Abstract
Background
A relationship between hospitalization for respiratory syncytial virus (RSV) bronchiolitis and asthma development has been suggested in case-control studies.
Objective
The aim of this study was to assess the risk of current wheeze, asthma, and lung function at school age in infants previously hospitalized for RSV bronchiolitis compared to non-hospitalized children.
Methods
For this study, data from a prospective birth cohort of unselected, term-born infants (n = 553), of whom 4 (0.7%) were hospitalized for RSV bronchiolitis, and a prospective patient cohort of 155 term infants hospitalized for RSV bronchiolitis were used. Respiratory outcomes at age 6 in children hospitalized for RSV bronchiolitis were compared to non-hospitalized children.
Results
The risk of current wheeze was higher in hospitalized patients (n = 159) compared to non-hospitalized children (n = 549) (adjusted odds ratio (OR) 3.2 (95% CI 1.2–8.1). Similarly, the risk of current asthma, defined as a doctor’s diagnosis of asthma plus current symptoms or medication use, was higher in hospitalized patients (adjusted OR 3.1 (95% CI 1.3–7.5). Compared to non-hospitalized children, RSV bronchiolitis hospitalization was associated with lower lung function (mean difference FEV1% predicted −6.8 l (95% CI (−10.2 to −3.4).
Conclusions and Clinical Relevance
This is the first study showing that hospitalization for RSV bronchiolitis during infancy is associated with increased risk of wheezing, current asthma, and impaired lung function as compared to an unselected birth cohort at age 6.
Introduction
Respiratory syncytial virus (RSV) infection is a common cause of severe bronchiolitis in infants. The annual global incidence of RSV infection in children younger that 5 years, has been estimated at 34 million per year, with at least 10% episodes representing severe infections that require hospitalization [1]. Over the last 20 years, epidemiologic studies have shown an association between RSV bronchiolitis and the subsequent development of wheeze and asthma up to school age [2]. Most studies had a case-control design [3]–[7], which bears the challenge of control selection. In order to prevent selection bias, sampling of controls needs to be independent of the determinant studied [8]. The ALSPAC study retrospectively analyzed the relationship between a discharge code of hospitalization for RSV bronchiolitis and long-term airway disease up to age 7 years within in the study registry [9]. In that study there was an excess of subjects with missing data among RSV cases. A recent meta-analysis concluded that study quality of follow-up studies after RSV bronchiolitis was generally poor [10]. For that reason Stein and Martinez argued that the association between RSV hospitalization during infancy and asthma at school has still not been established [11]. To our knowledge, no study has prospectively compared wheezing and lung function at school age between term hospitalized RSV bronchiolitis patients and a large birth cohort of healthy term children. We performed a prospective cohort study of healthy infants, including a group of well characterized, hospitalized RSV infants with bronchiolitis [12]. Our aim was to determine whether the risk of wheeze and asthma at age 6 is related to RSV bronchiolitis hospitalization during infancy.
Materials and Methods
Participants
The WHISTLER project is an ongoing population based, prospective birth cohort on determinants of wheezing illnesses in children, which started in December 2001. Participants are healthy, term-born infants born in Leidsche Rijn, a residential area near the city of Utrecht. Infants are enrolled at the age of 2 weeks, and are prospectively followed with (1) a diary for respiratory symptoms in the first year of life, and with (2) study visits including lung function at age 5 and 8 [12]. For each participant, data from the most recent study visit was used (either from the visit at age 5 or age 8), resulting in 553 children of similar age for analysis. Children that were hospitalized during infancy for a proven RSV bronchiolitis (positive immunofluorescence test or PCR) were identified. This birth cohort was compared to a cohort of children that had been hospitalized for RSV bronchiolitis. Summarized, between 2004 and 2006, 243 previously healthy infants aged less than 13 months were admitted to the hospital with an RSV infection, as described earlier in detail [13]. Patients with a proven RSV bronchiolitis by a positive immunofluorescence test for RSV in epithelial cells from nasopharyngeal aspirates were randomly assigned to receive either high dose extra fine HFA beclomethasone dipropionate or placebo. At age 6, no differences in lung function or presence of respiratory symptoms were found between groups, and therefore we combined both groups for the current report. All children were invited to participate in the follow-up study visit at 6 years of age, including a questionnaire, and lung function assessment. From April 2010 to November 2011, 185 (76%) children attended this study visit (Figure S1). Only term born infants (155/185) were included, and added to the 4 hospitalized RSV bronchiolitis patients in the Whistler cohort. Respiratory outcomes of hospitalized RSV bronchiolitis patients within the complete cohort (n = 159, hereafter called hospitalized patients), were compared to non-hospitalized children (n = 549). Parents gave written informed consent for study participation.
