Abstract
Since the discovery of the CTR1 protein kinase and the endoplasmic reticulum (ER)-localized EIN2 protein nearly 20 y ago, plant biologists have wondered how these proteins respectively serve as negative and positive regulators of ethylene-mediated signal transduction in plants.1,2 Now with the publication of four studies, it can be concluded that in the absence of ethylene (ET) in Arabidopsis thaliana, CTR1 phosphorylates EIN2 thereby inactivating ET signal transduction, while in the presence of ET, CTR1 no longer phosphorylates EIN2 and the cytosolic C-terminus of EIN2 is released from the ER to translocate to the nucleus to promote gene transcription.3-6 Chen et al. (2011) showed that EIN2 is differentially phosphorylated at amino acids (a.a.) S645 and S924 after ET treatment.6 Ju et al. (2012) then proved that CTR1 phosphorylates EIN2 at those positions and that the lack of phosphorylation at S645 and S924 leads to the translocation of an EIN2 C-terminus peptide.5 Wen et al. (2012) and Qiao et al. (2012) also demonstrated ET-induced translocation of an EIN2 C-terminus peptide, while Qiao et al. (2012) proved that EIN2 has a nuclear localization signal sequence required for translocation, confirmed phosphorylation at S645 and said that proteolytic cleavage occurs at S645 in absence of phosphorylation there.3,4 Despite the revelation of this elegant switch, there are contradictory indications for specific cleavage at EIN2 S645. This article investigates the data and concludes that EIN2 may be cleaved at alternative positions.
Keywords: ethylene signaling, triple response, mass spectrometry, semi-tryptic peptide, phosphorylation
The first sign of inconsistency has to do with the theoretical molecular weight of the EIN2 C-terminus from S645 to the stop codon which is approximately 70 kDa. A 70 kDa fragment could migrate to the nucleus if cleavage occurred at S645, but the Qiao et al. immunoblots (see Fig. 3A and B in ref.3) showed an ~80 kDa EIN2 C-terminus peptide. The EIN2 C-terminus-YFP fusion peptide (Fig. 4G and H in ref. 3) was also larger than expected from an S645 cleavage site. Although protein sizes can be difficult to estimate by SDS-PAGE, a cleavage site upstream of S645 could reasonably explain the ~10 kDa size excess. Wen et al. also revealed a product larger than predicted by cleavage at S645 and several smaller fragments. Hence, the immunoblots do not appear to be consistent with a single cleavage position at S645.
Qiao et al. reasoned that S645 is the cleavage site because EIN2 is differentially phosphorylated at S645 as originally shown by Chen et al. Subsequently, Qiao et al. used pseudo-multiple reaction monitoring (pMRM) mass spectrometry to detect changes in abundance between EIN2 tryptic peptides and their phosphorylated analogs before and after ET treatment. According to the Qiao et al. model (rendered in Fig. 1), if EIN2 is cleaved at S645 after ET treatment, then the C-terminus moves from the ER to the nucleus. This means that the abundance of peptides downstream of S645 should decrease in ER membrane fractions after ET treatment and concomitantly increase in the nucleus. Immunoblots showing a preponderance of EIN2 antigen in nuclear preparations after ET treatment were consistent with the model (Fig. 3B in ref.3; Fig. 3K in ref. 4), but the abundances of downstream peptides measured by pMRM were inconsistent (Table S1A in ref. 3): Assuming that pMRM precisely measured a 10-fold change for phosphopeptide a.a. 648–662 in ER membranes, then the data unexpectedly showed no decrease in abundance of the analogous nonphosphorylated peptide a.a. 648–662 after ET treatment.
