Abstract
Background
Several observational studies have shown that statin use may modify the risk of haematological malignancies. To quantify the association between statin use and risk for haematological malignancies, we performed a detailed meta-analysis of published studies regarding this subject.
Methods
We conducted a systematic search of multiple databases including PubMed, Embase, and Cochrane Library Central database up to July 2013. Fixed-effect and random-effect models were used to estimate summary relative risks (RR) and the corresponding 95% confidence intervals (CIs). Potential sources of heterogeneity were detected by meta-regression. Subgroup analyses and sensitivity analysis were also performed.
Results
A total of 20 eligible studies (ten case-control studies, four cohort studies, and six RCTs) reporting 1,139,584 subjects and 15,297 haematological malignancies cases were included. Meta-analysis showed that statin use was associated with a statistically significant 19% reduction in haematological malignancies incidence (RR = 0.81, 95% CI [0.70, 0.92]). During subgroup analyses, statin use was associated with a significantly reduced risk of haematological malignancies among observational studies (RR = 0.79, 95% CI [0.67, 0.93]), but not among RCTs (RR = 0.92, 95% CI [0.77, 1.09]).
Conclusions
Based on this comprehensive meta-analysis, statin use may have chemopreventive effects against haematological malignancies. More studies, especially definitive, randomized chemoprevention trials are needed to confirm this association.
Introduction
Hematologic malignancies, including three major groups: leukemia, lymphoma, and plasma cell neoplasms, derive from cells of the bone marrow and the lymphatic system [1]. In general, the overall incidence of hematological malignancies appears to be rising in Western countries, however, it is very difficult to describe their epidemiological behavior in a consistent and uniform way. In the USA, the number of estimated new cases of hematological malignancies in 2011 was 140,310 and it was predicted to have 53,010 deaths due to hematological malignancies [2].
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) are used for primary and secondary prevention of cardiovascular diseases, and their efficacy on cardiovascular events has been proven irrefutably for both reduction of morbidity and mortality [3], [4]. Statins are also found to be associated with decreased risk of certain cancers [5], [6] and reduce cancer-related mortality [7]. In vitro and animal studies have shown that statins have anti-proliferative, pro-apoptotic, anti-angiogenic and immunomodulatory effects, which prevent cancer development, growth, and metastasis [8]–[12].
Several randomized controlled trials(RCTs) and epidemiologic studies have evaluated the association between statin use and the risk of haematological malignancies; however, the existing results are inconsistent. To better understand this issue, we carried out a meta-analysis of existing RCTs and observational studies that investigated the association between statin use and the risk of developing haematological malignancies.
Methods
Literature Search
This meta-analysis was conducted following the guidance provided by the Cochrane Handbook and was reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE)guidelines [13]. A systematic literature search of PubMed, Embase, and Cochrane Library Central database was conducted for all relevant articles investigating the effect of statin use on the risk of haematological malignancies between January 1966 and July 2013. Search terms included: “hydroxymethylglutaryl-CoA reductase inhibitor(s)” or “statin(s)” or “lipid-lowering agent(s)” and “tumour(s)” or “cancer(s)” or “neoplasm(s)” or “malignancy(ies)” and “lymphatic” or “haematopoietic” or “hematopoietic” or “leukemia” or “lymphoma” or “haematological” or “blood” or “multiple myeloma”. In addition, we reviewed the reference lists from all relevant articles to identify additional studies.
Study Selection
We first excluded all irrelevant papers based on the titles and abstracts of the articles, and then the full texts of the remaining articles were read to determine whether they contained information on the topic of interest. Studies considered in this meta-analysis were either RCTs or observational studies that met the following inclusion criteria: (i) evaluated and clearly defined exposure to statins, (ii) reported haematological malignancies incidence and (iii) presented odds ratio (OR), relative risk (RR), or hazard ratio (HR) estimates with its 95% confidence interval (CI), or provided data for their calculation. There were no restrictions of origin, study size, language or publication type. Exclusion criteria was (i) lack of available data (ii) reviews, editorials, comments, reports from scientific sessions or discussions.When there were multiple publications from the same population, only data from the most recent comprehensive report was included.
Data Extraction
Data was independently abstracted onto a standardized form by two authors. The following data was collected from each study: name of the first author, publishing time, study design, country of the population studied, study period, follow-up time, statin type, RR, OR, HR and their 95% CIs, confounding factors for matching or adjustments.
