AIDS, likely due to the introduction of HAART in 1996.4,5 Among HIV-infected individuals, HAART use resulted in substantial declines in the risk of death and AIDS events, including ADCs.1,5,19 HAART improves immune function and control of the oncogenic viruses that cause KS (ie, KS-associated herpesvirus), AIDS-defining NHLs (Epstein-Barr virus for CNS lymphoma and a large fraction of DLBCLs), and cervical cancer (human papillomavirus).20
Our results confirm findings of previous studies that have partially attributed general population trends in the rates of KS, NHL overall, and CNS lymphoma to the HIV epidemic.7–9 It is interesting to note, however, that the long-term temporal increase in overall NHL rates in the US general population8,21 is only partly explained by the HIV epidemic. For example, a rise in cases of the AIDS-defining NHL subtypes is still seen even when cases in persons with AIDS are removed (Table 1 and Figure 1). The rise in overall NHL incidence over time among HIV-uninfected persons remains unexplained.
Age patterns in the United States for KS, CNS lymphoma, and BL are also influenced by cases with AIDS. Strongly bimodal age patterns for KS and CNS lymphoma have been observed previously.7,9 For these 2 malignancies, our results clarify that the age peaks among 30- to 39-year-olds are primarily associated with AIDS, while the age peaks among 70- to 79-year-olds include almost no AIDS cases (Figure 3 and Figure 4). Older-age, non-AIDS KS cases include the “classic” variant, initially described in men of Mediterranean descent, and the iatrogenic variant among persons receiving immunosuppressive medication.22
CNS lymphoma may represent separate disease entities in HIV-infected persons and in elderly individuals, in that the tumors arising in HIV-infected persons are almost always positive for Epstein-Barr virus, while cases in elderly individuals are frequently negative for Epstein-Barr virus.23,24 Consistent with this etiologic heterogeneity, US rates for CNS lymphoma dropped steeply in 20- to 59-year-olds after 1995, likely reflecting decreasing rates in HIV-infected persons, while rates among those 60 years and older continued to increase.9
The age-specific incidence pattern of BL has recently been described as trimodal.25 Our analysis suggests that the middle-age peak is largely composed of cases with AIDS, whereas the age peaks in children/adolescents and older adults include few cases with AIDS (Figure 3 and Figure 4).
Despite the aging of the AIDS population over time, age patterns in the most recent time period (2001–2007) were similar to those in the overall cohort; ie, the proportion of cases of KS, DLBCL, and CNS lymphoma with AIDS remains high in younger age groups and very low in older age groups. The very low proportion of ADC cases with AIDS in the older age groups is associated with relatively low AIDS prevalence in persons 60 years and older, even in more recent years (0.08% in 20076,14) and also reflects the large number of ADC cases that occur in persons without AIDS in this age group.
Although women with AIDS experience elevated cervical cancer risk,1,19 their contribution to the total numbers of cervical cancer in the United States has been modest. Among females without AIDS, cervical cancer incidence was higher than the incidence of the other cancers we evaluated. Further, AIDS prevalence in females was 4-fold lower than in males, resulting in a low proportion of US cervical cancer cases with AIDS. However, the number of cervical cancer cases with AIDS (as well as the proportion) has increased over time, due to the increasing prevalence of AIDS among US females.
A strength of our analyses was the availability of HACM Study and CDC surveillance data. The HACM Study encompasses a large representative sample of US AIDS cases,1 and rates from this study were applied to CDC data for the entire US AIDS population. A limitation is the imperfect sensitivity of the data match in the HACM Study to identify cancer cases with AIDS. We applied a uniform correction across cancers to adjust for an estimated 81% sensitivity. Results were very similar when we used alternative assumptions (no correction or assuming 70% sensitivity), with the exception of KS, where we estimated that only 62.0% would have AIDS, assuming no correction (eTable).