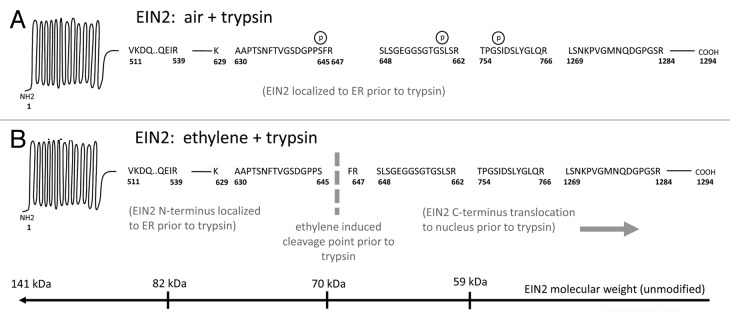
Figure 1. EIN2 phosphorylation and cleavage products based on Qiao et al.3(A) EIN2 in absence of ET (ambient air exposure) and digested with trypsin. EIN2 is phosphorylated (circled p) and remains anchored at the ER. (B) EIN2 in the presence of ET. EIN2 is not phosphorylated and is cleaved in vivo at S645 and the EIN2 C-terminus translocates to the nucleus (prior to trypsin digestion). The bottom bar shows the approximate molecular weight of native EIN2 as measured from the C-terminus. The author contends that the ET-induced cleavage site in model in B is not sufficiently proven by published results.
Interestingly, Qiao et al. observed a 19-fold decrease for nonphosphorylated peptide a.a. 630–647 after ET treatment, but they attributed this to cleavage at S645 even though upstream cleavage would also explain the observation. It may be unintuitive why Qiao et al. reached that conclusion, so Figure 1 is provided for clarity. The amino acids K629 and R647 are trypsin digestion sites that flank S645 and after ET treatment and tryptic digestion the semi-tryptic peptide a.a. 630–645 should become more prevalent with increasing amounts of nonphosphorylated EIN2. Thus, Qiao et al. concluded that tryptic peptide a.a. 630–647 decreased because of prior, ET-mediated proteolytic cleavage at S645. Thus, they expected this would lead to an increased semi-tryptic variant after ET treatment and trypsin digestion and they found evidence of this by pMRM, which they cited as proof of cleavage at S645. Since the model dictates that the semi-tryptic peptide is prevalent after ET treatment, I re-examined the mass spectrometry data from Chen et al. which were sufficient to reveal differential phosphorylation of EIN2 at S645.6 Mascot searches for semi-tryptic termini and error-tolerant searches for hundreds of mass deviations reconfirmed the phosphorylated tryptic peptide a.a. 630–647 in ambient air control seedlings and the nonphosphorylated form in ET-treated seedlings (Table 1). There was, however, no other prevalent mass modification in EIN2 peptides and the semi-tryptic peptide a.a. 630–645 was not apparent (Table 1). Of course, not finding a peptide by shotgun mass spectrometry rarely invalidates its existence,7 but it is suspect that an essential nonphosphorylated semi-tryptic peptide predicted by Qiao et al. was more difficult to observe than its inherently-difficult-to-detect phosphorylated precursor. Thus, it can be argued that the available pMRM and shotgun proteomics data do not conclusively support EIN2 cleavage at S645.
Table 1. Peptide-tandem mass spectrum matches from air (control) and ET-treated A. thaliana etiolated seedlings.
| Air treated (control) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spectrum query | EIN2 amino acid start | EIN2 amino acid end | Observed m/z | Mr (expt) | Mr (calc) | Parent ion ppm error | Mascot Ions score | Mascot expect value | EIN2 peptide sequence match |
| 329 | 502 | 506 | 603.345 | 602.3378 | 602.3388 | −1.67 | 25 | 0.063 | D.ESIVR.L |
| 1221474 | 511 | 539 | 1104.5542 | 3310.6408 | 3309.6286 | 306 | 51 | 0.00039 | R.VKDQLDTTSVTSSVYDLPENILMTDQEIR.S |