Ethics Statement
The medical ethics committee of the University Medical Centre Utrecht approved both studies. Written informed parent consent was obtained from all parents. The study was conducted according to the principles of the Declaration of Helsinki (version 2000) and in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO). Good Clinical Practice (GCP) guidelines were followed.
Outcomes
Questionnaire and definitions
The questionnaire contained standardized questions about atopic diseases from the ISAAC questionnaire [14], medication use (e.g. antihistamines, inhalation steroids and beta-mimetics) in the last 12 months and known risk factors for atopic diseases (i.e. positive family history). All questions were filled in by the parents of the hospitalized patients and non-hospitalized children. Parental allergy was defined as a questionnaire-reported allergy to pollen, house dust mite, pets or food. Smoke exposure was defined as smoking of one of the parents in the last 5 years of life, and data from the first year of life were used to obtain information about smoke exposure in the first year of life to prevent recall bias. A non-western origin was defined as a country of birth in Asia (including Turkey), Africa, Latin America, excluding Indonesia and Japan. In the Netherlands, children regularly visit child healthcare centers for standardized anthropometry. These measurements are recorded in a personal file for every child, kept by parents. Parents were asked to use this file to report these anthropometric measures in the questionnaire. Current wheeze, in both the hospitalized patients and non-hospitalized children, was defined as a positive response to the question “Has your child had wheezing or whistling in the chest in the last 12 months?” Current asthma was defined as a history of doctor’s diagnosis of asthma plus asthma symptoms or medication (beta-mimetics or inhaled corticosteroids) use in the last 12 months.
A doctor’s diagnosis of asthma ever was defined as an ever recorded asthma diagnosis from the general practitioner after a primary care visit (R03 wheezing, R96 asthma), according to the International Classification of Primary Care (ICPC) [15].
Lung function
Spirometry was performed using a calibrated spirometer (Zan 100 pulmonary spirometer system (nSpire, USA). Maximal flow-volume curves were measured according to the ATS/ERS standards [20]. The largest forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and peak expiratory flow (PEF) were selected from three correctly performed manoeuvres. Results were related to Dutch normative values [16]. Expiratory measurements of respiratory system resistance (Rint) were obtained using MicroRint (Micro Medical Limited, Kent, UK). Mean Rint was calculated from at least 5 acceptable interruptions. FeNO was measured in exhaled breath using the NioxMino (NIOX; Aerocrine AB, Solna, Sweden). All children withheld their rescue medication for at least 12 hours beforehand. If the child had suffered from a respiratory tract infection in the last 2 weeks, the test was postponed.
Statistical analysis
Logistic regression was used to investigate the effect of RSV bronchiolitis hospitalization on current wheeze and asthma. Hereafter, multivariable analysis was used to adjust for possible confounders influencing both current wheeze or asthma (outcomes) as well as RSV bronchiolitis hospitalization (determinant). Results are presented as odds ratio (OR) with a 95% confidence interval (CI). Linear regression was used to investigate the effect of RSV bronchiolitis hospitalization on lung function. Again, multivariable analyses was used to adjust for possible confounders, and results are presented as crude and adjusted mean differences with a 95% CI. Data were analyzed using PASW Statistics 18 (version 18.0.0, SPSS Inc., 2009, Chicago USA).
