Abstract
Recognition of pathogens by plants initiates defense responses including activation of defense-related genes and production of antimicrobial compounds. Recently, we reported that Phytophthora capsici can successfully infect Arabidopsis and revealed interaction specificity among various accession-isolate combinations. We used this novel pathosystem to demonstrate that camalexin, indole glucosinolates (iGS) and salicylic acid (SA) have a role in defense against P. capsici. To further investigate the role of camalexin-, iGS- and SA-related pathways in the differential interaction between Arabidopsis and P. capsici, we monitored expression of marker genes over time during infection. In both compatible and incompatible interactions, induction of expression was detected, but in compatible interactions transcript levels of camalexin and iGS marker genes were higher.
Keywords: Phytophthora capsici, Arabidopsis, defense signaling, salicylic acid, jasmonic acid, ethylene, camalexin, indole glucosinolates
Phytophthora capsici is a pathogen with a broad host range and is particularly devastating in vegetable crops worldwide. Recently, we showed that P. capsici can also infect Arabidopsis and we anticipate that such a model pathosystem will facilitate the genetic dissection of complex traits responsible for P. capsici resistance in crops.1 Microscopic investigation of the infection process revealed that inoculation of Arabidopsis Col-0 with zoospores of the highly virulent P. capsici isolate LT263 resulted in colonization of leaf and root tissue and formation of haustoria followed by sporulation. In contrast, inoculation of Col-0 with zoospores of the intermediate virulent isolate LT123 resulted in an incompatible interaction characterized by callose deposition, accumulation of active oxygen species and cell death.1 We also observed natural variation among Arabidopsis accessions in response to the two isolates; LT263 caused severe disease symptoms on 29 out of 35 Arabidopsis accessions tested, while LT123 could infect only 2 out of the 35, i.e., Ler-0 and Wei-0. Moreover, Arabidopsis mutants deficient in SA signaling and the biosynthesis of camalexin and iGS showed compromised resistance against P. capsici, indicating a role for these components in defense. The aim of this study was to find additional support for the involvement of these secondary metabolites in defense against P. capsici. Therefore we monitored the expression of several defense-related genes in Arabidopsis upon inoculation with P. capsici over time using real-time PCR. For inoculation we used mycelial plugs instead of zoospores; the outcome is similar but disease development is faster thus shortening the time course.1 Based on previous work,1 we selected two P. capsici isolates and two Arabidopsis accessions. This allowed us to compare an incompatible and compatible interaction on either the same accession (Col-0) inoculated with different isolates (LT123 and LT263, respectively) or different accessions (Col-0 and Ler-0, respectively) inoculated with the same isolate (LT123). In these interactions the disease severity index (DSI) as defined previously,1 ranged from 0.2 with hardly any visible symptoms, to 3.9 showing complete collapse of the inoculated leaves (Fig. 1).

Figure 1. Symptoms on Arabidopsis accessions Col-0 and Ler-0 after plug-inoculation with P. capsici isolate LT263 or LT123 at 3 dpi. The disease severity index (DSI) determined according to Wang et al.,1 is indicated. The white arrowheads point to inoculated leaves.
