Abstract
Introduction
Polycystic Ovary Syndrome (PCOS) has a strong genetic background and the majority of patients with PCOS have elevated BMI levels. The aim of this study was to determine to which extent BMI-increasing alleles contribute to risk of PCOS when contemporaneous BMI is taken into consideration.
Methods
Patients with PCOS and controls were recruited from the United Kingdom (563 cases and 791 controls) and The Netherlands (510 cases and 2720 controls). Cases and controls were of similar BMI. SNPs mapping to 12 BMI-associated loci which have been extensively replicated across different ethnicities, i.e., BDNF, FAIM2, ETV5, FTO, GNPDA2, KCTD15, MC4R, MTCH2, NEGR1, SEC16B, SH2B1, and TMEM18, were studied in association with PCOS within each cohort using the additive genetic model followed by a combined analysis. A genetic allelic count risk score model was used to determine the risk of PCOS for individuals carrying increasing numbers of BMI-increasing alleles.
Results
None of the genetic variants, including FTO and MC4R, was associated with PCOS independently of BMI in the meta-analysis. Moreover, no differences were observed between cases and controls in the number of BMI-risk alleles present and no overall trend across the risk score groups was observed.
Conclusion
In this combined analysis of over 4,000 BMI-matched individuals from the United Kingdom and the Netherlands, we observed no association of BMI risk alleles with PCOS independent of BMI.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting up to 10% of females of reproductive age. [1] PCOS itself as well as its separate phenotypic characteristics demonstrate familial aggregation [2], [3] and its heritability has been estimated as high as 65%. [4] Key features of the syndrome include ovulatory dysfunction, hyperandrogenism and polycystic ovarian morphology. Moreover, the majority of patients with PCOS have overweight or obesity. [5] It has been well established that obesity worsens the phenotype of PCOS. Lifestyle interventions and weight-loss not only improve body composition and insulin resistance in patients with PCOS, they also ameliorate the reproductive phenotype.[6]–[8] Although this close relationship between PCOS and obesity clearly exists, underlying mechanisms are far from being understood.
Obesity is also known to be a highly heritable trait [9], [10] and its genetics have been widely and successfully investigated using genome wide association studies (GWASs).[11]–[15] Since patients with PCOS have increased BMI levels compared to controls, genetic variants influencing PCOS might well include BMI risk alleles such that GWAS signals identified influencing PCOS might in fact be driven by primary effects on BMI. Therefore, it is of importance to evaluate whether adjustment or matching for BMI would eradicate the potential for variants influencing BMI to have an apparent effect on PCOS risk. If these effects remain after taking case-control differences in BMI into consideration, it might suggest either that a single BMI measurement is not an adequate proxy for lifelong BMI when it comes to specifying the effects of BMI on PCOS, or that the BMI risk alleles have pleiotropic effects on BMI as well as PCOS. The latter has been suggested for SNPs mapping to the FTO gene in association with obesity and type 2 diabetes mellitus in Asians. [16], [17].
Previous studies observed association of risk-alleles mapping to the FTO and MC4R gene with PCOS and its phenotypic characteristics.[18]–[23] However, these studies did not include BMI-matched case-control sets and had relatively small sample sizes (number of cases ranging from 65 to 800 and less than 1000 controls).
Therefore, we studied twelve BMI-associated loci in BMI-matched case-control sets from two large university medical centers to determine the effect on PCOS-susceptibility independently of current BMI.
Materials and Methods
Ethics Statement
All clinical investigations were conducted according to the guidelines in the Declaration of Helsinki. The study was approved by the medical ethics committee from the Erasmus MC University Medical Centre. Approval for the UK study was obtained from the North Thames Multicenter Research Ethics Committee [MREC/99/2/45]). All subjects provided fully written informed consent.