Confounders
Baseline characteristics between the two cohorts were likely to be different with respect to risk factors for RSV bronchiolitis hospitalization. These risk factors were considered to be potential confounders of the association between RSV hospitalization and current wheeze or asthma. The following confounders were corrected for in our analysis: male gender, breastfeeding, siblings, day care attendance, smoke exposure, birth weight, being born between April and September, year of birth, and educational level of the mother.
Results
Of the 243 hospitalized RSV bronchiolitis patients that participated in the initial trial, 185 participated in the follow-up study. 155 Previously healthy, term infants were selected for further analyses, of whom 113 (72.9%) agreed to take part in the lung function test (figure S1). Non-participants were more often of non-Caucasian ethnicity compared to participants or the excluded premature born participants (table S1). Participants with a successful lung function measurement were significantly younger compared to participants with unsuccessful lung function measurement (5.8 versus 6.3 years, p<0.001, table S2). Children hospitalized for RSV bronchiolitis had lower birth weight, were more often born between September and March, more often exposed to maternal smoking in pregnancy, less often breastfed, did go to daycare less often, had more often siblings, had lower educated parents and more often Caucasian parents compared to non-hospitalized children (table 1).
Table 1. Group characteristics at age 6 for 159 hospitalized RSV bronchiolitis patients [13], and non-hospitalized children [12].
| Hospitalized RSVbronchiolitis patients(n = 159) | Non-hospitalized (n = 549) | p-value | |
| Sex (male) | 87/159 (54.7) | 265/549 (48.3) | 0.153 |
| Median age at follow-up in yrs (IQR) | 5.88 (5.67–6.25) | 5.33 (5.17–7.75) | 0.923 |
| Median age at hospitalisation (months) | 2.0 (1.0–4.0) | – | – |
| Birth weight (g) | 3250 (3250–3750) | 3557 (3260–3860) | <0.001 |
| Gestational age (weeks) | 40.0 (39.0–40.6) | 40.0 (39.1–40.9) | 0.081 |
| Born September-March | 99/159 (62.3) | 270/549 (49.2) | 0.002 |
| Smoking during pregnancy | 27/155 (17.0) | 26/548 (4.7) | <0.001 |
| Smoke exposure up to age 6 | 42/159 (26.4) | 126/451 (27.9) | 0.721 |
| Breastfed in first 3 months | 97/155 (62.6) | 3768/515 (71.5) | 0.036 |
| Daycare in first 3 months | 52/155 (33.5) | 227/515 (44.1) | 0.020 |
| Pets in first year of life | 79/154 (51.3) | 274/475 (57.7) | 0.166 |
| Siblings | 134/155 (86.5) | 297/548 (54.2) | <0.001 |
| Maternal atopy | 66/154 (42.9) | 224/466 (48.1) | 0.262 |
| Maternal ethnicity Caucasian* | 149/154 (96.8) | 386/469 (82.3) | <0.001 |
| Maternal high educational level | 53/153 (34.6) | 301/470 (64.0) | <0.001 |
| Median height at age 6 (cm, IQR) | 117.2 (113.0–126.10 | 118.2 (114.3–121.8) | 0.094 |
| Median weight at age 6 (kg, IQR) | 21.0 (19.0–25.0) | 21.6 (19.7–24.0) | 0.567 |
Data are numbers (percentages) unless stated otherwise.
Caucasian = Not born in Africa, Latin America and Asia (Japan and Indonesia excluded) or Turkey.
Current Wheeze, Asthma and Allergic Diseases
In the univariable logistic regression analysis hospitalized RSV bronchiolitis patients had a 3.2 fold increased odds for wheeze in the last 12 months (table 2). Hospitalized RSV patients more often were diagnosed with asthma at any point in life, and a higher proportion had current asthma defined as a history of doctor’s diagnosis of asthma plus asthma symptoms or medication use in the last 12 months. Hospitalized patients more often used airway medication compared to non-hospitalized children, however this did not reach statistical significance. In multivariable analysis we initially adjusted the risk of current wheeze and asthma for sex and age only, but this did not change our results. We repeated our analysis by adjusting for sex, age, birth weight, season of birth, year of birth, smoke exposure during pregnancy and during life, breastfeeding, daycare, siblings, maternal atopy and maternal ethnicity. This slightly changed the odds for current wheeze and current asthma to 3.2 (95% CI 1.2 to 8.1) and 3.1 (95%CI 1.3 to 7.5) respectively.