| 1221420 | 511 | 539 | 1104.2208 | 3309.6406 | 3309.6286 | 3.63 | 41 | 0.0035 | R.VKDQLDTTSVTSSVYDLPENILMTDQEIR.S |
| 1221418 | 511 | 539 | 1104.2197 | 3309.6374 | 3309.6286 | 2.64 | 36 | 0.0091 | R.VKDQLDTTSVTSSVYDLPENILMTDQEIR.S |
| 811749 | 552 | 567 | 886.933 | 1771.8515 | 1771.8476 | 2.23 | 44 | 0.00092 | K.YSTSQVSSLKEDSDVK.E |
| 812432 | 552 | 567 | 887.4352 | 1772.8558 | 1771.8476 | 569 | 45 | 0.00098 | K.YSTSQVSSLKEDSDVK.E |
| 811742 | 552 | 567 | 886.9304 | 1771.8463 | 1771.8476 | −0.73 | 34 | 0.006 | K.YSTSQVSSLKEDSDVK.E |
| 812434 | 552 | 567 | 887.4361 | 1772.8576 | 1771.8476 | 570 | 33 | 0.0073 | K.YSTSQVSSLKEDSDVK.E |
| 812425 | 552 | 567 | 887.4332 | 1772.8519 | 1771.8476 | 567 | 25 | 0.091 | K.YSTSQVSSLKEDSDVK.E |
| 878122 | 630 | 647 | 944.4185 | 1886.8224 | 1886.82 | 1.24 | 92 | 0.00000033 | K.AAPTSNFTVGSDGPPSFR.S + Phospho S (S) |
| 878123 | 630 | 647 | 944.4192 | 1886.8238 | 1886.82 | 2.01 | 78 | 0.0000028 | K.AAPTSNFTVGSDGPPSFR.S + Phospho S (S) |
| 878470 | 630 | 647 | 944.9172 | 1887.8199 | 1886.82 | 530 | 64 | 0.000017 | K.AAPTSNFTVGSDGPPSFR.S + Phospho S (S) |
| 878121 | 630 | 647 | 944.4182 | 1886.8219 | 1886.82 | 0.98 | 56 | 0.000034 | K.AAPTSNFTVGSDGPPSFR.S + Phospho S (S) |
| 878126 | 630 | 647 | 944.4207 | 1886.8269 | 1886.82 | 3.63 | 62 | 0.00005 | K.AAPTSNFTVGSDGPPSFR.S + Phospho S (S) |
| 878117 | 630 | 647 | 944.4091 | 1886.8036 | 1886.82 | −8.72 | 58 | 0.000087 | K.AAPTSNFTVGSDGPPSFR.S + Phospho S (S) |
| 878472 | 630 | 647 | 630.2815 | 1887.8227 | 1886.82 | 531 | 26 | 0.096 | K.AAPTSNFTVGSDGPPSFR.S + Phospho S (S) |
| 936929 | 648 | 666 | 668.6009 | 2002.7807 | 2001.7847 | 498 | 33 | 0.06 | R.SLSGEGGSGTGSLSRLQGL.G + 2 Phospho S (S); Phospho T (T) |
| 423230 | 700 | 710 | 646.8458 | 1291.6771 | 1291.6772 | −0.094 | 44 | 0.0026 | K.KLDQLFGTDQK.S |
| 423193 | 700 | 710 | 646.8409 | 1291.6673 | 1291.6772 | −7.65 | 30 | 0.075 | K.KLDQLFGTDQK.S |
| 542771 | 754 | 766 | 703.8643 | 1405.714 | 1405.7201 | −4.39 | 69 | 0.000033 | R.TPGSIDSLYGLQR.G |
| 542775 | 754 | 766 | 703.8674 | 1405.7203 | 1405.7201 | 0.12 | 59 | 0.000064 | R.TPGSIDSLYGLQR.G |
| 542786 | 754 | 766 | 703.8703 | 1405.726 | 1405.7201 | 4.2 | 50 | 0.00038 | R.TPGSIDSLYGLQR.G |
| 542779 | 754 | 766 | 703.8681 | 1405.7217 | 1405.7201 | 1.13 | 44 | 0.0011 | R.TPGSIDSLYGLQR.G |
| 608871 | 754 | 766 | 743.8513 | 1485.6881 | 1485.6865 | 1.09 | 31 | 0.04 | R.TPGSIDSLYGLQR.G + Phospho S (S) |
| 608879 | 754 | 766 | 743.8526 | 1485.6907 | 1485.6865 | 2.87 | 35 | 0.068 | R.TPGSIDSLYGLQR.G + Phospho S (S) |
| 274965 | 835 | 843 | 551.3035 | 1100.5925 | 1100.5938 | −1.16 | 45 | 0.0099 | K.ERLEALQSR.G |
| 754564 | 922 | 936 | 841.355 | 1680.6954 | 1680.6888 | 3.91 | 31 | 0.0072 | K.YSSMPDISGLSMSAR.N + Phospho S (S) |
| 52751 | 1211 | 1217 | 699.45 | 698.4427 | 698.4439 | −1.65 | 20 | 0.062 | A.AKPAKGK.C |
| 790762 | 1269 | 1284 | 868.8923 | 1735.77 | 1735.7713 | −0.74 | 31 | 0.062 | R.LSNKPVGMNQDGPGSR.K + Phospho S (S) |
| Ethylene treated | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum query | EIN2 amino acid start | EIN2 amino acid end | Observed m/z | Mr (expt) | Mr (calc) | Parent ion ppm error | Mascot Ions score | Mascot expect value | EIN2 peptide sequence match | |
