Abstract
Auxin response factors (ARFs), together with auxin/indole acetic acid proteins (Aux/IAAs), are transcription factors that play key roles in regulating auxin-responsive transcription in plants. Current models for auxin signaling predict that auxin response is dependent on ARF-Aux/IAA interactions mediated by the related protein-protein interaction domain (i.e., referred to as the CTD) found in the ARF and Aux/IAA C-terminal regions. When auxin concentrations in a cell are low, ARF activators residing on the promoters of auxin response genes are thought to be inactive because of the association with dominant Aux/IAA repressors. When auxin concentrations are elevated, the Aux/IAA repressors are recruited to auxin receptors and degraded via the ubiquitin-proteasome pathway. Destruction of the Aux/IAA repressors allows the ARF activators to function in derepressing/activating auxin response genes. While this auxin signaling pathway is simple and attractive, it is unclear whether auxin-regulated gene expression is solely dependent on ARF-Aux/IAA interactions. Here we show that auxin can affect the expression of auxin response genes in a manner that is independent of the ARF activator CTD.
Keywords: auxin, auxin response factors, auxin response gene, DNA-binding domain, activation domain, carboxyl terminal domain, protoplast transfection
Introduction
A major auxin signaling pathway is known to involve the rapid regulation of auxin response genes.1 This pathway consists of several well-known components, including auxin response factors (ARFs) that function as either transcriptional activators or repressors, auxin/indole acetic acid (Aux/IAA) transcriptional repressors, the topless (TPL) co-repressor and the transport inhibitor response1 (TIR1) receptor families of proteins. ARFs contain a conserved N-terminal DNA-binding domain (DBD or D) that targets TGTCTC and related auxin response elements (AuxREs), a non-conserved middle region (MR or M) that functions as a portable activation (AD) or repression domain (RD), and in most cases, a conserved C-terminal protein-protein interaction domain (CTD or C) that resembles PB1 domains found throughout eukaryotes and facilitates interactions among ARF and Aux/IAA proteins.2,3 Aux/IAA proteins contain an N-terminal EAR-like repression domain (e.g., Glu/AspLeuXLeuXLeu, where X may be one of several different amino acids,4,5 an adjacent degron domain that targets Aux/IAA proteins to the ubiquitin-proteasome pathway in an auxin-dependent manner and a CTD that resembles the CTD found in ARFs and facilitates protein-protein interactions among ARF and Aux/IAA proteins.3,6 TPL is a Groucho/Tup1-like co-repressor that interacts with the EAR-like repression domain in Aux/IAA proteins and other repressors and presumably modifies chromatin to a repressive state.7-9 TIR1 is both an auxin receptor and a part of the SCF ubiquitin ligase complex that binds both auxin and the degron of Aux/IAA proteins and facilitates the entry of Aux/IAA repressors into the ubiquitin-proteasome pathway.6
Models for auxin signaling predict that the auxin response is dictated by ARF-Aux/IAA interactions, which are mediated by the related CTD protein-protein interaction domain found in both families of transcription factors.3 It has been proposed that ARFs are targeted to AuxREs in promoters of auxin response genes independently of auxin levels in cells.2,10,11 ARF activators residing on promoters of auxin response genes are thought to be inactive when cells contain low amounts of free auxin, because they are associated with dominant Aux/IAA repressors. When the amount of auxin within cells rises, the ARFs are freed from the Aux/IAA repressors and allowed to derepress/activate auxin response genes.2 In turn, the Aux/IAA repressors are recruited to the TIR1 SCF-ubiquitin ligase complex where they become ubiquitinated and shuttled to the proteasome for destruction.6 While experimental evidence is largely consistent with this model for auxin signaling, it remains possible that additional complexity for auxin-mediated transcription remains to be discovered. Here we show that ARF activators lacking a CTD can nevertheless regulate gene expression in an auxin-dependent manner, suggesting that ARF-Aux/IAA interactions are not strictly required for auxin-responsive gene expression.