Table 2. Respiratory morbidity of hospitalized RSV bronchiolitis patients and non-hospitalized children at the age of 6 years.
| Respiratory morbidityat age 6 | HospitalizedRSVbronchiolitispatients | Non hospitalized | Crude | Adjusted* | Adjusted** | ||||||
| (n = 159) | (n = 549) | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Respiratory symptoms | |||||||||||
| Current wheeze | 33/155 (21.3) | 42/516 (8.1) | 3.0 | 1.9–5.0 | <0.001 | 3.0 | 1.9–5.1 | <0.001 | 3.2 | 1.2–8.1 | 0.002 |
| Current use of asthmamedication | |||||||||||
| Inhaled steroids | 11/159 (6.9) | 25/549 (6.9) | 1.6 | 0.7–3.2 | 0.235 | 1.5 | 0.7–3.2 | 0.255 | 3.4 | 1.0–11.3 | 0.292 |
| Betamimetics | 17/159 (10.7) | 46/549 (8.4) | 1.3 | 0.7–2.4 | 0.368 | 1.3 | 0.7–2.3 | 0.378 | 2.1 | 0.8–5.7 | 0.109 |
| Asthma | |||||||||||
| Doctor’s diagnosisasthma ever | 63/158 (39.9) | 58/517 (11.2) | 5.2 | 3.5–8.0 | <0.001 | 5.3 | 3.4–8.0 | <0.001 | 5.8 | 2.8–11.8 | <0.001 |
| Current asthma*** | 34/159 (21.4) | 29/549 (5.3) | 4.9 | 2.9–8.3 | <0.001 | 4.8 | 2.8–8.2 | <0.001 | 3.1 | 1.3–7.5 | 0.010 |
Data are numbers (percentages) unless stated otherwise;
Adjusted for sex and age;
Adjusted for sex, age, birth weight, birth season, smoke exposure during pregnancy and during life, breastfeeding, daycare, siblings, maternal atopy, ethnicity, year of birth, and maternal educational level;
Defined as combination of a history of doctor’s diagnosed asthma plus asthma symptoms or medication use in the last 12 months (beta-mimetics or inhaled corticosteroids).
Comparable adjusted odds ratios were found for current wheeze in atopic children (OR 3.7 (95% CI 0.8 to 15.6) and non-atopic children (2.9 (95%CI 0.8 to 10.5), as well as for current asthma in atopic (OR 3.3 (95% CI 0.8 to 13.6) and non-atopic children (2.6 (95%CI 0.8 to 8.8)). Proportions of parent-reported allergic diseases in their children were not significantly different between the hospitalized patients and non-hospitalized children (hay fever 5.7% versus 5.5%, and eczema 34.0% versus 27.5%).
Lung Function
Compared to the non-hospitalized children, lung function was significantly impaired in the hospitalized RSV patients (table 3, figure 1). Hospitalized patients had lower FEV1 (mean difference (MD) −6.8 l. %predicted (95% CI −10.2 to −3.4), lower FVC (MD −6.5 l. %predicted) (95% CI −10.4 to −2.7), a lower Tiffeneau index (MD −2.0% (95% CI −3.6 to −0.3), higher FeNO (MD 4.0 ppb (95% CI 1.9 to 6.2) and increased resistance of the respiratory system (MD 15.6 kPa/L/s (95% CI 6.5 to 24.6) compared to the non-hospitalized children.