| 902435 | 511 | 539 | 1104.2163 | 3309.6271 | 3309.6286 | −0.46 | 44 | 0.0012 | R.VKDQLDTTSVTSSVYDLPENILMTDQEIR.S | |
| 902516 | 511 | 539 | 1104.5494 | 3310.6265 | 3309.6286 | 302 | 34 | 0.016 | R.VKDQLDTTSVTSSVYDLPENILMTDQEIR.S | |
| 902436 | 511 | 539 | 1104.2182 | 3309.6327 | 3309.6286 | 1.24 | 29 | 0.056 | R.VKDQLDTTSVTSSVYDLPENILMTDQEIR.S | |
| 902520 | 511 | 539 | 1104.5546 | 3310.6419 | 3309.6286 | 306 | 31 | 0.087 | R.VKDQLDTTSVTSSVYDLPENILMTDQEIR.S | |
| 518666 | 552 | 567 | 591.6218 | 1771.8437 | 1771.8476 | −2.22 | 26 | 0.06 | K.YSTSQVSSLKEDSDVK.E | |
| 536908 | 630 | 647 | 904.4321 | 1806.8497 | 1806.8537 | −2.2 | 55 | 0.00013 | K.AAPTSNFTVGSDGPPSFR.S | |
| 241596 | 700 | 710 | 646.8446 | 1291.6747 | 1291.6772 | −1.98 | 59 | 0.00026 | K.KLDQLFGTDQK.S | |
| 242470 | 700 | 710 | 647.3521 | 1292.6897 | 1291.6772 | 784 | 35 | 0.013 | K.KLDQLFGTDQK.S | |
| 241600 | 700 | 710 | 646.8446 | 1291.6747 | 1291.6772 | −1.93 | 32 | 0.04 | K.KLDQLFGTDQK.S | |
| 778132 | 805 | 826 | 819.3732 | 2455.0977 | 2454.1022 | 406 | 65 | 0.000017 | R.APSSSEGWEHQQPATVHGYQMK.S | |
| 778133 | 805 | 826 | 819.3735 | 2455.0987 | 2454.1022 | 406 | 71 | 0.000056 | R.APSSSEGWEHQQPATVHGYQMK.S | |
| 777982 | 805 | 826 | 819.0399 | 2454.0977 | 2454.1022 | −1.84 | 39 | 0.0054 | R.APSSSEGWEHQQPATVHGYQMK.S | |
| 142268 | 835 | 843 | 551.3033 | 1100.592 | 1100.5938 | −1.61 | 37 | 0.065 | K.ERLEALQSR.G | |
| 309469 | 839 | 850 | 698.3436 | 1394.6727 | 1393.6715 | 718 | 23 | 0.096 | E.ALQSRGEIPTSR.S + Phospho S (S) | |
| 461623 | 1269 | 1284 | 828.9051 | 1655.7956 | 1655.8049 | −5.63 | 58 | 0.000044 | R.LSNKPVGMNQDGPGSR.K | |
| 461625 | 1269 | 1284 | 828.9077 | 1655.8009 | 1655.8049 | −2.46 | 38 | 0.0029 | R.LSNKPVGMNQDGPGSR.K | |
| 461621 | 1269 | 1284 | 828.9038 | 1655.7931 | 1655.8049 | −7.18 | 37 | 0.0054 | R.LSNKPVGMNQDGPGSR.K | |
| 461619 | 1269 | 1284 | 552.9373 | 1655.7901 | 1655.8049 | −8.95 | 31 | 0.051 | R.LSNKPVGMNQDGPGSR.K | |
Qiao et al. and Ju et al. genetically assessed EIN2 phosphosites and showed that these are crucial for regulating EIN2 nuclear translocation, but these experiments may not have provided conclusive insight on positions of cleavage. For example, Qiao et al. substituted S645 with alanine (S645A), expressed EIN2S645A-YFP in transgenic plants, observed the translocation of YFP to the nucleus, and found an ET phenotype in the absence of ET treatment. Their results implied that the loss of phosphorylation is a regulatory signal that sends EIN2 to the nucleus. But while the S645A mutation will indeed inhibit phosphorylation at that position, it seems plausible that the mutation could also change the recognition site for the unknown protease that Qiao et al. concluded catalyzed hydrolysis there. Qiao et al. did not test by pMRM for the abundance of peptides with S645A termini, so it remains unknown whether the existence of such peptides were adversely affected. Notwithstanding, it is reasonable to suspect that the S645A mutation may have not inhibited potential upstream cleavage positions (evidenced by the larger-than-predicted size of the EIN2 fragment in Fig. 4G in ref. 3). Thus, these genetic experiments supported a functional role of phosphorylation, but did not validate cleavage at S645.