Results
ARF5 and ARF7 lacking a CTD show an auxin response when tested on integrated auxin-responsive reporter genes in transfected protoplasts
To assess whether an ARF activator CTD was strictly required for auxin-responsive gene expression, full-length ARF effector genes, 35S:ARF5 and 35S:ARF7, and ARF effector genes lacking a CTD, 35S:5D5M and 35S:7D7M, were transfected into Arabidopsis nph4-1/arf7 mutant mesophyll protoplasts containing an integrated auxin-responsive 2XD0:GUS reporter gene.11,12 Effector genes lacking the CTD are designated with a D and M for the DNA-binding domain and middle region containing the AD, respectively. The 2XD0 promoter consists of two copies of a 72 bp region in the soybean GH3 promoter that confers auxin responsiveness when fused to a minimal CaMV 35S promoter.13
Protoplasts isolated from nph4-1/arf7 mutant were used for transfection assays because these protoplasts display very low levels of auxin-responsive gene expression unless they are transfected with an ARF activator.11Figure 1A shows that protoplasts transfected with effector genes encoding full-length ARF5 or ARF7 had reporter gene expression that was strongly inducible by auxin (i.e., 5-to 30-fold), while protoplasts transfected with effector genes encoding ARF5 or ARF7 CTD truncations (5D5M and 7D7M) had reporter gene expression that was reduced in terms of an auxin response (i.e., 4-to 8-fold), but, nevertheless, still showed a response to auxin. These results indicated that the ARF activator CTD is not absolutely required for auxin response with an integrated 2XD0:GUS reporter gene in protoplast transfection assays.
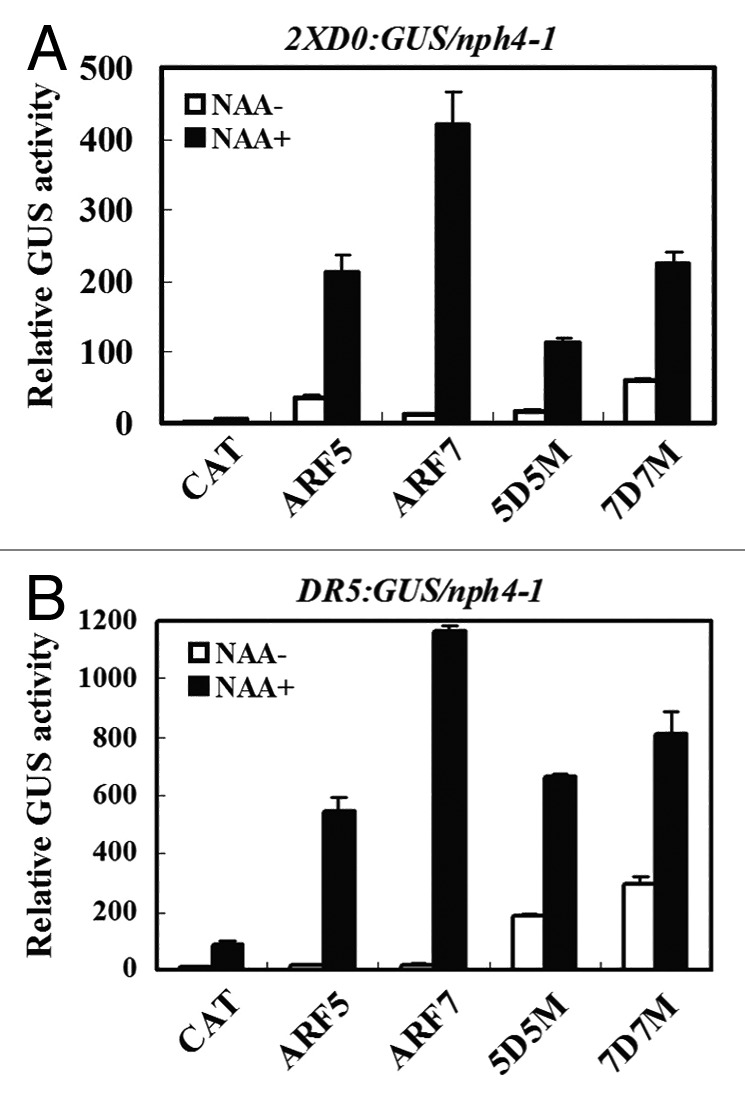
Figure 1. Expression of an integrated auxin-responsive reporter gene in Arabidopsis nnph4-1/arf7 protoplasts transfected with full-length or CTD truncated ARF5 and ARF7 proteins. Truncated constructs are denoted as 5D5M and 7D7M. Arabidopsis mesophyll protoplasts were isolated from nph4-1/arf7 leaves containing a stably integrated 2XD0:GUS (A) or DR5:GUS (B) reporter gene, full-length or CTD truncated ARF5 and ARF7 effector genes were transfected into protoplasts and incubated in darkness in the presence (black columns) and absence (white columns) of 1 μM 1-NAA (1-naphthalene acetic acid) for 20–22 h and then GUS activities were measured.11 A 35S:CAT (chloramphenicol acetyltransferase) plasmid was used as a control for the amount of effector plasmid DNA (10 μg) introduced into protoplasts. Data represent mean ± SD of three replicates. In some case, error bars may not visible because of their small size.