Table 3. Lung functions of hospitalized RSV bronchiolitis patients and non-hospitalized children at the age of 6 years.
| Hospitalized RSVbronchiolitis patients | Non-hospitalized | Crude | Adjusted* | |||||||||
| (n = 117) | (n = 549) | |||||||||||
| Absolute# | % predicted | Absolute# | % predicted | Mean difference (95% CI) | p-value | Mean difference(95% CI) | p-value | |||||
| FEV1 (l) | 1.24 (0.20) | 93.3 (12.19) | 1.43 (0.33) | 100.27 (13.90) | −6.97 (−9.69 to −4.24) | <0.001 | −6.82 (−10.20 to −3.44) | <0.001 | ||||
| FVC (l) | 1.35 (0.24) | 98.16 (13.81) | 1.53 (0.39) | 104.31 (15.55) | −6.16 (−9.21 to −3.11) | <0.001 | −6.54 (−10.43 to −2.65) | 0.001 | ||||
| FEV1/FVC (%) | 92.32 (8.68) | – | 93.68 (5.96) | −1.36 (−2.75 to 0.04) | 0.057 | −1.97 (−3.61 to −0.33) | 0.019 | |||||
| PEF (l/s) | 2.86 (0.49) | 98.09 (15.19) | 3.17 (0.89) | 101.36 (21.45) | −3.28 (−7.71 to 1.17) | 0.148 | −2.93 (−8.59 to 2.73) | 0.310 | ||||
| Rint (kPa L−1 s) | 0.76 (0.23) | 124.72 (35.69) | 0.66 (0.17) | 112.73 (31.88) | 11.99 (4.54 to 19.43) | 0.002 | 15.59 (6.55 to 24.64) | 0.001 | ||||
| FeNO (ppb)a | 10.85 (9.01) | – | 8.12 (5.47) | – | 2.73 (1.15 to 4.29) | 0.001 | 4.02 (1.88 to 6.16) | 0.001 | ||||
Values are expressed as % of the predicted values (mean (SD)); Presented mean differences are differences in mean %predicted values;
Adjusted for sex, age, birth weight, birth season, smoke exposure during pregnancy and during life, breastfeeding, daycare, siblings, maternal atopy, ethnicity, year of birth, and maternal educational level.
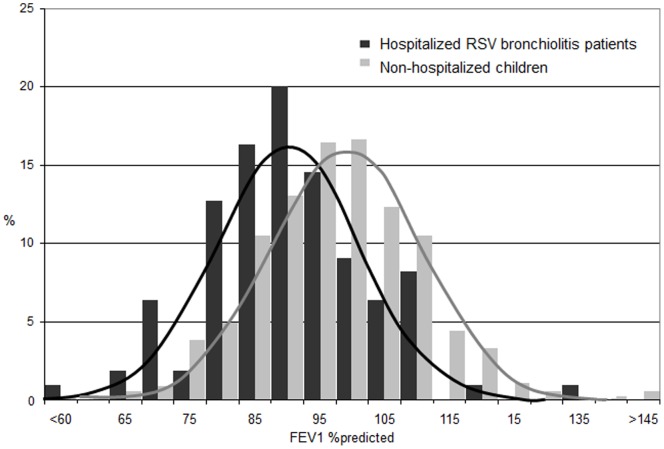
Figure 1. FEV1 values presented as % predicted values for hospitalized RSV bronchiolitis patients and non-hospitalized children measured at the age of 6 years.
Hospitalized patients had a lower mean FEV1% predicted compared to non hospitalized children (93.3 (SD12.2) versus 100.3% (SD 13.9), mean difference −7.0 (95% CI (−9.7 to −4.2)).
Discussion
In this prospective study we studied the risk of wheeze and asthma after RSV bronchiolitis hospitalization during infancy. We established a 3.2 fold increased risk of current wheeze and a 3.1 fold increased risk of current asthma in hospitalized RSV bronchiolitis patients compared to non-hospitalized children. In hospitalized RSV bronchiolitis patients the mean FEV1 percentage predicted was 6.8% lower compared to non-hospitalized children.