In fact, there are other sites of phosphorylation on EIN2 shown by Chen et al. and Ju et al. that were not fully investigated by Qiao et al. Independent mutations on two different CTR1-regulated phosphosites revealed that ambient-air grown A. thaliana seedlings transgenic for EIN2S645A expressed by the native EIN2 promoter exhibited little ET-response phenotype, whereas EIN2S942A transgenic seedlings exhibited a much stronger phenotype. Since the S645 site retained the potential to be phosphorylated in the EIN2S924A seedlings in ambient air, it is likely that specific cleavage was blocked at S645 under the Qiao et al. model. But because EIN2S924A produced a strong phenotype whereas EIN2S645A did not, cleavage likely occurred elsewhere.
So what explains the strong phenotype for S645A observed by Qiao et al. when the same mutation conferred a weak phenotype for Ju et al.? Transgenic expression and protein accumulation may be the difference. Ju et al. revealed that transgenic seedlings overexpressing wild-type EIN2 from the constitutive CaMV 35S promoter exhibited an unexpected, abnormal, strong ET-response phenotype in ambient air. Consequently, Ju et al. switched to using the native EIN2 promoter. When they did, their EIN2 transgenics more closely resembled nontransgenic wild-type plants. Hence, expression and accumulation differences also likely explain why Ju et al. observed a slight ET phenotype for EIN2S645A transgenic seedlings with the native EIN2 promoter but a stronger ET phenotype for the EIN2S645A transgenic seedlings with a 35S promoter. Therefore, it is possible that the same strong phenotype for the same S645A mutation observed by Qiao et al. may have been due to their use of the 35S promoter as well. In that case, excessive and constant accumulation of EIN2S645A may have short-circuited CTR1 control, preventing phosphorylation at the unexamined S924 site (mimicking S924A in Ju et al.) and leading to an inadvertent but stronger phenotype that masked the weaker effect of S645A.
The results from four papers reveal that differential phosphorylation of EIN2 controls EIN2-mediated activation of transcription at the nucleus and leads to ET-regulated proteomic changes.3-6 Nevertheless, on the basis of the conflicting evidence, specific cleavage at S645 is controversial.
Glossary
Abbreviations:
- ER
endoplasmic reticulum
- ET
ethylene
- a.a.
amino acid
- pMRM
pseudo-multiple reaction monitoring
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24721
References
- 1.Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–41. doi: 10.1016/0092-8674(93)90119-B. [DOI] [PubMed] [Google Scholar]
- 2.Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–52. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 3.Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, et al. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science. 2012;338:390–3. doi: 10.1126/science.1225974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, et al. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 2012;22:1613–6. doi: 10.1038/cr.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA. 2012;109:19486–91. doi: 10.1073/pnas.1214848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R, Binder BM, Garrett WM, Tucker ML, Chang C, Cooper B. Proteomic responses in Arabidopsis thaliana seedlings treated with ethylene. Mol Biosyst. 2011;7:2637–50. doi: 10.1039/c1mb05159h. [DOI] [PubMed] [Google Scholar]
- 7.Cooper B, Feng J, Garrett WM. Relative, label-free protein quantitation: spectral counting error statistics from nine replicate MudPIT samples. J Am Soc Mass Spectrom. 2010;21:1534–46. doi: 10.1016/j.jasms.2010.05.001. [DOI] [PubMed] [Google Scholar]