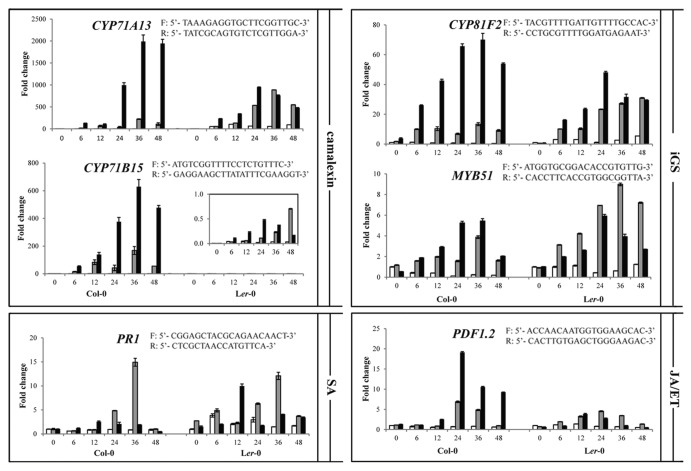
The cytochrome P450 monooxygenase (CYP) encoding genes CYP71B15 (PAD3) and CYP71A13 were selected as marker genes for the biosynthesis of camalexin.2,3 Another CYP gene, CYP81F2 and the gene encoding the transcription factor MYB51 were used as markers for the iGS biosynthesis pathway.4,5 In Col-0, inoculation with either P. capsici LT123 or LT263 leads to an increase in transcript levels of these four genes over time with a peak at 36 h post inoculation (hpi), regardless of the compatible and incompatible nature of the interaction. In particular for CYP71B15 and CYP71A13, drastic increases in transcript levels were observed (Fig. 2) whereas this increase did not occur in mock-inoculated leaves. As shown previously1 loss-of-function mutants of these four genes showed gain of susceptibility upon infection with P. capsici LT123, suggesting a role in resistance and thus in incompatible interactions. Remarkably, at all time points the transcript levels were significantly higher in the compatible interaction than in the incompatible interaction (Fig. 2). This might be due to the fact that in LT263-infected Col-0 leaves, many more cells are colonized thus resulting in a stronger response to infection, especially for the camalexin-related marker genes. In several pathosystems, camalexin accumulation as well as transcripts of relevant biosynthesis genes were found to be strictly limited to the infection sites and correlated with the spatial expansion of the pathogen.6,7 To investigate if this holds for the P. capsici-Arabidopis pathosystem, we checked expression of these marker genes during infection of Arabidopsis Ler-0. This accession is susceptible to LT123 (DSI 2.1), but even more susceptible to LT263 (DSI 3.9). In these compatible interactions transcript levels of CYP71A13 were higher than in the incompatible interaction of Col-0 with LT123 (DSI 0.2) but overall lower than in the compatible interaction of Col-0 with LT263 (DSI 3.7). Remarkably, in Ler-0 basal expression of CYP71B15 is extremely low when compared with Col-0. Nevertheless, also in infected Ler-0 leaves the CYP71B15 transcript levels increased dramatically (more than 100-fold after 24 hpi), as shown in the inset in Figure 2. During time, the peak in expression in LT263-infected Ler-0 leaves (with a higher DSI) was at 24 hpi, so slightly earlier than in LT123-infected Ler-0 leaves (with a lower DSI) and in Col-0, but overall the transcript changes followed a similar pattern in all interactions. Taken together we found no evidence for a strict correlation between transcript levels and DSI. We do however, observe a consistent pattern for the two camalexin marker genes, namely induction of expression upon inoculation and overall higher transcript levels in compatible compared with incompatible interactions. A similar strong increase in the compatible interaction was found for one of the iGS biosynthesis marker genes, i.e., CYP81F2. For the other one, MYB51, the differences between transcript levels in the Col-0 compatible and incompatible interaction were less pronounced and in Ler-0, MYB51 transcript levels in leaves with the lower DSI (2.1) were overall higher than in those with a higher DSI (3.9).
Figure 2. Transcript levels of CYP71A13, CYP71B15, CYP82F2, MYB51, PR1 and PDF1.2 genes during compatible and incompatible Arabidopsis-P. capsici interactions.Four-to-five-week-old Arabidopsis leaves were plug-inoculated with P. capsici LT123 (gray bars) and LT263 (black bars). Six circular leaf discs (Ø 1 cm), with the inoculation spot in the center, were collected from three plants at the time points indicated on the X-axes (hours post inoculation). Subsequently, total RNA was isolated and used as template for cDNA synthesis. Transcript levels were quantified by real-time PCR using gene-specific primers of which the sequences are shown in the figure and were normalized with ArabidopsisActin 2 (F: 5′-TAACTCTCCCGCTATGTATGTCGC-3′; R: 5′-GAGAGAAACCCTCGTAGATTGGC-3′). Values on the Y-axes are expressed as mean fold changes (± SD) relative to the transcript level in mock-inoculated Col-0 leaves (white bars) at time point 0 which was arbitrary set as 1. Experiments were repeated twice with similar results.