To our knowledge, this is the first study comparing prospectively followed hospitalized infants with RSV bronchiolitis to a normal birth cohort, and obtaining valid risk estimates on respiratory morbidity in childhood. Late effects of RSV bronchiolitis requiring hospitalization have previously been studied by others [3]–[5], [9], [17]. A study by Sigurs et al. [3] reported a 2 fold increased risk of “any wheezing” in prospectively followed hospitalized RSV bronchiolitis patients without concomitant chronic disease, whereas Henderson et al. reported a 3.2–6.6 fold increased risk of wheeze in hospitalized RSV patients selected from a large cohort study [9]. Asthma prevalence in the control population was 3% in the Sigurs study, which is much lower than in the general Swedish population [18]. In our non-hospitalized group the prevalence of wheeze and asthma was 8.1% and 5.3% respectively. These numbers are comparable to the prevalence of wheeze and asthma in 6–7 year old children in western surrounding countries [18], indicating that this group reflects the general population of the Netherlands with respect to wheeze and asthma.
Although symptoms may subside with age, lung function has been shown to track over life [19]. Lung functions in the hospitalized RSV patients were impaired compared to the non-hospitalized children, and differences between groups were larger than described in previous reports [4], [20], [21]. Lung function decline could be of vital importance because low lung function in adulthood is one of the strongest predictors of chronic airway obstructive pulmonary disease later on in life [19].
The mechanism underlying long-term consequences of severe RSV bronchiolitis is intriguing. Here, we confirm the results from the Tucson study showing that atopy does not play a major role in the development of wheeze and loss of lung function following RSV infection. Numerous hypotheses explaining the association between RSV bronchiolitis and asthma development have been published, including innate immune mechanisms, adaptive immune mechanisms as well as neurogenic mechanisms [22]. RSV bronchiolitis is associated with a strong local neutrophil response. Production of proteases and radical oxygen species may cause major damage to the airways resulting in abnormal development of lung architecture and function. An alternative hypothesis is based on our previous observations that children with severe RSV bronchiolitis have a stronger local IL-10 response, which may partially be genetically determined [23]–[25]. Increased local IL-10 production is associated with decreased production of type I and type 2 interferons, leaving patients susceptible to respiratory viral infections.
Previous studies as well as ours were not designed to determine whether RSV bronchiolitis causes airway morbidity at school age or reflects a common predisposition, probably abnormal lung function at birth [26]. It is clear that both hypotheses are not mutually exclusive. We have recently shown that RSV infection is causally related to recurrent wheeze in the first year of life in otherwise healthy preterm infants 33–35 weeks gestational age [27]. RSV immunoprophylaxis prevented 60% of all cause wheeze, even after end of therapy. Similarly, intervention studies are required to assess to what extent RSV hospitalization causes asthma at school age, which will prove instrumental in estimating the potential long-term benefit of new preventive and therapeutic interventions against RSV, of which many are currently under development [28].
In this study we assessed the unbiased risk of wheeze and asthma in childhood after RSV bronchiolitis hospitalization. However, some limitations need to be discussed. First, although our study has some advantages over previous retrospective or case-control studies, the optimal study design was not met. In our cohort, we have actively enriched the group of children hospitalized for RSV bronchiolitis. This led to the limitation that we were not able to assess absolute respiratory risks of hospitalization for RSV, but we were able to validly estimate relative respiratory risks. Second, lung function measurement was not successful in 42 patients (27%) in the RSV hospitalization group. Surprisingly, children with a successful lung function were younger compared to those without a successful lung function. As the remaining baseline characteristics (demographics) in these children were similar to patients with successful lung function measurements, we think that the lung functions measured are representative of all RSV patients. Third, we were able to obtain follow up data from 76% of the hospitalized RSV cohort. Non-participants were more often non-Caucasian compared to participants, which may limit the generalizability of our results to the non-Caucasian group. Fourth, residual confounding may have influenced our conclusions, even though we aimed to correct for all known potential confounders. Fifth, cohort effects may have influenced our outcomes. Although asthma prevalence has increased over the last decades, the prevalence of wheeze and asthma in our study is most likely not affected by the slight variation in time periods between our cohorts. Finally, we did not assess post bronchodilator reversibility which is thought to have added value to make an asthma diagnosis in children [29].