Subjects
Independent European PCOS populations from the United Kingdom (UK) and the Netherlands were included in this study. The UK case-control set included a total of 1354 women, of whom 563 were diagnosed with PCOS and 791 served as controls. The case-control set from the Netherlands consisted of 510 patients diagnosed with PCOS and 2720 control women from the general population. BMI levels between cases and controls in both studies were similar (p-value >0.05). Patients in both cohorts were diagnosed according to 2003 Rotterdam criteria. [24] In agreement with these criteria two of the following three symptoms should be present: oligo-ovulation and/or anovulation with gonadotropins levels within the normal limits, biochemical and/or clinical hyperandrogenism and polycystic morphology of the ovaries (PCOM). Oligomenorrhea was defined as a cycle length over 35 days and amenorrhea as absence of menstrual bleeding. Biochemical hyperandrogenism was determined by calculation of the Free Androgen Index (FAI) as: 100 x T (nmol/L)/SHBG (nmol/L). A FAI exceeding 4.5 was used as a cut-off. Clinical hirsutism was assessed using the modified Ferriman-Gallwey score and defined as an FG-score of at least 8. PCOM was assessed by transvaginal ultrasound and defined as the presence of at least 12 follicles in one or both ovaries and/or increased ovarian volume >10 ml. Exclusion criteria were presence of related disorders with similar presentations such as Cushing’s disease and congenital adrenal hyperplasia. The controls from the UK were population-based and recruited as part of the UK Blood Services (UKBS) set up by the Wellcome Trust Case Control Consortium (WTCCC). [25] Control women from the Netherlands were derived from the Rotterdam study, a population-based prospective cohort study. [26] In brief, this is a large population-based study of elderly subjects from a specific area near Rotterdam (Ommoord). All women aged 45 years or older at onset of menopause and with available DNA were included in the present analyses. These population-based control groups provided reference groups of allele frequencies which reflect the local general European population, rather than being control groups wherein PCOS specifically was excluded. Patients and controls were of European descent.
Genotyping and Quality Control
Table S1 summarizes the studied SNPs mapping to BMI-associated loci as identified by Frayling et al [11], Loos et al. [13], Thorleifsson et al [14] and Willer et al. [15] These 12 loci were established as genome wide significant between the years 2007–2009 during the first waves of GWAS and have been replicated across several ethnic populations ever since. [27] The studied SNPs were the lead SNPs mapping to the BMI-associated loci, as described in the aforementioned papers.[11], [13]–[15] Genotyping was carried out using Taqman “on demand”-assays (Applied Biosystems, Warrington, UK). The genotyping success rate was >95%. The Rotterdam Study controls were genotyped using the Illumina 550k array and imputed using HapMap2 CEU reference panel. [28] Genotypes of six of the SNPs were derived from this data: rs4074134 mapping to BDNF, rs7138803 mapping to FAIM2, rs7647305 mapping to ETV5, rs10838738 mapping to MC4R, rs10913469 mapping to SEC16B, rs7498665 mapping to SH2B1. SNP rs11084753 had an imputation quality of 94%, rs6548238 of 95% and all other SNPs had an imputation quality of >99%. The other SNPs were genotyped using Taqman “on demand”-assays (Applied Biosystems, Warrington, UK). None of the SNPs deviated from Hardy-Weinberg equilibrium (HWE).
Statistical Analysis and Power Calculation
Association analyses were initially carried out within each case-control set separately. The additive genetic model was tested using PLINK (v.1.07) [29] and IBM SPSS version 20 (IBM Statistical Package for the Sociological Sciences Inc., Chicago, USA). The combined effect of the BMI-increasing alleles in the two populations was evaluated using a fixed-effects meta-analysis in GWAMA [30] for SNPs with heterogeneity (I2) less than 25%. When I2 exceeded 25%, a random effect meta-analysis was performed using statistical software package R (http://www.r-project.org). Moreover, we studied the association of BMI-increasing alleles with PCOS when only including individuals with a BMI ≥30 kg/m2 and in a second analysis when only including individuals with a BMI <30 kg/m2.
Using Genetic Power Calculator software we determined that with the sample size of the total case control set (cases: n = 1073; controls: n = 3511), we reached approximately 95% power to detect association of a risk allele of frequency ≥0.2 having an odds ratio of ≥1.3 and an alpha of 0.05 (http://pngu.mgh.harvard.edu/~purcell/gpc/). [31] Since we selected the genetic variants, we did not correct for multiple testing and a P value <0.05 was considered statistically significant.