PR1, the gene that encodes a pathogenesis-related protein, was selected as marker gene for the SA pathway.8 In Col-0, PR1 expression was found to be induced during the incompatible interaction with P. capsici isolate LT123 with a maximum at 36 hpi followed by a rapid decline to the basal level. In the compatible interaction with isolate LT263, PR1 transcript accumulation was triggered faster, but to a lesser extent. Also in the compatible interactions of Ler-0 with LT123 and LT263, PR1 expression was triggered rather fast and the fold increase approached the levels observed in the incompatible interaction in Col-0. As a marker gene for the JA/ET-pathway we used PDF1.2.8 In Col-0, the PDF1.2 transcript levels started to increase from 12 hpi, reaching a peak at 24 hpi and then decreasing steadily in both the compatible and incompatible interaction. Transcript levels in the compatible interaction, however, are significantly higher than in the incompatible interaction. Also in Ler-0 PDF1.2 transcript levels increase upon inoculation but to a much lesser extent than in Col-0.
In summary and in line with our recent report1 in which we screened the response of Arabidopsis mutants to P. capsici and revealed requirement of SA, camalexin and iGS for resistance to this pathogen, we found induced expression of relevant marker genes in Arabidopsis challenged with P. capsici. Variations in expression levels were detected in different accession-isolate combinations and for nearly all marker genes expression was higher in compatible interactions as compared with incompatible interaction. The expression profiles, however, do not reveal any obvious correlation between expression levels and disease phenotype. These results raise the question why in the absence of proteins encoded by the camalexin and iGS marker genes, so in the mutants, the plants are susceptible and can no longer stop the pathogen while in wild-type plants these genes are highly induced upon successful infection. When assuming that expression indeed leads to protein production, one would expect that in the compatible interaction camalexin and iGS are produced but apparently not in time or at sufficient levels to block disease development.
Acknowledgments
This project was financially supported by a Wageningen University sandwich PhD fellowship and a Huygens scholarship (Y.W.) and by the Centre for BioSystems Genomics (CBSG) (K.B., F.G.), which is part of the Netherlands Genomics Initiative /Netherlands Organisation for Scientific Research.
Glossary
Abbreviations:
- SA
salicylic acid
- JA
jasmonic acid
- ET
ethylene
- iGS
indole glucosinolates
- DSI
disease severity index
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24618
References
- 1.Wang Y, Bouwmeester K, van de Mortel JE, Shan W, Govers F. A novel Arabidopsis-oomycete pathosystem; differential interactions with Phytophthora capsici reveal a role for camalexin, indole glucosinolates and salicylic acid in defense. Plant Cell Environ. 2013;36(1):224–3. doi: 10.1111/pce.12052. [DOI] [PubMed] [Google Scholar]
- 2.Nafisi M, Goregaoker S, Botanga CJ, Glawischnig E, Olsen CE, Halkier BA, et al. Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis. Plant Cell. 2007;19:2039–52. doi: 10.1105/tpc.107.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuhegger R, Nafisi M, Mansourova M, Petersen BL, Olsen CE, Svatos A, et al. CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol. 2006;141:1248–54. doi: 10.1104/pp.106.082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gigolashvili T, Berger B, Mock HP, Müller C, Weisshaar B, Flügge UI. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007;50:886–901. doi: 10.1111/j.1365-313X.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- 5.Pfalz M, Vogel H, Kroymann J. The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell. 2009;21:985–99. doi: 10.1105/tpc.108.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glawischnig E, Hansen BG, Olsen CE, Halkier BA. Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:8245–50. doi: 10.1073/pnas.0305876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuhegger R, Rauhut T, Glawischnig E. Regulatory variability of camalexin biosynthesis. J Plant Physiol. 2007;164:636–44. doi: 10.1016/j.jplph.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SCM, et al. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta. 2010;232:1423–32. doi: 10.1007/s00425-010-1265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]