In summary, this is the first prospective study comparing previously healthy term infants hospitalized for RSV bronchiolitis with an unselected healthy, term population. We established that hospitalization for RSV bronchiolitis was associated with a 3.2-fold increased risk of wheeze, a 3.1-fold increased risk of asthma and a 6.8% decrease in FEV1% predicted at age 6 compared to non-hospitalized children. Intervention studies will have to determine to what extent RSV bronchiolitis is causally related to long-term airway disease.
Supporting Information
RSV study population at 6 years follow up.
(TIF)
Baseline characteristics of participants, non-participants and premature participants that were excluded from analyses.
(DOC)
General characteristics of the population with and without a successful lung function measurement.
(DOCX)
Acknowledgments
The authors thank all the parents and children who participated, the lung function analists Mrs. R. Bekkema, Mrs. H. Faber, Mrs. V. Haneveer-van Maanen, Mrs. Tersmette from the department of paediatric pulmonology, for collecting the data, and Mrs. E.M. Bloemen-Carlier in assisting in recruiting the subjects and collecting the data.
In addition to the authors, the following paediatricians participated in the Respiratory Syncytial Virus Corticosteroid Study Group: M ten Berge- Kuipers, St Antonius Hospital, Nieuwegein; J de Bie, Hofpoort Hospital, Woerden; D Blom, Academic Medical Centre, Amsterdam; R Bruinsma, Gelre Hospital, Apeldoorn; F Brus, Rijnstate Hospital, Arnhem; M Colombijn, Beatrix Hospital, Gorinchem; G van Enk, Gelderse Vallei Hospital, Ede; R van Gent, Maxima Medical Centre, Veldhoven; M Hoetjer, Meander Medical Centre, Amersfoort; A van der Kaaden, Hospital Hilversum, Hilversum; E van Leer, Groene Hart Hospital, Gouda; R de Moor, Twee Steden Hospital, Tilburg; A Nieuwenhuis, Jeroen Bosch Hospital, ‘s-Hertogenbosch; M Pekelharing, Diakonessen Hospital, Utrecht; C Smeets, St Elisabeth Hospital, Tilburg; E de Vries, Jeroen Bosch Hospital, ‘s-Hertogenbosch; A van Wermeskerken, Flevoziekenhuis, Almere; D van der Zwet-Fandri, Mesos Medical Centre, Utrecht.
Funding Statement
LB received a grant for this study from Teva Pharmaceutical industries inc., and from the Dutch Asthma Foundation. MR received an educational grant from Abbott, to provide financial support without restrictions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nair H, Nokes DJ, Gessner BD, Dherani M, Shabir AM, et al. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375: 1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Szabo SM, Levy AR, Gooch KL, Bradt P, Wijaya H, et al. (2013) Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev 13 Suppl 2: S9–15. [DOI] [PubMed] [Google Scholar]
- 3. Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B (2000) Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 161: 1501–7. [DOI] [PubMed] [Google Scholar]
- 4. Mikalsen IB, Halvorsen T, Øymar K (2012) The outcome after severe bronchiolitis is related to gender and virus. Pediatr Allergy Immunol 23: 391–8. [DOI] [PubMed] [Google Scholar]
- 5. Murray M, Webb MSC, Callaghan CO, Swarbrick AS, Milner AD (1992) Respiratory status and allergy after bronchiolitis. Arch Dis Child 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juntti H, Kokkonen J, Dunder T, Renko M, Uhari M (2003) Association of an early respiratory syncytial virus infection and atopic allergy. Allergy 878–84. [DOI] [PubMed] [Google Scholar]
- 7. Fjaerli H-O, Farstad T, Rød G, Ufert GK, Gulbrandsen P, et al. (2005) Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC pediatr 5: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sedgwick P (2011) Case-control studies: sources of bias. BMJ 343: d6284–d6284. [Google Scholar]
- 9. Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, et al. (2005) Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 16: 386–92. [DOI] [PubMed] [Google Scholar]