Statistical Analysis
In our meta-analysis, we pooled data using the fixed or random effect models depending on heterogeneity between studies. Heterogeneity was assessed using the Cochran Q and I2 statistics. For the Q statistic, a P value<0.10 was considered statistically significant for heterogeneity; for the I2 statistic, heterogeneity was interpreted as absent (I2∶0%–25%), low (I2∶25.1%–50%), moderate (I2∶50.1%–75%), or high (I2∶75.1%–100%) [14]. When substantial heterogeneity was detected, the summary estimate based on the random-effect model (DerSimonian–Laird method) [15] was reported, which assumed that the studies included in the meta-analysis had varying effect sizes. Otherwise, the summary estimate based on the fixed-effect model (the inverse variance method) [16] was reported, which assumed that the studies included in the meta-analysis had the same effect size.
The overall analysis including all eligible studies was performed first, and subgroup analyses were performed according to (i) study design(observational studies, RCTs), (ii) study location(Western countries, Asian countries), (iii) study setting (population-based, hospital-based), (iv) subtypes of haematological malignancies(leukemia, lymphoma, multiple myeloma) to examine the impact of these factors on the association. To test the robustness of association and characterize possible sources of statistical heterogeneity, sensitivity analysis was carried out by excluding studies one-by-one and analyzing the homogeneity and effect size for all of rest studies. To better investigate the possible sources of between-study heterogeneity, a meta-regression analysis was performed [17]. Publication bias was assessed using Begg and Mazumdar adjusted rank correlation test and the Egger regression asymmetry test [18], [19]. All analyses were performed using Stata version 11.0 (StataCorp, College Station, TX).
Results
Search Results
We identified 2,630 potentially relevant articles through database searching and other sources(shown in Fig 1). Of these, 2,603 articles were excluded after the first screening based on abstracts or titles, leaving 27 articles for full-text review. After further evaluation, five studies were excluded for lack of available data, and two studies were excluded because they were from the same population. At last, a total of 20 eligible studies published between 1996 and 2012 were identified, including ten case-control studies [20]–[29], four cohort studies [30]–[33], and six RCTs [34]–[39] (Baseline data and other details of included studies were shown in Table 1). A total of 1,139,584 subjects, including 15,297 haematological malignancies cases were involved. Of the 20 included studies, eight studies were conducted in Europe [20], [25], [27], [29], [33]–[36], nine studies in America [21]–[23], [26], [28], [31], [32], [38], [39], and remaining three studies in other countries [24], [30], [37]. Nine studies were hospital-based [21], [22], [24], [34]–[39], and 11 studies were population-based [20], [23], [25]–[33].
Figure 1. Flow diagram of screened, excluded, and analysed publications.
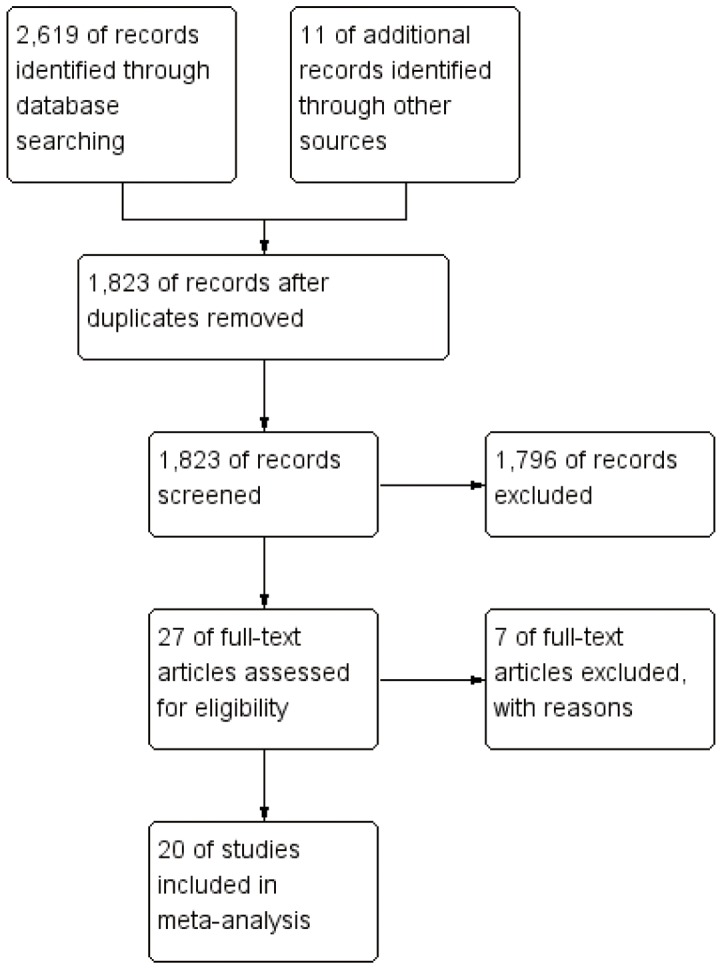
Table 1. Characteristics of included studies assessing the risk of haematological malignancies with statin use.