To test for the combined effect of all the BMI-associated alleles on PCOS susceptibility and to estimate the genetic risk of having PCOS for these women dependent on the number of BMI-increasing alleles present, we calculated the Genetic Risk Score (GRS). The GRS was modeled as a continuous variable and the calculation was carried out using R (http://www.r-project.org/). Using the GRS we assume that each SNP in the panel contributes equally to PCOS risk and that each individual allele has an equal and additive effect on risk. To obtain accurate counts of BMI-increasing alleles, only individuals with genotypes for at least 90% of SNPs (11 out of 12) were included. Based on this criterion, a total of 1264 individuals, i.e., 512 cases and 752 controls, from the UK and 3150 individuals from the Netherlands, i.e., 502 cases and 2648 controls, were included in the GRS-analysis. This method was described previously. [32], [33] Missing genotypes were replaced with the average risk score for each SNP in the total population. The maximum attainable score was 24 BMI-increasing alleles (12 SNPs * 2 alleles). The reference group was defined as 12 to 13 BMI-increasing alleles, which was the mean number of BMI-increasing alleles present in the controls. Analyses were carried out within the separate case-control sets as well as in the combined set. Finally, we calculated the overall trend across the GRS-groups using the Kruskal Wallis trend test (IBM SPSS version 20).
Results
The UK cases had a mean BMI of 26.0 kg/m2 (±10.9 SD) and the controls had a BMI of 25.9 kg/m2 (±4.57 SD), whereas the cases from the Netherlands had a median BMI of 26.4 kg/m2 (IQR 22.4–31.7) and the controls had a BMI of 26.3 kg/m2 (IQR 23.97–29.1). The prevalence rates of normal weight, overweight en obese individuals are depicted in Table 1. Although the BMI levels of the total group of cases and controls were similar in the samples from the UK as well as the Netherlands, these prevalences were significantly different (P<0.001). Therefore, we also ran separate analyses including only individuals with BMI ≥30 kg/m2 and including only individuals with a BMI <30 kg/m2. Allele frequencies of the BMI-risk alleles in the cases and controls of both case-control sets are reported in Table S2.
Table 1. The prevalence rates of normal weight, overweight en obese patients with PCOS and controls.
| The United Kingdom | P | The Netherlands | P | ||||
| % cases | % controls | % cases | % controls | ||||
| Normal weight | BMI <25 | 42.7 | 50.3 | <0.001 | 43.9 | 35.2 | <0.001 |
| Overweight | BMI ≥25 and <30 | 17.6 | 32.4 | 22.2 | 45.5 | ||
| Obese | BMI ≥30 | 39.7 | 17.3 | 33.9 | 19.3 | ||
BMI Body Mass Index; P p-value.
First, we tested whether carrying BMI-associated alleles influenced PCOS susceptibility in cases and controls who were of similar BMI (Table 2). SNP rs7498665 mapping to the SH2B1 locus was significantly associated with a decreased risk of having PCOS (OR = 0.79; 95%CI: 0.69–0.90, P = 0.001) in the case-control set from the Netherlands. No such association was evident in the case-control set from the UK (OR = 1.04; 95% CI: 0.88–1.22, P = 0.64) or in the random effects meta-analysis. Moreover, none of the other SNPs was significantly associated with PCOS, neither in the separate UK and Dutch analysis nor in the meta-analysis. The BMI-increasing alleles were not associated with PCOS in the meta-analysis including only individuals with BMI<30 kg/m2, nor were they associated with PCOS in the BMI ≥30 kg/m2 group (Table S3 and Table S4, respectively).