To rule out the possibility that something special about the 2XD0:GUS reporter gene might be responsible for the auxin response observed with ARF activator CTD truncated proteins, transfection assays were also performed with nph4/arf7 protoplasts containing an integrated DR5:GUS reporter gene. Figure 1B shows that transfection of protoplasts with effector genes encoding full-length ARF5 or ARF7 enhanced reporter gene expression in a strongly auxin-dependent manner, as described previously.11 In contrast, transfection with effector genes encoding ARF5 or ARF7 CTD truncations (5D5M and 7D7M) restored reporter gene expression, but the restoration in expression was not fully dependent on auxin. Nevertheless, addition of auxin enhanced expression of the DR5:GUS reporter gene by 2–3-fold, supporting results observed with the 2XD0:GUS reporter gene (Fig. 1A). Thus, results with both auxin response reporter genes indicate that an auxin response is not fully dependent on the CTDs of ARF5 and ARF7.
An AD is sufficient to confer an auxin response with an ARF5 DBD
We next tested whether the glutamine-rich AD of the ARF5 CTD truncated protein could be substituted with a heterologous AD or RD in maintaining an auxin response. To carry out these experiments, the ARF7 glutamine-rich AD, the herpes simplex virus VP16 acidic AD and the proline-rich ARF1 RD were swapped for the ARF5 AD to create 35S:5D7M, 35S:5DVP and 35S:5D1M, respectively. Figure 2 shows that nph4-1/arf7 protoplasts transfected with either the 35S:5D7M or 35S:5DVP effector gene showed an auxin response as did 35S:5D5M, but the 35S:5D1M effector gene showed no response, similar to the CAT control. These results suggest that an AD is required for an auxin response with the ARF5 CTD truncated protein, but ARF7 glutamine-rich AD and the VP16 acidic AD can substitute for the ARF5 glutamine-rich AD.
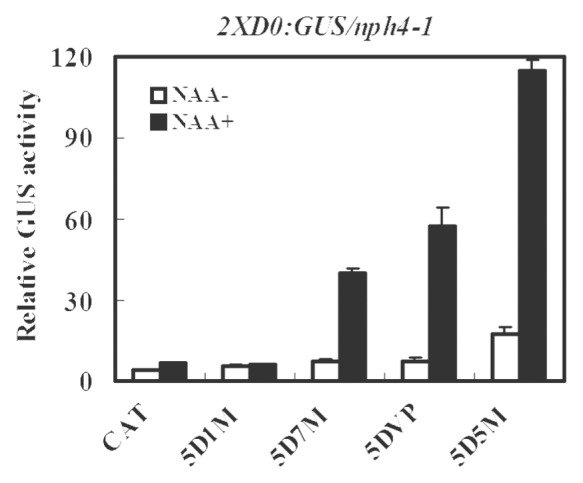
Figure 2. Expression of an integrated 2XD0:GUS auxin-responsive reporter gene in Arabidopsis nph4-1/arf7 protoplasts transfected with CTD truncated ARF5 (5D5M) or the ARF5 DBD fused to ARF7 (5D7M) or VP16 AD (5DVP) or the ARF1 RD (5D1M). Assays were conducted as described in Figure 1.