| Study | Year of publication | Study design | Country | Statin | Follow up (years) | Time Period | Sex | Study setting | Cases/Subjects | Cancer outcome | Confounding variables adjusted |
| Lutski M | 2012 | cohort | Israel | A,P,S | 4.7(mean) | 1998–2006 | M/F | Population-based | 681/202,648 | Haematological malignancies HR: 0.69 (0.55–0.88)Leukemia HR: 0.58 (0.37–0.91)Lymphoma HR: 0.69 (0.51–0.94) | Age, sex, marital status, area of residence, nationality, socioeconomic level, years of stay in Israel, obesity, diabetes mellitus, hypertension, cardiovascular disease, efficacy, hospitalizations and visits to physicians a year before first statin dispensation, and asthma |
| HPS | 2011 | RCT | England | S | 5.3(mean) | 1994–2001 | M/F | Hospital-based | 327/20,536 | Haematological malignancies RR: 1.01 (0.81–1.25) | Randomization |
| Vinogradova Y | 2011 | case-control | England | A,P,S | 2.3(median) | 1998–2008 | M/F | Population-based | 7,185/29,162 | Haematological malignancies OR: 0.78 (0.71–0.86) | Townsend quintile, BMI, smoking status, myocardial infarction, coronary heart disease, diabetes, hypertension, stroke, rheumatoid arthritis, use of NSAIDs, Cox2-inhibitors, aspirin |
| Jacobs EJ | 2011 | cohort | America | F,L,P,S | ≥5(mean) | 1997–2007 | M/F | Population-based | 1,005/133,255 | Non-Hodgkin lymphoma RR: 0.74 (0.62–0.89) | Age, sex, race, education, smoking, use of NSAIDs, BMI, physical activity, history of elevated cholesterol, diabetes, heart disease, hypertension |
| Chao C | 2011 | case-control | America | A,L,P,S | NR | 1996–2008 | M/F | Hospital-based | 259/1,554 | Non-Hodgkin lymphoma HR: 0.55 (0.31–0.95) | Age, sex, race, index year, known duration of HIV infection, Kaiser Permanente region (Northern or Southern California), clinical AIDS diagnosis prior to index date (yes/no), duration of antiretroviral therapy (ART) use (years), baseline CD4 cell count level (<200, 201–500, and>500/m l), and history of selected co-morbidity (yes/no), history of hepatitis B and C, diabetes, and obesity |
| Friedman GD | 2008 | cohort | America | A, C, F, L, P, R, S | ≥5(mean) | 1994–2003 | M/F | Population-based | 312/361,859 | Hodgkin lymphoma HR: 1.08 (0.26–4.42)Non-Hodgkin lymphoma HR: 1.02 (0.71–1.45)Multiple myeloma HR: 0.81 (0.42–1.58)Lymphocytic leukemia HR: 0.86 (0.41–1.84)Myeloid leukemia HR: 0.40 (0.15–1.09) | Smoking, use of NSAIDs, calendar year |
| Coogan PF | 2007 | case-control | America | NR | 3–6(median) | 1991–2005 | M/F | Hospital-based | 25/379 | Leukemia OR: 1.1 (0.6–2.0)Non-Hogdkin lymphoma OR: 1.2 (0.6–2.4) | Age, sex, BMI, interview year, study center, alcohol consumption, race, years of education, smoking, use of NSAID |
| Landgren O | 2006 | case-control | America | NR | 1.8–11.2(median) | 1996–2002 | F | Population-based | 179/870 | Multiple myeloma OR:0.4(0.2–0.8) | Age, race, education, and BMI |