- 10. Régnier S, Huels J (2013) Association between Respiratory Syncytial Virus Hospitalizations in Infants and Respiratory Sequelae: Systematic Review and Meta-Analysis. Pediatr Infect Dis J 3–37. [DOI] [PubMed] [Google Scholar]
- 11. Stein RT, Martinez FD (2010) Respiratory syncytial virus and asthma: still no final answer. Thorax 65: 1033–34. [DOI] [PubMed] [Google Scholar]
- 12. Katier N, Uiterwaal CSPM, de Jong BM, Kimpen JLL, Verheij TJ, et al. (2004) The Wheezing Illnesses Study Leidsche Rijn (WHISTLER): rationale and design. Eur J Epidemiol 19: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ermers MJJ, Rovers MM, Van Woensel JB, Kimpen JLL, Bont LJ (2009) The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomised double blind placebo controlled trial. BMJ 338: b897–b897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zomer-Kooijker K, Van Erp FC, Balemans WA, Van Ewijk BE, Van der Ent CK (2013) The expert network and electronic portal for children with respiratory and allergic symptoms: rationale and design. BMC pediatr 13: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verbeke M, Schrans D, Deroose S, De Maeseneer J (2006) The International Classification of Primary Care (ICPC-2): an essential tool in the EPR of the GP. Stud Health Technol Inform 124. [PubMed] [Google Scholar]
- 16. Koopman M, Zanen P, Kruitwagen CLJJ, Van der Ent CK, Arets HGM (2011) Reference values for paediatric pulmonary function testing: The Utrecht dataset. Respir Med 105: 15–23. [DOI] [PubMed] [Google Scholar]
- 17. Pullan ACR, Hey EN (1982) Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. BMJ 284: 1665–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J 12: 315–35. [DOI] [PubMed] [Google Scholar]
- 19. Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD (2007) Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet 370: 758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, et al. (1999) Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354: 541–45. [DOI] [PubMed] [Google Scholar]
- 21. Korppi M, Piippo-Savolainen E, Korhonen K, Remes S (2004) Respiratory morbidity 20 years after RSV infection in infancy. Pediatric Pulm 38: 155–60. [DOI] [PubMed] [Google Scholar]
- 22. Mohapatra SS, Boyapalle S (2008) Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev 21: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F (2000) Monocyte IL-10 production during respiratory syncytial virus bronchiolitis is associated with recurrent wheezing in a one-year follow-up study. Am J Respir Crit Care Med 161: 1518–1523. [DOI] [PubMed] [Google Scholar]
- 24. Schuurhof A, Janssen R, De Groot H, Janssen R, de Groot H (2011) Hodemaekers (2011) Local interleukin-10 production during respiratory syncytial virus bronchiolitis is associated with post-bronchiolitis wheeze. Respir Res 12: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoebee B, Bont L, Rietveld E, van Oosten M, Hodemaekers HM (2004) Influence of promoter variants of interleukin-10, interleukin-9, and tumor necrosis factor-alpha genes on respiratory syncytial virus bronchiolitis. J Infect Dis 189: 239–47. [DOI] [PubMed] [Google Scholar]
- 26. Van der Zalm MM, Uiterwaal CSPM, Wilbrink B, Koopman M, Verheij TJM, et al. (2011) The influence of neonatal lung function on rhinovirus-associated wheeze. Am J Respir Crit Care Med 183: 262–7. [DOI] [PubMed] [Google Scholar]
- 27. Blanken MO, Rovers MR, Molenaar JM, Winkler-Seinstra PL, Meijer A (2013) Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 368: 1791–9. [DOI] [PubMed] [Google Scholar]
- 28. Empey KM, Peebles RS, Kolls JK (2010) Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis 50: 1258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM (2008) Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008 31: 143–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RSV study population at 6 years follow up.
(TIF)
Baseline characteristics of participants, non-participants and premature participants that were excluded from analyses.
(DOC)
General characteristics of the population with and without a successful lung function measurement.
(DOCX)