Table 2. Genetic association results for BMI-increasing risk alleles with PCOS in the United Kingdom and The Netherlands.
| United Kingdom | The Netherlands | Meta-analysis | ||||||||||
| cases n = 563 controls n = 791 | cases n = 510 and control n = 2720 | cases n = 1073 and control n = 3511 | ||||||||||
| SNP | CHR | position | Locus name | BMI-increasing risk allele | Overall frequencyrisk allele | OR per risk allele (95% CI) | P | Overall frequencyrisk allele | OR per risk allele (95% CI) | P | OR per risk allele (95% CI) | P |
| rs4074134 | 11 | 27603861 | BDNF | G | 0.79 | 1.10 (0.91–1.33) | 0.34 | 0.79 | 0.86 (0.74–1.02) | 0.08 | 0.97 (0.76–1.23)* | 0.79 |
| rs7138803 | 12 | 48533735 | FAIM2 | A | 0.38 | 1.05 (0.90–1.23) | 0.53 | 0.37 | 1.02 (0.89–1.17) | 0.80 | 1.03 (0.93–1.14) | 0.54 |
| rs7647305 | 3 | 187316984 | ETV5 | C | 0.78 | 1.09 (0.90–1.31) | 0.38 | 0.80 | 0.96 (0.82–1.14) | 0.66 | 1.01 (0.90–1.15) | 0.82 |
| rs9939609 | 16 | 52378028 | FTO | A | 0.42 | 1.17 (1.00–1.37) | 0.05 | 0.36 | 1.01 (0.88–1.16) | 0.87 | 1.08 (0.93–1.25)* | 0.29 |
| rs10938397 | 4 | 44877284 | GNPDA2 | G | 0.45 | 0.99 (0.85–1.15) | 0.87 | 0.42 | 1.15 (1.00–1.31) | 0.05 | 1.06 (0.92–1.24)* | 0.35 |
| rs11084753 | 19 | 39013977 | KCTD15 | G | 0.68 | 0.95 (0.80–1.11) | 0.44 | 0.66 | 0.99 (0.86–1.14) | 0.91 | 0.97 (0.87–1.08) | 0.61 |
| rs17782313 | 18 | 56002077 | MC4R | C | 0.24 | 1.06 (0.89–1.27) | 0.51 | 0.25 | 1.12 (0.96–1.31) | 0.14 | 1.09 (0.97–1.23) | 0.13 |
| rs10838738 | 11 | 47619625 | MTCH2 | G | 0.36 | 1.00 (0.85–1.17) | 0.99 | 0.33 | 1.09 (0.95–1.26) | 0.20 | 1.05 (0.94–1.17) | 0.37 |
| rs2815752 | 1 | 72585028 | NEGR1 | A | 0.60 | 0.99 (0.85–1.17) | 0.93 | 0.61 | 1.04 (0. 91–1.19) | 0.56 | 1.02 (0.92–1.13) | 0.72 |
| rs10913469 | 1 | 176180142 | SEC16B | C | 0.21 | 0.91 (0.75–1.11) | 0.37 | 0.20 | 0.91 (0.77–1.08) | 0.30 | 0.90 (0.80–1.03) | 0.15 |
| rs7498665 | 16 | 28790742 | SH2B1 | G | 0.39 | 1.04 (0.88–1.22) | 0.64 | 0.40 | 0.79 (0.69–0.90) | 0.001 | 0.90 (0.73–1.18)* | 0.45 |
| rs6548238 | 2 | 624905 | TMEM18 | C | 0.84 | 1.05 (0.85–1.30) | 0.64 | 0.83 | 1.00 (0.83–1.19) | 0.95 | 1.02 (0.89–1.17) | 0.77 |
random effect meta-analysis (I2>25%), otherwise fixed effect meta-analysis was performed. CHR chromosome, SNP single nucleotide polymorphism, OR odds ratio, CI confidence interval, P p-value.