| Iwata H | 2006 | case-control | Japan | F,P,S | 4(median) | 1995–2001 | M/F | Hospital-based | 221/1100 | Lymphoma OR: 2.06(0.88–4.8)Multiple myeloma OR: 3.99(1.75–9.10) | Age, sex, year of visit, serological status for anti-Hepatitis B surface antigens (HBsAg) and anti-Hepatitis C virus antibodies (HCVAb) |
| Fortuny J | 2006 | case-control | Czech Republic, France, Germany, Ireland, Italy, and Spain | >6.25(mean) | 1998–2004 | M/F | Population-based | 2,362/4,568 | Lymphoma OR: 0.61 (0.33–1.15) | Age, gender, and country | |
| Friis S | 2005 | cohort | Denmark | A, C, F, L, P, S | 3.3(mean) | 1989–2002 | M/F | Population-based | 1,626/334,754 | Haematological malignancies RR: 0.88 (0.60–1.29) | Age, sex, calendar period, use of NSAIDs, use of hormone, use of cardiovascular drugs |
| Zhang Y | 2004 | case-control | America | NR | NR | 1996–2000 | F | Population-based | 601/1,318 | Non-Hodgkin lymphoma OR: 0.5(0.4–0.8) | Age, BMI, menopausal status, and family history of non-Hodgkin lymphoma |
| Strandberg TE | 2004 | RCT | Nordic countries | S | 5.4(median) | 1988–1994 | M/F | Hospital-based | 36/4,444 | Haematological malignancies RR: 1.12 (0.58–2.14) | Randomization |
| Graaf MR | 2004 | case-control | Netherlands | A, C, F, P, S | 7.2(mean) | 1995–1998 | M/F | Population-based | 93/20,105 | Lymphoma OR: 0.28 (0.06–1.30) | Age, sex, geographic region, follow-up time, calendar time, diabetes mellitus, chronic use of diuretics, use of ACE inhibitors,use of calcium antagonists, use of NSAIDs, use of hormones, other lipid-lowering therapies, familiar hypercholesterolemia |
| Holdaas H | 2003 | RCT | Belgium, Denmark, Finland, Germany, Norway,Sweden, Switzerland, the UK, and Canada | F | 5.1(mean) | 1996–1997 | M/F | Hospital-based | 29/2,102 | Haematological malignancies RR: 0.61 (0.29–1.29) | Randomization |
| LIPID Study Group | 2002 | RCT | Australia and New Zealand | P | ≥8(mean) | 1990–1992 | M/F | Hospital-based | 89/7,680 | Haematological malignancies RR: 0.70 (0.46–1.07) | Randomization |
| Blais L | 2000 | case-control | Canada | L, P, S | 2.7(median) | 1988–1994 | M/F | Population-based | 24/264 | Lymphoma RR: 2.17 (0.38–12.36) | Age, sex, use of fibric acid, use of other lipid-reducing agents, previous benign neoplasm, year of cohort entry, the score of comorbidity |
| Downs JR | 1998 | RCT | America | L | 5.2(mean) | 1990–1997 | M/F | Hospital-based | 23/6,605 | Lymphoma RR: 0.92 (0.41–2.08) | Randomization |
| Traversa G | 1998 | case-control | Italy | NR | NR | 1992–1994 | M/F | Population-based | 202/2,222 | Leukemia OR: 1.3 (0.6–3.0) | Age,gender |
| Sacks FM | 1996 | RCT | Canada and America | P | 5(mean) | 1989–1991 | M/F | Hospital-based | 18/4,159 | Haematological malignancies RR: 0.80(0.32–2.02) | Randomization |
NR = not reported; RR = Relative risk; HR = Hazard ratio; OR = Odds ratio; M = male; F = female; BMI = body mass index; RCT = randomized controlled trial.