Expression of ARF5 CTD truncated proteins with substituted DBDs show an auxin response
We then tested whether the DBD of the ARF5 CTD truncated protein could be substituted with a heterologous DBD from an activator or repressor ARF. To carry out these experiments, the ARF1, ARF6 or ARF7 DBD was used to substitute the ARF5 DBD in the ARF5 CTD truncated protein to generate 35S:1D5M, 35S:6D5M and 35S:7D5M respectively. Figure 3 shows that protoplasts transfected with 35S:1D5M, 35S:6D5M and 35S:7D5M all showed an auxin response as did 35S:5D5M. These results show that a repressor ARF1 DBD or activator ARF6 or ARF7 DBD can be substituted for the ARF5 DBD in ARF CTD truncated proteins and suggest that a specific type of ARF DBD may not be important for the auxin response with ARF activator CTD truncated proteins (i.e., there is not something special about ARF5 or ARF7 DBD in targeting the CTD truncated ARF to 2XD0 auxin response elements and conferring an auxin response).
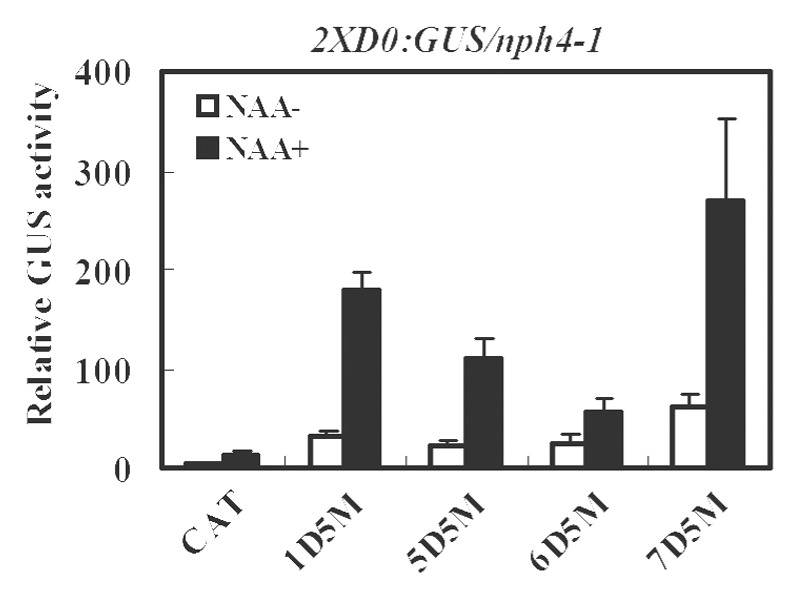
Figure 3. Expression of an integrated 2XD0:GUS auxin-responsive reporter gene in Arabidopsisnph4-1/arf7 protoplasts transfected with CTD truncated ARF5 (5D5M) or the ARF1 (1D5M), ARF6 (6D5M) or ARF7 DBD (7D5M) fused to ARF5 AD. Assays were conducted as described in Figure 1.
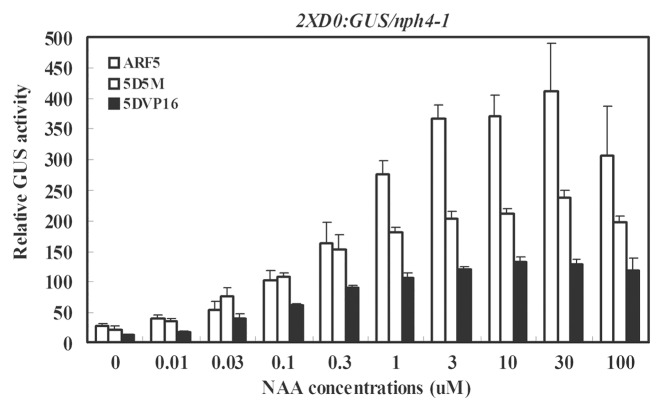
The auxin dose response shows a similar pattern for full-length ARF5 and ARF5 CTD truncated proteins in protoplast transfection assays
To determine if the dose response to exogenous auxin is similar or different for protoplasts transfected with the full-length ARF5 effector gene and CTD-truncated versions of the ARF5 effector genes, 35S:ARF5, 35S:5D5M and 35S:5DVP were transfected into protoplasts. Figure 4 shows that the dose response for the three effector genes follows a similar pattern with a maximal response between 3 to 100 μM NAA. Thus, a similar amount of auxin is required for an auxin response on the 2XD0:GUS reporter gene whether the response is driven by a full-length ARF activator or an ARF activator lacking a CTD.
Figure 4. Dose response for 1-NAA on the expression of an integrated 2XD0:GUS auxin-responsive reporter gene in Arabidopsisnph4-1/arf7 protoplasts transfected with full-length or CTD truncated ARF5 or the ARF5 DBD fused to the VP16 AD. Assays were conducted as described in Figure 1 using the concentration of 1-NAA indicated.