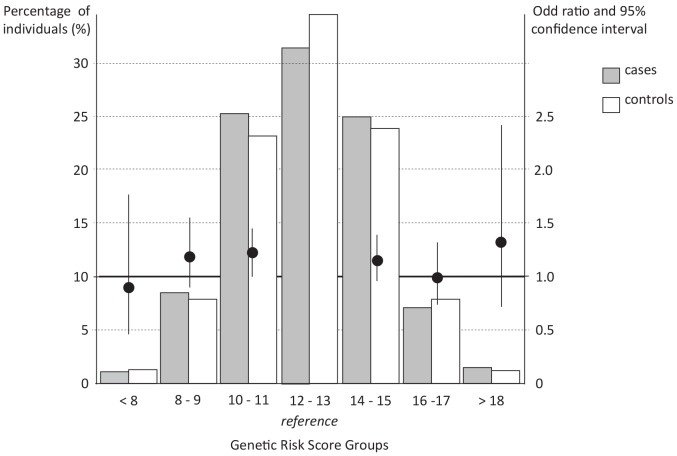
To determine whether the overall burden of BMI-increasing alleles was associated with PCOS status in these samples, a GRS was constructed (Figure 1, Table 3). The individuals carrying less than 8 BMI-associated alleles and individuals carrying over 18 BMI-associated alleles together account for a very small proportion of the total population, i.e., 2.6%. None of the GRS-groups was attributed more often to patients with PCOS compared with the reference GRS-group. Moreover, no overall trend in carrying an increasing number of BMI-associated alleles on PCOS susceptibility was observed neither for the total case-control set (P = 0.44), nor for the separate case-control sets from the UK (P = 0.97) or the Netherlands (P = 0.17).
Figure 1. Combined impact of risk alleles on the risk of having PCOS compared to the reference risk group.
Along the X-axis the risk categories are shown based on the number of BMI-increasing alleles. The histogram (Y-axis on the left) indicates the percentage of individuals for each risk-score group. The odds ratio and confidence intervals calculated based on the risk of having PCOS compared to the reference risk group are plotted on the Y axis on the right.
Table 3. Combined impact of BMI-increasing alleles on the risk of having PCOS.
| United Kingdom | The Netherlands | Combined | |||||||
| GRS | Casesn (%) | Controlsn (%) | OR (95%CI) | Casesn (%) | Controlsn (%) | OR (95%CI) | Cases n (%) | Controlsn (%) | OR (95%CI) |
| <8 | 7 (1.4) | 14 (1.9) | 0.73 (0.29–1.86) | 4 (0.8) | 31 (1.2) | 0.82 (0.28–2.34) | 11 (1.1) | 45 (1.3) | 0.90 (0.46–1.76) |
| 8 to 9 | 49 (9.6) | 78 (10.4) | 0.92 (0.61–1.38) | 37 (7.4) | 191 (7.2) | 1.22 (0.83–1.81) | 86 (8.5) | 269 (7.9) | 1.18 (0.90–1.55) |
| 10 to 11 | 121 (23.6) | 177 (23.5) | 1.00 (0.74–1.36) | 136 (27.1) | 611 (23.1) | 1.41 (1.09–1.81) | 257 (25.3) | 788 (23.2) | 1.20 (1.00–1.45) |
| 12 to 13 | 173 (33.8) | 254 (33.8) | Reference | 146 (29.1) | 922 (34.8) | Reference | 319 (31.5) | 1176 (34.6) | Reference |
| 14 to 15 | 125 (24.4) | 171 (22.7) | 1.07 (0.79–1.45) | 129 (25.7) | 641 (24.2) | 1.27 (0.98–1.64) | 254 (25.0) | 812 (23.9) | 1.15 (0.96–1.39) |
| 16 to 17 | 32 (6.3) | 52 (6.9) | 0.90 (0.56–1.46) | 40 (8.0) | 216 (8.2) | 1.17 (0.80–1.71) | 72 (7.1) | 268 (7.9) | 0.99 (0.74–1.32) |
| >18 | 5 (1.0) | 6 (0.8) | 1.22 (0.37–4.01) | 10 (2.0) | 36 (1.4) | 1.75 (0.85–3.61) | 15 (1.5) | 42 (1.2) | 1.32 (0.72–2.41) |
Data are presented for pools of BMI-increasing alleles as odds ratio and confidence intervals. Only individuals with data of >90% of the SNPs available were included. The mean number of BMI-increasing alleles in the controls was used as the reference group. GRS genetic risk score; n number of individuals; OR odds ratio.