Risk of Haematological Malignancies
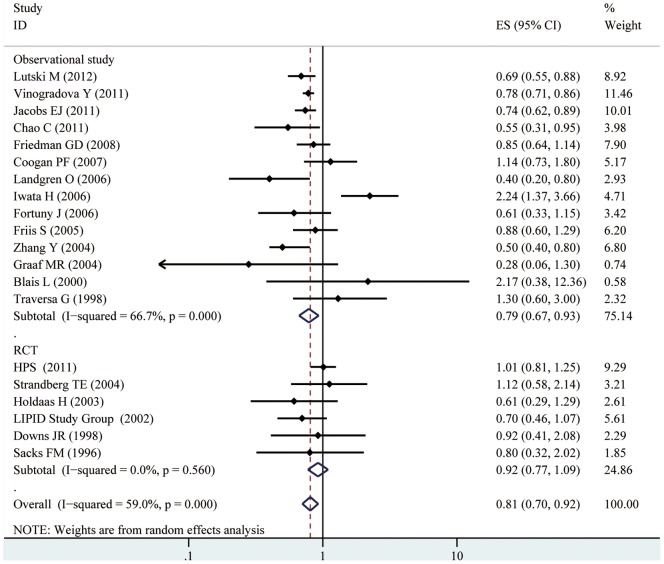
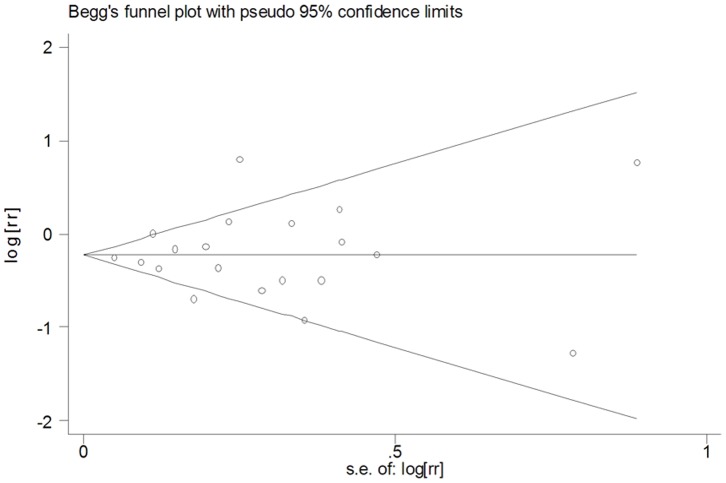
Based on data from 20 studies assessing the risk of haematological malignancies, the use of statins was associated with a statistically significant 19% reduction in haematological malignancies incidence (RR = 0.81, 95% CI [0.70, 0.92]). There was, however, considerable heterogeneity observed across studies (I2 = 59.0%, p<0.001). Both multivariable adjusted RR estimates with 95% CIs of each study and combined RR were shown in Fig.2. In the present meta-analysis, no publication bias was observed among studies using?Begg’s P value(P = 0.95); Egger’s(P = 0.78) test, which suggested there was no evidence of publication bias (Fig. 3).
Figure 2. Forest plot: estimates (95% CIs) of statin use and risk of haematological malignancies.
Squares indicated study-specific risk estimates (size of square reflects the study-statistical weight, i.e. inverse of variance); horizontal lines indicate 95% confidence intervals; diamond indicates summary relative risk estimate with its corresponding 95% confidence interval.
Figure 3. Funnel plot for publication bias in the studies investigating the association between statin use and the risk of haematological malignancies.
Subgroup Analysis
We carried out subgroup analyses of studies based on study design, study location, study setting, and subtypes of haematological malignancies (Table 2). Statin use was associated with a significantly reduced risk of haematological malignancies among observational studies(RR = 0.79, 95% CI [0.67, 0.93]), but not among RCTs(RR = 0.92, 95% CI [0.77, 1.09]). When stratified the various studies by study location, we found a significant association among studies conducted in Western countries (RR = 0.78, 95%CI [0.69, 0.88]), but not among studies conducted in Asian countries(RR = 1.22, 95%CI [0.38, 3.86]). When we examined whether the associations differed by study setting, statin use was significantly associated with a reduced risk of haematological malignancies among population-based studies(RR = 0.73, 95% CI [0.64, 0.83]), but not among hospital-based studies(RR = 0.96, 95% CI [0.73, 1.25]). When we stratified the various studies by cancer subtype, we found that statin therapy was associated with a significantly reduced risk of lymphoma(RR = 0.76, 95% CI [0.62, 0.95]), and a borderline significantly reduced risk of leukemia(RR = 0.77, 95% CI [0.57, 1.02]), but not multiple myeloma(RR = 0.86, 95% CI [0.19, 4.0]). We then divided the studies investigating statin use and risk of lymphoma to two subgroups(Non-Hodgkin lymphoma and Hodgkin lymphoma), and found that statin therapy was associated with a significantly reduced risk of Non-Hodgkin lymphoma(RR = 0.72, 95% CI [0.59, 0.87]), but not Hodgkin lymphoma(RR = 0.84, 95% CI [0.37, 1.95]).