Discussion
As pointed out in the Introduction, current models for auxin-regulated gene expression suggest that auxin response is dictated by ARF-Aux/IAA interactions, and these interactions are dependent on related CTD protein-protein interaction domains found in ARF activator and Aux/IAA repressor proteins.3,10,11,14 Our results presented here suggest, however, that these models may not fully explain how auxin-responsive gene expression is conferred, because an auxin response is still observed with two different reporter genes that are targeted by ARFs lacking a CTD.
Parts of the current model for auxin-responsive gene expression are based on results of Tiwari et al.,10 who observed that effector genes encoding 35S:5D5M, 35S:5DVP, 35S:1D5M and 35S:1DVP conferred high level constitutive expression (in the presence or absence of exogenous auxin, with little or no auxin enhancement) when transfected along with a P3(4X):GUS reporter gene into wild type carrot suspension cell protoplasts. This same study showed that the CTD of ARF5 was able to confer auxin-responsiveness to an effector gene encoding the yeast Gal4 DBD (GD) fused to the ARF5 MC, creating 35S:GD5M5C, when tested in carrot protoplasts with a transfected Gal(4X):GUS reporter gene. Co-transfection of a second effector gene, 35S:IAA17mII (i.e., a stabilized version of Arabidopsis IAA17), resulted in complete loss of Gal(4X):GUS expression in the presence or absence of auxin. In total, these results are consistent with the ARF5 CTD being required for an auxin response and supported the current model for auxin-regulated gene expression. Conclusions reached from the above protoplast transfection experiments do not, however, rule out additional mechanisms for auxin-responsive gene expression, as suggested from results presented in this manuscript.
Why might the results in the Tiwari et al.10 studies differ from those reported here? The current studies were conducted with Arabidopsis nph4-1/arf7 mesophyll protoplasts that have no ARF7 gene expression and show very little expression of the stably integrated, single copy 2XD0:GUS and DR5:GUS reporter genes whether or not auxin is applied to the protoplasts.11,15 With the nph4-1/arf7 protoplasts, the integrated reporter genes are tightly regulated in response to auxin, and expression of the integrated reporter genes as well as selected endogenous auxin response genes require transfection of an ARF activator or an ARF DBD fused to an AD to activate the auxin response genes.11,15 In contrast, the Tiwari et al.10 experiments relied on wild type carrot protoplasts and a transfected P3(4X):GUS reporter gene (i.e., an auxin-responsive promoter consisting of inverted repeats of the TGTCTC AuxRE) that, when transfected, might not be present or might be present at one or more copies within protoplasts. The carrot protoplast transfection assays were less tightly regulated than the nph4-1/arf7 protoplast assays in response to auxin and did not require an ARF activator effector gene to observe an auxin response. For these reasons, the nph4-1/arf7 protoplast system along with the two types of integrated auxin response reporter genes described by Wang et al.11 is a more sensitive and auxin-responsive system than protoplast systems like those used by Tiwari et al.,10 and this might explain why auxin-responsive gene expression that was not dependent on the CTD was revealed in the current experiments, but not in earlier experiments reported by Tiwari et al.10
How does one explain the auxin responsiveness observed with ARFs that lack CTD domains? It is possible that a proper chromatin environment and promoter composition are required to observe an effect on auxin-responsive gene expression that does not involve the ARF CTD. There is compelling evidence for the importance of the ARF and Aux/IAA CTDs in conferring auxin response gene expression in transient expression systems10,11,16 as well as in transformed plants.5,17-19 There is little evidence to date that suggests auxin response genes might have additional levels of regulation that go beyond the CTD; however, Walcher and Nemhauser20 have recently reported that ARF5 becomes enriched on the SAUR15 promoter in response to auxin as well as brassinolide in ChIP (chromatin immunoprecipitation) assay experiments. These results suggest that auxin levels might influence the targeting of ARFs to at least some AuxREs in promoters of auxin response genes. This might occur through direct effects on ARF binding to DNA target sites or through interactions with other transcription factors or chromatin-associated proteins.