An additional limitation was our inability to quantify the proportion of all US NHL cases with AIDS. DLBCL, BL, and CNS lymphoma are classified as ADCs; therefore, by definition, an HIV-infected individual with 1 of these NHLs has AIDS and would be captured as an AIDS case by an HIV/AIDS registry. In contrast, non–AIDS-defining NHL subtypes can occur in HIV-infected persons without AIDS. Because US registry data are more limited for persons with HIV who have not developed AIDS,26,27 we were unable to calculate reliable rates for this group or apply the rates to national population estimates. Limited availability of data on persons with HIV prior to AIDS also precluded us from including other cancers that are not considered AIDS-defining, such as anal cancer, in this analysis. Of note, the burden of non–AIDS-defining cancers occurring in the US AIDS population has increased over time.28
From a clinical perspective, persons with HIV diagnosed late after infection receive delayed HIV treatment and are more likely to develop AIDS compared with HIV-infected persons diagnosed shortly after infection. In the United States, an estimated 21% of HIV-infected persons are undiagnosed,29 and 54% of HIV-infected individuals have CD4 cell counts less than 350/μL at first presentation for care,30 a level below the currently recommended threshold for HAART initiation.31,32 Currently, the CDC recommends routine HIV screening in all health care settings for persons aged 13 to 64 years, unless prevalence of undiagnosed HIV infection in patients has been documented to be less than 0.1%.33 Improvements in early HIV detection may increase the number of persons in treatment and thereby reduce risk of AIDS (including ADCs). Further, early HIV diagnoses would improve prevention of cervical cancer by allowing clinicians to target HIV-infected women for more frequent Pap testing.34 In addition to having a role in preventing ADCs, HIV testing is important for all persons diagnosed with an ADC, particularly KS or NHL, given the high prevalence of HIV infection in persons with these cancers. Knowledge of a cancer patient’s HIV status provides an opportunity for clinicians to offer anti-retroviral treatment and prophylaxis for opportunistic infections.10 For NHL, knowledge of HIV status is also important for selection of the appropriate chemotherapy regimen.35–37
In conclusion, our study quantified the association between the HIV epidemic and US counts of KS, DLBCL, BL, CNS lymphoma, and cervical cancer. The proportion of cancer cases with AIDS has been especially high among young adults and males. These results highlight the importance of prevention of ADCs in HIV-infected persons and the need for HIV testing for individuals diagnosed with ADCs.
Acknowledgments
Funding/Support: This study was funded by the Intramural Research Program of the National Cancer Institute.
Role of the Sponsor: The sponsor reviewed and approved final submission but did not have a role in design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation of the manuscript.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Online-Only Material: The eFigure and eTable are available at http://www.jama.com.
Additional Contributions: For providing data for the HACM Study, we thank the HIV/AIDS and cancer registry staff in the states of Colorado, Connecticut, Florida, Illinois, Georgia, Maryland, Massachusetts, Michigan, New Jersey, and Texas and the metropolitan areas of New York, New York; Los Angeles, San Diego, and San Francisco, California; Seattle, Washington; and Washington, DC. We also thank Tim McNeel, BA (Information Management Systems), who was compensated for database management.
Author Contributions: Dr Shiels had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shiels, Pfeiffer, Goedert, Engels.
Acquisition of data: Hall, Li, Engels.
Analysis and interpretation of data: Shiels, Hall, Pfeiffer, Li, Morton, Hartge, Engels.
Drafting of the manuscript: Shiels, Hall, Pfeiffer, Li.
Critical revision of the manuscript for important intellectual content: Shiels, Hall, Li, Goedert, Morton, Hartge, Engels.
Statistical analysis: Shiels, Hall, Pfeiffer, Li, Hartge, Engels.
Obtained funding: Goedert.
Administrative, technical, or material support: Hall, Li.
Study supervision: Engels.