Table 2. Subgroup analysis of all studies.
| Grouping variable | Subgroups | No. of studies | Pooled estimate | Tests of heterogeneity | ||
| RR | 95% CI | P value | I2(%) | |||
| All studies | 20 | 0.81 | 0.70–0.92 | <0.001 | 59.00 | |
| Study design | Observational study | 14 | 0.79 | 0.67–0.93 | <0.001 | 66.70 |
| RCT | 6 | 0.92 | 0.77–1.09 | 0.56 | 0.00 | |
| Study location | Western countries | 18 | 0.78 | 0.69–0.88 | 0.05 | 38.80 |
| Asian countries | 2 | 1.22 | 0.38–3.86 | <0.001 | 94.40 | |
| Study setting | Population-based | 11 | 0.73 | 0.64–0.83 | 0.09 | 38.40 |
| Hospital-based | 9 | 0.96 | 0.73–1.25 | 0.01 | 59.70 | |
| Cancer subtypes | Leukemia | 4 | 0.77 | 0.57–1.02 | 0.19 | 37.50 |
| Lymphoma | 11 | 0.76 | 0.62–0.95 | 0.02 | 51.60 | |
| Non-Hodgkin lymphoma | 6 | 0.72 | 0.59–0.87 | 0.04 | 55.80 | |
| Hodgkin lymphoma | 2 | 0.84 | 0.37–1.95 | 0.67 | 0.00 | |
| Multiple myeloma | 3 | 0.86 | 0.19–4.0 | <0.001 | 90.10 | |
No, number; RR, relative risks; CIs, confidence intervals; RCTs, randomized, controlled trials.
Sensitivity Analysis and Meta-regression Analysis
To test the robustness of association and characterize possible sources of statistical heterogeneity, sensitivity analysis was carried out by excluding studies one-by-one and analyzing the homogeneity and effect size for all of the rest studies. Sensitivity analysis indicated that no significant variation in combined RR by excluding any of the study, confirming the stability of present results. To better investigate the possible sources of between-study heterogeneity, a meta-regression analysis was performed. Geographic area, publication year, follow-up time, study design, and study setting, which may be potential sources of heterogeneity, were tested by a meta-regression method. Finally, we found that study design and study setting had statistical significance in a multivariate model (P<0.05).
Discussion
In this comprehensive meta-analysis of all existing studies(ten case-control studies, four cohort studies, and six RCTs) involving a total of 1,139,584 subjects with 15,297 cases of haematological malignancies, we found that statin use was inversely related to the risk for haematological malignancies, with a 19% reduction in the risk of haematological malignancies. There was statistically significant heterogeneity among the 20 included studies investigating the association between statin use and haematological malignancies risk, so a random-effect model was chosen over a fixed-effect model. Meta-regression analysis revealed that study design and study setting may be the source of heterogeneity. Our sensitivity analysis yielded similar and robust results, indicating that no study considerably influenced the overall risk estimate between statin use and haematological malignancies risk. Moreover, the results of Begg’ s test and Egger’ s test did not support the existence of major publication bias.
Previous in vitro studies have suggested anti-inflammatory and immunomodulatory properties of statins, including selective blockage of LFA-1-mediated adhesion and costimulation of lymphocytes [40], down regulation of class II major histocompatibility complexes on antigen-presenting cells [41], and reduction of chemokine synthesis in peripheral blood mononuclear cells [42]. In cell line and animal models, statins showed anticancer effects for haematological malignancies. Researchers have found that statins could induce apoptosis and inhibit proliferation of human acute myeloid leukemia cells and multiple myeloma cells [43]–[45]. Further, inhibitory effect of statins on spontaneous metastases derived from lymphoma was found in animal experiment [46]. So it is biologically plausible that statin use has protective effect upon haematological malignancies risk.