Potential involvement of chromatin modifications in negatively regulating auxin response gene expression is suggested from studies with TPL, which likely plays a role in inducing a repressive chromatin state through posttranslational histone modifications (i.e., histone deacetylation and methylation/demethylation) on auxin response genes.9 Other results suggest that the reversal of this process (i.e., induction of an active chromatin state through histone acetylation and methylation/demethylation) plays an important role in the activation of auxin response genes.21-23 It seems likely that chromatin modifications could impact on the binding of ARFs to their AuxRE target sites. Auxin might play a direct or indirect role in altering the state of chromatin and thus influence the binding of ARFs to auxin response promoters. Phosphorylation of ARF proteins (e.g., ARF2) might also influence their DNA-binding activity and phosphorylation of ARF2 may be an auxin-dependent process.24,25 The study reported here along with the chromatin and phosphorylation studies summarized above suggest that it would be worthwhile to launch investigations into mechanisms involved in auxin-responsive gene expression that explore areas beyond ARF-Aux/IAA CTD interactions on promoters of auxin response genes. Our results further suggest that employing integrated reporter genes in protoplast transfection assays11,15 may provide additional insight into how transcription factors might regulate hormone-responsive genes and other genes through multiple levels of control.
Materials and Methods
Plant materials and growth conditions
Arabidopsisnph4-1/arf7 mutant plants with an integrated auxin response reporter gene 2XD0:GUS or DR5:GUS were used in this study.12,26Arabidopsis seeds were germinated in 3×3-inch pots containing Pro-Mix soil (Premier Horticulture) and grown at 20°C under constant illumination. Leaves from 3–5-wk-old plants were used for protoplast isolation, as described previously.11,15
Effector constructs
Constructs including full-length ARF5 and ARF7 and their CTD truncated protein constructs ARF5DBD5MR (5D5M) and ARF7DBD7MR (7D7M) were described previously.10,11 Domain swap constructs ARF5DBDARF1MR (5D1M), ARF5DBDARF7MR (5D7M), ARF1DBDARF5MR (1D5M) and ARF6DBDARF5MR (6D5M) were made by fusing the designated ARF DBD in-frame with the designated ARF AD or RD. The constructs were cloned into pUC19 vector as described in which all of the constructs had an N-terminal HA tag.10,16 The plasmids were sequenced to confirm PCR and cloning fidelity.
Effector plasmids were prepared using the EndoFree Plasmid Maxi Kit (Qiagen) following the manufacturer’s protocols.
Protoplast isolation and transfection
Protoplast isolation, transfection and GUS activity assays were performed as described previously.11 In all transfection assays, the 35S:CAT gene was used in place of the ARF effector genes as control for the amount of effector plasmid DNA (10 μg) introduced into protoplasts. Two independent protoplast preparations were tested and each construct was assayed with three replications for the independent protoplast preparations. Similar results were obtained with the independent protoplast preparations. Data in figures represent one of the preparations.
Acknowledgments
This work was supported by the National Science Foundation (grant no. MCB 00800096 and IOB 0550417 (to T.J.G. and G.H.) and the University of Missouri Food for the 21st Century Program (to T.J.G.).
Glossary
Abbreviations:
- AD
activation domain
- ARF
auxin response factor
- Aux/IAA
auxin/indole acetic acid
- AuxREs
auxin response elements
- CAT
chloramphenicol acetyltransferase
- CTD
carboxyl terminal domain
- ChIP
chromatin immunoprecipitation
- DBD
DNA-binding domain
- GD
gal4 DNA-binding domain
- MR
middle region
- RD
repression domain
- 1-NAA
1-naphthalene acetic acid
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24526
References
- 1.Hagen G, Guilfoyle TJ. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol. 2002;49:373–85. doi: 10.1023/A:1015207114117. [DOI] [PubMed] [Google Scholar]
- 2.Guilfoyle TJ, Hagen G. Auxin response factors. Curr Opin Plant Biol. 2007;10:453–60. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Guilfoyle TJ, Hagen G. Getting a grasp on domain III/IV responsible for Auxin Response Factor-IAA protein interactions. Plant Sci. 2012;190:82–8. doi: 10.1016/j.plantsci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Tiwari SB, Hagen G, Guilfoyle TJ. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell. 2004;16:533–43. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Tiwari SB, Hagen G, Guilfoyle TJ. Identical amino acid substitutions in the repression domain of AUX/IAA proteins have contrasting effects on auxin signaling. Plant Physiol. 2011;155:1252–63. doi: 10.1104/pp.110.171322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi K-I. The interaction and integration of auxin signaling components. Plant Cell Physiol. 2012;53:965–75. doi: 10.1093/pcp/pcs035. [DOI] [PubMed] [Google Scholar]