References
- 1.Engels EA, Pfeiffer RM, Goedert JJ, et al. for the HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 3.Gatter KC, Warnke RA. Diffuse large B-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics: Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. pp. 171–174. [Google Scholar]
- 4.Bedimo R, Chen RY, Accortt NA, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989–2002. Clin Infect Dis. 2004;39(9):1380–1384. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 5.Shiels MS, Cole SR, Wegner S, et al. Effect of HAART on incident cancer and noncancer AIDS events among male HIV seroconverters. J Acquir Immune Defic Syndr. 2008;48(4):485–490. doi: 10.1097/QAI.0b013e31817dc42b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HIV/AIDS Surveillance Report. Vol. 17. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Accessed March 10, 2011]. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/ [Google Scholar]
- 7.Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the US: an analysis of 12,114 cases. Cancer. 2008;113(3):616–627. doi: 10.1002/cncr.23571. [DOI] [PubMed] [Google Scholar]
- 8.Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi’s sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94(16):1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 9.Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002;95(1):193–202. doi: 10.1002/cncr.10643. [DOI] [PubMed] [Google Scholar]
- 10.Chiao EY, Dezube BJ, Krown SE, et al. Time for oncologists to opt in for routine opt-out HIV testing? JAMA. 2010;304(3):334–339. doi: 10.1001/jama.2010.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HIV/AIDS Cancer Match Study. National Cancer Institute; [Accessed February 2, 2011]. http://www.hivmatch.cancer.gov. [Google Scholar]
- 12.Frisch M, Biggar RJ, Engels EA, Goedert JJ AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) program. National Cancer Institute; [Accessed September 1, 2010]. SEER*stat database: incidence: SEER 9 regs research data, November 2009 sub (1973–2007): single ages to 85+: Katrina/Rita population adjustment: linked to county attributes: total US 1969–2007 counties. http://seer.cancer.gov/ [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) program. National Cancer Institute; [Accessed March 10, 2011]. SEER*stat database: populations: total US (1969–2007): single ages to 85+: linked to county attributes: total US, 1969–2007 counties [published 2011] http://seer.cancer.gov/ [Google Scholar]
- 15.HIV/AIDS Statistics and Surveillance. Centers for Disease Control and Prevention; [Accessed February 2, 2011]. http://www.cdc.gov/hiv/topics/surveillance/ [Google Scholar]
- 16.Harrison KM, Kajese T, Hall HI, Song R. Risk factor redistribution of the national HIV/AIDS surveillance data: an alternative approach. Public Health Rep. 2008;123(5):618–627. doi: 10.1177/003335490812300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green TA. Using surveillance data to monitor trends in the AIDS epidemic. Stat Med. 1998;17 (2):143–154. doi: 10.1002/(sici)1097-0258(19980130)17:2<143::aid-sim757>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Goedert JJ, Coté TR, Virgo P, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351(9119):1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 19.Patel P, Hanson DL, Sullivan PS, et al. Adult and Adolescent Spectrum of Disease Project and HIV Out-patient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 20.Bellan C, De Falco G, Lazzi S, Leoncini L. Pathologic aspects of AIDS malignancies. Oncogene. 2003;22(42):6639–6645. doi: 10.1038/sj.onc.1206815. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 22.Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88(3):500–517. [PubMed] [Google Scholar]
- 23.Bashir R, McManus B, Cunningham C, Weisenburger D, Hochberg F. Detection of Eber-1 RNA in primary brain lymphomas in immunocompetent and immunocompromised patients. J Neurooncol. 1994;20(1):47–53. doi: 10.1007/BF01057960. [DOI] [PubMed] [Google Scholar]
- 24.Larocca LM, Capello D, Rinelli A, et al. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood. 1998;92(3):1011–1019. [PubMed] [Google Scholar]
- 25.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973–2005. Int J Cancer. 2010;126(7):1732–1739. doi: 10.1002/ijc.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300 (5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 28.Shiels M, Pfeiffer R, Gail M, et al. The burden of cancer among HIV-infected persons in the US population [abstract WEAB0101]. Paper presented at: 18th Annual International AIDS Conference; Vienna, Austria. July 18–23, 2010. [Google Scholar]
- 29.Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53(5):619–624. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 30.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50(11):1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson MA, Aberg JA, Cahn P, et al. International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 32.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; [Accessed February 2, 2011]. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [January 10, 2011] http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 33.Branson BM, Handsfield HH, Lampe MA, et al. Centers for Disease Control and Prevention (CDC) Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 34.Kaplan JE, Benson C, Holmes KH, et al. Centers for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR Recomm Rep. 2009;58(RR-4):1–207. [PubMed] [Google Scholar]
- 35.Levine AM. HIV-associated lymphoma. Blood. 2010;115(15):2986–2987. doi: 10.1182/blood-2010-01-262717. [DOI] [PubMed] [Google Scholar]
- 36.Sparano JA, Lee JY, Kaplan LD, et al. AIDS Malignancy Consortium. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115(15):3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15):3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]