In our subgroup analyses, the results were substantially affected by study design. The chemopreventive effect of statins was seen primarily in observational studies, however, RCTs included in the present study did not demonstrate any significant chemopreventive effect of statins though there was a trend toward statistical significance (RR = 0.92, 95% CI [0.77, 1.09]). Importantly, the RCTs included in the meta-analysis were carried out mainly to investigate the effect of statins on cardiovascular morbidity. By design, the patients enrolled in these RCTs were not at high risk of development of haematological malignancies. And there were only six RCTs with a small number of participants and haematological malignancies cases, so it was not adequately powered to detect a significant difference in haematological malignancies incidence. Moreover, since the occurrence of cancer was not the primary objective of these trials, patients were not routinely screened for development of haematological malignancies; this might have affected the detection rate of haematological malignancies. These factors may explain why current clinical trials of statins did not demonstrate a statistically significant chemopreventive effect of statins against haematological malignancies. When stratified the various studies by study location, we found a significantly reduced risk in haematological malignancies among studies conducted in western countries, however, statin use had no significant association with haematological malignancies risk among studies conducted in Asian countries. The exact reason for the difference was unclear. The differences in genetic susceptibility, culture, and lifestyles may explain part of the inconsistency of the results. Further, we should notice that there were only two studies investigating the association between statin use and haematological malignancies risk. So more studies conducted in Asia are needed to confirm this association in the future. During subgroup analyses, we found that study setting also affected the association between statin use and haematological malignancies risk. A significant association was observed in population-based studies, but not in the hospital-based studies. The reason may be that the hospital-based studies have some inherent selection biases as such controls may just represent a sample of ill-defined reference population and may not be very representative of the study population or the general population. For the subgroup analysis of statin use and haematological malignancies risk by cancer subtype, we observed a statistically significant inverse association between statin use and Non-Hodgkin lymphoma, but not other subtypes of haematological malignancies, though there was a trend(we can see in Table2). More studies with more participants are needed to get a narrow confidence interval of RR and draw firm conclusions.
The strength of the present meta-analysis lies in a large sample size (1,139,584 subjects and 15,297 cases of haematological malignancies) and no significant evidence of publication bias. Two investigators independently performed the article identification, data extraction, and verification and resolved all discrepancies. Furthermore, our findings were stable and robust in sensitivity analysis. However, several limitations of this meta-analysis should be noted. Firstly, we did not search for unpublished studies, so only published studies were included in our meta-analysis. Therefore, publication bias may have occurred although no publication bias was indicated from both visualization of the funnel plot and Egger’s test. Secondly, the included studies were different in terms of study design and definition of drug exposure. Finally, the RCTs included in the meta-analysis were carried out mainly to investigate the effect of statins on cardiovascular morbidity. So, definitive, randomized chemoprevention trials are needed to more rigorously assess the effects of statins on incident haematological malignancies, but would be lengthy, logistically challenging and resource intensive.
Based on this comprehensive meta-analysis, statin use may have chemopreventive effects against haematological malignancies. More studies, especially definitive, randomized chemoprevention trials are needed to confirm this association.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
This work was supported by Natural Science Foundation of Hubei Province (No. 2012FFB02435) and the central university special funding (No. 2013QN191). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rodriguez-Abreu D, Bordoni A, Zucca E (2007) Epidemiology of hematological malignancies. Ann Oncol 18 Suppl 1i3–i8. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61: 212–236. [DOI] [PubMed] [Google Scholar]
- 3. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. (2012) The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, et al. (2008) Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 52: 1769–1781. [DOI] [PubMed] [Google Scholar]
- 5. Bansal D, Undela K, D'Cruz S, Schifano F (2012) Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One 7: e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W (2013) Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144: 323–332. [DOI] [PubMed] [Google Scholar]
- 7. Nielsen SF, Nordestgaard BG, Bojesen SE (2012) Statin use and reduced cancer-related mortality. N Engl J Med 367: 1792–1802. [DOI] [PubMed] [Google Scholar]
- 8. Dimitroulakos J, Marhin WH, Tokunaga J, Irish J, Gullane P, et al. (2002) Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia 4: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, et al. (2002) 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology 122: 308–317. [DOI] [PubMed] [Google Scholar]