- 7.Causier B, Ashworth M, Guo W, Davies B. The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiol. 2012;158:423–38. doi: 10.1104/pp.111.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Causier B, Lloyd J, Stevens L, Davies B. TOPLESS co-repressor interactions and their evolutionary conservation in plants. Plant Signal Behav. 2012;7:325–8. doi: 10.4161/psb.19283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szemenyei H, Hannon M, Long JA. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science. 2008;319:1384–6. doi: 10.1126/science.1151461. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari SB, Hagen G, Guilfoyle TJ. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell. 2003;15:533–43. doi: 10.1105/tpc.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Tiwari SB, Hagen G, Guilfoyle TJ. AUXIN RESPONSE FACTOR7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell. 2005;17:1979–93. doi: 10.1105/tpc.105.031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murfett J, Wang X-J, Hagen G, Guilfoyle TJ. Identification of Arabidopsis histone deacetylase HDA6 mutants that affect transgene expression. Plant Cell. 2001;13:1047–61. doi: 10.1105/tpc.13.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulmasov T, Liu Z-B, Hagen G, Guilfoyle TJ. Composite structure of auxin response elements. Plant Cell. 1995;7:1611–23. doi: 10.1105/tpc.7.10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari SB, Wang X-J, Hagen G, Guilfoyle TJ. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell. 2001;13:2809–22. doi: 10.1105/tpc.010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari SB, Wang S, Hagen G, Guilfoyle TJ. Transfection assays with protoplasts containing integrated reporter genes. Methods Mol Biol. 2006;323:237–44. doi: 10.1385/1-59745-003-0:237. [DOI] [PubMed] [Google Scholar]
- 16.Ulmasov T, Hagen G, Guilfoyle TJ. Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci USA. 1999;96:5844–9. doi: 10.1073/pnas.96.10.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Cheng Y, Murphy A, Hagen G, Guilfoyle TJ. Constitutive repression and activation of auxin signaling in Arabidopsis. Plant Physiol. 2009;149:1277–88. doi: 10.1104/pp.108.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau S, De Smet I, Kolb M, Meinhardt H, Jürgens G. Auxin triggers a genetic switch. Nat Cell Biol. 2011;13:611–5. doi: 10.1038/ncb2212. [DOI] [PubMed] [Google Scholar]
- 19.Krogan NT, Ckurshumova W, Marcos D, Caragea AE, Berleth T. Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. New Phytol. 2012;194:391–401. doi: 10.1111/j.1469-8137.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 20.Walcher CL, Nemhauser JL. Bipartite promoter element required for auxin response. Plant Physiol. 2012;158:273–82. doi: 10.1104/pp.111.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anzola JM, Sieberer T, Ortbauer M, Butt H, Korbei B, Weinhofer I, et al. Putative Arabidopsis transcriptional adaptor protein (PROPORZ1) is required to modulate histone acetylation in response to auxin. Proc Natl Acad Sci USA. 2010;107:10308–13. doi: 10.1073/pnas.0913918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornet N, Scheres B. Members of the GCN5 histone acetyltransferase complex regulate PLETHORA-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis. Plant Cell. 2009;21:1070–9. doi: 10.1105/tpc.108.065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saiga S, Möller B, Watanabe-Taneda A, Abe M, Weijers D, Komeda Y. Control of embryonic meristem initiation in Arabidopsis by PHD-finger protein complexes. Development. 2012;139:1391–8. doi: 10.1242/dev.074492. [DOI] [PubMed] [Google Scholar]
- 24.Vert G, Walcher CL, Chory J, Nemhauser JL. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc Natl Acad Sci USA. 2008;105:9829–34. doi: 10.1073/pnas.0803996105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Zhou H, Berke L, Heck AJR, Mohammed S, Scheres B, et al. Quantitative phosphoproteomics after auxin-stimulated lateral root induction identifies a SNX1 phosphorylation site required for growth. Mol Cell Proteomics. 2013 doi: 10.1074/mcp.M112.021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–71. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]