Discussion
In this combined analysis including >4,000 patients with PCOS and controls from the United Kingdom and the Netherlands, we observed no association of genetic BMI-risk loci with PCOS when contemporaneous BMI is similar in cases and controls.
The last two years, genome wide association studies (GWASs) have emerged to identify genetic risk factors for PCOS. Two large studies identifying such PCOS-susceptibility loci in Han-Chinese patients have been published [34], [35] while GWAS in patients from Northern European ancestry are in progress. In the current study, we observed no systematic effect of the BMI-associated alleles on PCOS susceptibility in our BMI-matched case-control set. This infers that adjustment or matching for BMI will disentangle BMI-associated genetic signals to show up in PCOS GWAS and seems therefore not a genuine concern in the previous and upcoming GWASs in PCOS.
Presence of an increasing number of BMI-raising alleles is associated with an increased genetic predisposition to obesity. To determine the overall burden of BMI-associated alleles on risk for PCOS we calculated a counted genetic risk score and compared PCOS risk across such BMI risk groups. By doing so, we assumed that each allele has an equal and additive effect on PCOS risk. In practice some SNPs will have stronger effects than others. However, when the ORs are small as they are in our study, using a counted genetic risk model is appropriate. [32], [36] None of the GRS-groups was attributed more often to the patients diagnosed with PCOS than controls compared to the reference GRS-group. Moreover, no overall trend across the consecutive GRS groups was observed, strengthening the results from the allelic-association analysis that BMI-associated alleles seem not to have pleotropic effects on PCOS risk. Increased BMI levels and weight in PCOS seem to be mediated by other genetic factors determining an individual’s susceptibility to become obese or through modifying environmental effects. It has been shown that although women with PCOS reported a better, more healthy, dietary intake they seem to have an increased energy intake in combination with an increased sitting time without any discernible differences in total physical activity compared to women without PCOS. [37] Moreover, also in patients with PCOS, higher energy intake and glycemic index and lower physical activity, as well as age, smoking, alcohol intake, occupation, education and country of birth, were independently associated with BMI. [38].
In the current study we included population-based controls. Selection of controls in genetic studies is an extensively discussed issue. The main argument for using so-called ‘super controls’, i.e., individuals who are known to be unaffected, instead of population-based controls, is that the power is expected to improve because the difference in allele frequency between cases and controls is increased. For a disease as frequent as PCOS this would indeed increase the power to detect effects. However, only small samples of control women for whom PCOS is excluded are available. Moreover, these women are selected based on other criteria, for example tubal infertility. Consequently, it is more feasible to use a larger sample which is randomly selected from the general population as controls [39]–[41] and thereby compensating for the fact that we might also have also included women diagnosed with PCOS.
A potential limitation of the current study is that we matched BMI based on a single measurement. In general the BMI of men as well as in women increases throughout life. Therefore, a single BMI measurement may not be an appropriate proxy for lifetime BMI and might be a poor estimate of the long-standing effects of BMI on PCOS risk. However, since we observed no systematic effect of BMI-associated alleles on PCOS risk in our matched case-control set, this seems not to have influenced our results tremendously. It has been previously observed that the association of genetic variants in FTO and MC4R with BMI and weight strengthen during childhood up to age 20 years and then become weaker with increasing age during adulthood. [42] Therefore, as has been suggested for phenotypes associated with type 2 diabetes mellitus [17], longitudinal studies are needed to adequately explore the complex and dynamic nature of BMI-associated alleles on cardiometabolic characteristics in PCOS.
In conclusion, we have shown in two independent large PCOS case-control sets matched for BMI, that there is no systematic effect of BMI-associated alleles on PCOS risk suggesting that these alleles do not have a pleiotropic effect on PCOS susceptibility. Hence, adjusting for BMI in PCOS case-control GWAS studies should be an effective strategy for removing confounding effects of BMI on the association of other genetic variants and PCOS.
Supporting Information
Studied BMI-associated loci. SNP Single Nucleotide Polymorphism.