- 10. Park HJ, Kong D, Iruela-Arispe L, Begley U, Tang D, et al. (2002) 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res 91: 143–150. [DOI] [PubMed] [Google Scholar]
- 11. Weis M, Heeschen C, Glassford AJ, Cooke JP (2002) Statins have biphasic effects on angiogenesis. Circulation 105: 739–745. [DOI] [PubMed] [Google Scholar]
- 12. Wong WW, Dimitroulakos J, Minden MD, Penn LZ (2002) HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16: 508–519. [DOI] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 16. Woolf B (1955) On estimating the relation between blood group and disease. Ann Hum Genet 19: 251–253. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG (2004) Controlling the risk of spurious findings from meta-regression. Stat Med 23: 1663–1682. [DOI] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vinogradova Y, Coupland C, Hippisley-Cox J (2011) Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer 11: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao C, Xu L, Abrams DI, Towner WJ, Horberg MA, et al. (2011) HMG-CoA reductase inhibitors (statins) use and risk of non-Hodgkin lymphoma in HIV-positive persons. AIDS 25: 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coogan PF, Rosenberg L, Strom BL (2007) Statin use and the risk of 10 cancers. Epidemiology 18: 213–219. [DOI] [PubMed] [Google Scholar]
- 23. Landgren O, Zhang Y, Zahm SH, Inskip P, Zheng T, et al. (2006) Risk of multiple myeloma following medication use and medical conditions: a case-control study in Connecticut women. Cancer Epidemiol Biomarkers Prev 15: 2342–2347. [DOI] [PubMed] [Google Scholar]
- 24. Iwata H, Matsuo K, Hara S, Takeuchi K, Aoyama T, et al. (2006) Use of hydroxy-methyl-glutaryl coenzyme A reductase inhibitors is associated with risk of lymphoid malignancies. Cancer Sci 97: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fortuny J, de Sanjose S, Becker N, Maynadie M, Cocco PL, et al. (2006) Statin use and risk of lymphoid neoplasms: results from the European Case-Control Study EPILYMPH. Cancer Epidemiol Biomarkers Prev 15: 921–925. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, et al. (2004) Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control 15: 419–428. [DOI] [PubMed] [Google Scholar]
- 27. Blais L, Desgagne A, LeLorier J (2000) 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med 160: 2363–2368. [DOI] [PubMed] [Google Scholar]
- 28. Traversa G, Menniti-Ippolito F, Da Cas R, Mele A, Pulsoni A, et al. (1998) Drug use and acute leukemia. Pharmacoepidemiol Drug Saf 7: 113–123. [DOI] [PubMed] [Google Scholar]
- 29. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ (2004) The risk of cancer in users of statins. J Clin Oncol 22: 2388–2394. [DOI] [PubMed] [Google Scholar]
- 30. Lutski M, Shalev V, Porath A, Chodick G (2012) Continuation with statin therapy and the risk of primary cancer: a population-based study. Prev Chronic Dis 9: E137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobs EJ, Newton CC, Thun MJ, Gapstur SM (2011) Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res 71: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 32. Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP Jr, et al. (2008) Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf 17: 27–36. [DOI] [PubMed] [Google Scholar]
- 33. Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, et al. (2005) Cancer risk among statin users: a population-based cohort study. Int J Cancer 114: 643–647. [DOI] [PubMed] [Google Scholar]
- 34. Bulbulia R, Bowman L, Wallendszus K, Parish S, Armitage J, et al. (2011) Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet 378: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strandberg TE, Pyorala K, Cook TJ, Wilhelmsen L, Faergeman O, et al. (2004) Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 364: 771–777. [DOI] [PubMed] [Google Scholar]
- 36. Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, et al. (2003) Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 361: 2024–2031. [DOI] [PubMed] [Google Scholar]
- 37. LIPID Study Group (2002) Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet 359: 1379–1387. [DOI] [PubMed] [Google Scholar]
- 38. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 39. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, et al. (1996) The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 335: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 40. Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, et al. (2001) Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 7: 687–692. [DOI] [PubMed] [Google Scholar]
- 41. Kwak B, Mulhaupt F, Myit S, Mach F (2000) Statins as a newly recognized type of immunomodulator. Nat Med 6: 1399–1402. [DOI] [PubMed] [Google Scholar]
- 42. Romano M, Diomede L, Sironi M, Massimiliano L, Sottocorno M, et al. (2000) Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest 80: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 43. Griner LN, McGraw KL, Johnson JO, List AF, Reuther GW (2013) JAK2-V617F-mediated signalling is dependent on lipid rafts and statins inhibit JAK2-V617F-dependent cell growth. Br J Haematol 160: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gronich N, Drucker L, Shapiro H, Radnay J, Yarkoni S, et al. (2004) Simvastatin induces death of multiple myeloma cell lines. J Investig Med 52: 335–344. [DOI] [PubMed] [Google Scholar]
- 45. Xia Z, Tan MM, Wong WW, Dimitroulakos J, Minden MD, et al. (2001) Blocking protein geranylgeranylation is essential for lovastatin-induced apoptosis of human acute myeloid leukemia cells. Leukemia 15: 1398–1407. [DOI] [PubMed] [Google Scholar]
- 46. Matar P, Rozados VR, Binda MM, Roggero EA, Bonfil RD, et al. (1999) Inhibitory effect of Lovastatin on spontaneous metastases derived from a rat lymphoma. Clin Exp Metastasis 17: 19–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)