(DOC)
Allele frequencies in cases and controls from the United Kingdom and the Netherlands. SNP Single Nucleotide Polymorphism.
(DOC)
Genetic association results for BMI-increasing risk alleles with PCOS in the United Kingdom and The Netherlands including non-obese cases and controls (BMI <30 kg/m2). * random effect meta-analysis (I2>25%), otherwise fixed effect meta-analysis was performed. chr chromosome, SNP single nucleotide polymorphism, OR odds ratio, CI confidence interval, P p-value.
(DOC)
Genetic association results for BMI-increasing risk alleles with PCOS in the United Kingdom and The Netherlands including obese cases and controls (BMI ≥30 kg/m2). * random effect meta-analysis (I2>25%), otherwise fixed effect meta-analysis was performed. chr chromosome, SNP single nucleotide polymorphism, OR odds ratio, CI confidence interval, P p-value.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, et al. (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89: 2745–2749. [DOI] [PubMed] [Google Scholar]
- 2. Lunde O, Magnus P, Sandvik L, Hoglo S (1989) Familial clustering in the polycystic ovarian syndrome. Gynecol Obstet Invest 28: 23–30. [DOI] [PubMed] [Google Scholar]
- 3. Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R (2001) Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril 75: 53–58. [DOI] [PubMed] [Google Scholar]
- 4. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI (2006) Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 91: 2100–2104. [DOI] [PubMed] [Google Scholar]
- 5. Barber TM, McCarthy MI, Wass JA, Franks S (2006) Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 65: 137–145. [DOI] [PubMed] [Google Scholar]
- 6.Moran LJ, Hutchison SK, Norman RJ, Teede HJ (2011) Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev: CD007506. [DOI] [PubMed]
- 7. Holte J, Bergh T, Berne C, Wide L, Lithell H (1995) Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 80: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 8. Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, et al. (1992) Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 36: 105–111. [DOI] [PubMed] [Google Scholar]
- 9. Maes HH, Neale MC, Eaves LJ (1997) Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 27: 325–351. [DOI] [PubMed] [Google Scholar]
- 10. Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, et al. (2003) Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res 6: 409–421. [DOI] [PubMed] [Google Scholar]
- 11. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, et al. (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindgren CM, Heid IM, Randall JC, Lamina C, Steinthorsdottir V, et al. (2009) Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS Genet 5: e1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, et al. (2008) Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40: 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, et al. (2009) Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41: 18–24. [DOI] [PubMed] [Google Scholar]
- 15. Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, et al. (2009) Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li H, Kilpelainen TO, Liu C, Zhu J, Liu Y, et al. (2012) Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 55: 981–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyre D (2012) Is FTO a type 2 diabetes susceptibility gene? Diabetologia 55: 873–876. [DOI] [PubMed] [Google Scholar]
- 18. Barber TM, Bennett AJ, Groves CJ, Sovio U, Ruokonen A, et al. (2008) Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia 51: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 19. Kowalska I, Adamska A, Malecki MT, Karczewska-Kupczewska M, Nikolajuk A, et al. (2012) Impact of the FTO gene variation on fat oxidation and its potential influence on body weight in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 77: 120–125. [DOI] [PubMed] [Google Scholar]
- 20. Kowalska I, Malecki MT, Straczkowski M, Skupien J, Karczewska-Kupczewska M, et al. (2009) The FTO gene modifies weight, fat mass and insulin sensitivity in women with polycystic ovary syndrome, where its role may be larger than in other phenotypes. Diabetes Metab 35: 328–331. [DOI] [PubMed] [Google Scholar]
- 21. Tan S, Scherag A, Janssen OE, Hahn S, Lahner H, et al. (2010) Large effects on body mass index and insulin resistance of fat mass and obesity associated gene (FTO) variants in patients with polycystic ovary syndrome (PCOS). BMC Med Genet 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wehr E, Schweighofer N, Moller R, Giuliani A, Pieber TR, et al. (2010) Association of FTO gene with hyperandrogenemia and metabolic parameters in women with polycystic ovary syndrome. Metabolism 59: 575–580. [DOI] [PubMed] [Google Scholar]
- 23. Yan Q, Hong J, Gu W, Zhang Y, Liu Q, et al. (2009) Association of the common rs9939609 variant of FTO gene with polycystic ovary syndrome in Chinese women. Endocrine 36: 377–382. [DOI] [PubMed] [Google Scholar]
- 24. ESHRE ASRM Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41–47. [DOI] [PubMed] [Google Scholar]
- 25. Wellcome Trust Case Control C (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447: 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hofman A, Breteler MM, van Duijn CM, Janssen HL, Krestin GP, et al. (2009) The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol 24: 553–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Day FR, Loos RJ (2011) Developments in obesity genetics in the era of genome-wide association studies. J Nutrigenet Nutrigenomics 4: 222–238. [DOI] [PubMed] [Google Scholar]
- 28. International HapMap C (2003) The International HapMap Project. Nature 426: 789–796. [DOI] [PubMed] [Google Scholar]
- 29. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magi R, Morris AP (2010) GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics 11: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Purcell S, Cherny SS, Sham PC (2003) Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19: 149–150. [DOI] [PubMed] [Google Scholar]
- 32. Lango H, Consortium UKTDG, Palmer CN, Morris AD, Zeggini E, et al. (2008) Assessing the combined impact of 18 common genetic variants of modest effect sizes on type 2 diabetes risk. Diabetes 57: 3129–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cornelis MC, Qi L, Zhang C, Kraft P, Manson J, et al. (2009) Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med 150: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen ZJ, Zhao H, He L, Shi Y, Qin Y, et al. (2011) Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet 43: 55–59. [DOI] [PubMed] [Google Scholar]
- 35. Shi Y, Zhao H, Cao Y, Yang D, Li Z, et al. (2012) Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet 44: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 36. Janssens AC, Moonesinghe R, Yang Q, Steyerberg EW, van Duijn CM, et al. (2007) The impact of genotype frequencies on the clinical validity of genomic profiling for predicting common chronic diseases. Genet Med 9: 528–535. [DOI] [PubMed] [Google Scholar]
- 37. Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, et al. (2013) Longitudinal weight gain in women identified With polycystic ovary syndrome: Results of an observational study in young women. Obesity (Silver Spring) 21: 1526–1532. [DOI] [PubMed] [Google Scholar]
- 38. Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, et al. (2013) The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum Reprod 28: 2276–2283. [DOI] [PubMed] [Google Scholar]
- 39. Colhoun HM, McKeigue PM, Davey Smith G (2003) Problems of reporting genetic associations with complex outcomes. Lancet 361: 865–872. [DOI] [PubMed] [Google Scholar]
- 40. Fradin DD, Fallin MD (2009) Influence of control selection in genome-wide association studies: the example of diabetes in the Framingham Heart Study. BMC Proc 3 Suppl 7S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hattersley AT, McCarthy MI (2005) What makes a good genetic association study? Lancet 366: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 42. Hardy R, Wills AK, Wong A, Elks CE, Wareham NJ, et al. (2010) Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet 19: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Studied BMI-associated loci. SNP Single Nucleotide Polymorphism.
(DOC)
Allele frequencies in cases and controls from the United Kingdom and the Netherlands. SNP Single Nucleotide Polymorphism.
(DOC)
Genetic association results for BMI-increasing risk alleles with PCOS in the United Kingdom and The Netherlands including non-obese cases and controls (BMI <30 kg/m2). * random effect meta-analysis (I2>25%), otherwise fixed effect meta-analysis was performed. chr chromosome, SNP single nucleotide polymorphism, OR odds ratio, CI confidence interval, P p-value.
(DOC)
Genetic association results for BMI-increasing risk alleles with PCOS in the United Kingdom and The Netherlands including obese cases and controls (BMI ≥30 kg/m2). * random effect meta-analysis (I2>25%), otherwise fixed effect meta-analysis was performed. chr chromosome, SNP single nucleotide polymorphism, OR odds ratio, CI confidence interval, P p-value.
(DOC)